Elements of an Integrated Phenotyping System for Monitoring Crop Status at Canopy Level
Abstract
:1. Introduction
2. A Review of the Steps Necessary for Collecting Science Quality Spectral Data in the Field
- (1)
- Configure the instrumentation slowly and carefully; focus on every step in the set-up. Test each piece of equipment before beginning the data-collection exercise. Carefully document instrument conditions (orientation, environment, etc.) and settings either manually or, if able, automatically as part of the data record.
- (2)
- Begin collecting data no earlier than 2.5 h before solar noon; stop no later than 2.5 h after local solar noon.
- (3)
- Clear skies are best for collecting spectral data, especially if one is using a single field radiometer and calibrating with a bright-white reference surface. It is sometimes necessary to collect data under diffuse conditions, but do so only when you are using a spectroradiometer system (or systems) that allow for the simultaneous measurement of the downwelling (incident) irradiance and the upwelling radiance from the target of interest. You may be able to execute target scans during periods when the overhead sky is partly cloudy by waiting for intervals when the direct-beam conditions prevail, but be aware that reflection from bright-white clouds may actually increase the intensity of the incoming radiation.
- (4)
- In the case of acquiring spectral data over a vegetated surface from a nadir position (the usual approach), orient the field radiometer so that you have a field of view at the top of the canopy of at least one meter (and more is generally better). It is important to collect spectral data from an area large enough to be truly representative of the target vegetation, and not just one plant, or a portion of one plant. In fact, if the fraction of vegetative cover is not 100% and the sensor field of view is inadequate, you run the risk of scanning the soil background, and even measuring no vegetation at all. Therefore, it is imperative that one measure the exact distance from the top of the vegetation canopy to the sensor optic, and calculate the precise field of view at the top of the target location.
- (5)
- With regard to the use of a single field radiometer and a bright-white reference surface for calibration on a clear day, it is advisable is to scan that panel at least once every 20 min. When collecting spectral data when the incoming solar energy is being diffused by clouds from time to time, one must calibrate even more frequently. The goal is to scan both the target (e.g., a vegetation canopy) and the calibration panel under the exact same illumination conditions. The frequency of calibrations can be minimized when using two field radiometers (or some similar derivation), with one looking upward and one looking downward, and operating them concurrently. This arrangement, however, is not a trivial matter, and it requires considerable work to precisely match the two sensors.
- (6)
- A bright-white (nearly 100% and diffuse) reference surface should be used to calibrate when making spectral measurements of vegetation. The reference panel should be in a level position during scanning, and one should watch for and eliminate shadows which may be cast on the surface of the panel. One may have to either increase or decrease the distance between the sensor optic and the panel itself to avoid shadowing. Where possible, attach the calibration panel to a tripod, and once it is positioned beneath the sensor optic, step away at least 5 m during scanning. If the calibration panel must be held by an individual, instruct that person to hold the panel level above his/her head. In any case, be sure the exact same procedure is followed with every calibration scan; this includes people standing nearby being in the same position each time. Keep the calibration surface clean, but be sure that the procedures for cleaning are well understood and executed properly.
- (7)
- As noted above, the sensor optic should generally be positioned at nadir above the target of interest. Use a level to verify the orientation of the instrument. Sometimes, circumstances may warrant off-nadir scanning, but if done in this way, be sure to measure the angle of view and record it. To be absolutely correct, the sensor, when attached to a tripod or any type of vehicle-mounted boom, should be pointed in the principal plane of the sun. To accomplish this, however, the operator will need solar-ephemeris data for the study location, and the orientation of the boom must be changed frequently during the field campaign. A simple, and usually acceptable, approach is to point the boom due-south (appropriate for the Northern hemisphere) at all times during scanning.
- (8)
- Other recommendations include replicating each spectral scan numerous times and taking the average. Note that the sensor should be at least 5 m away from nearby objects (e.g., people, trucks, etc.) during scanning to avoid extraneous reflections emanating from those objects. Check the quality of your data from time to time during the period of data collection. Whenever possible, keep expensive equipment (e.g., controllers, computers, etc.) out of direct sunlight. Handle all equipment with care.
3. Data-Collection Platforms and Instruments
3.1. Sensor Deployment and Operation over Vegetation Canopies: A Basic Procedural Philosophy

3.2. Acquiring Spectral-Reflectance Data at Canopy and Plant Community Levels: The Human-Transported Backpack Sensor System
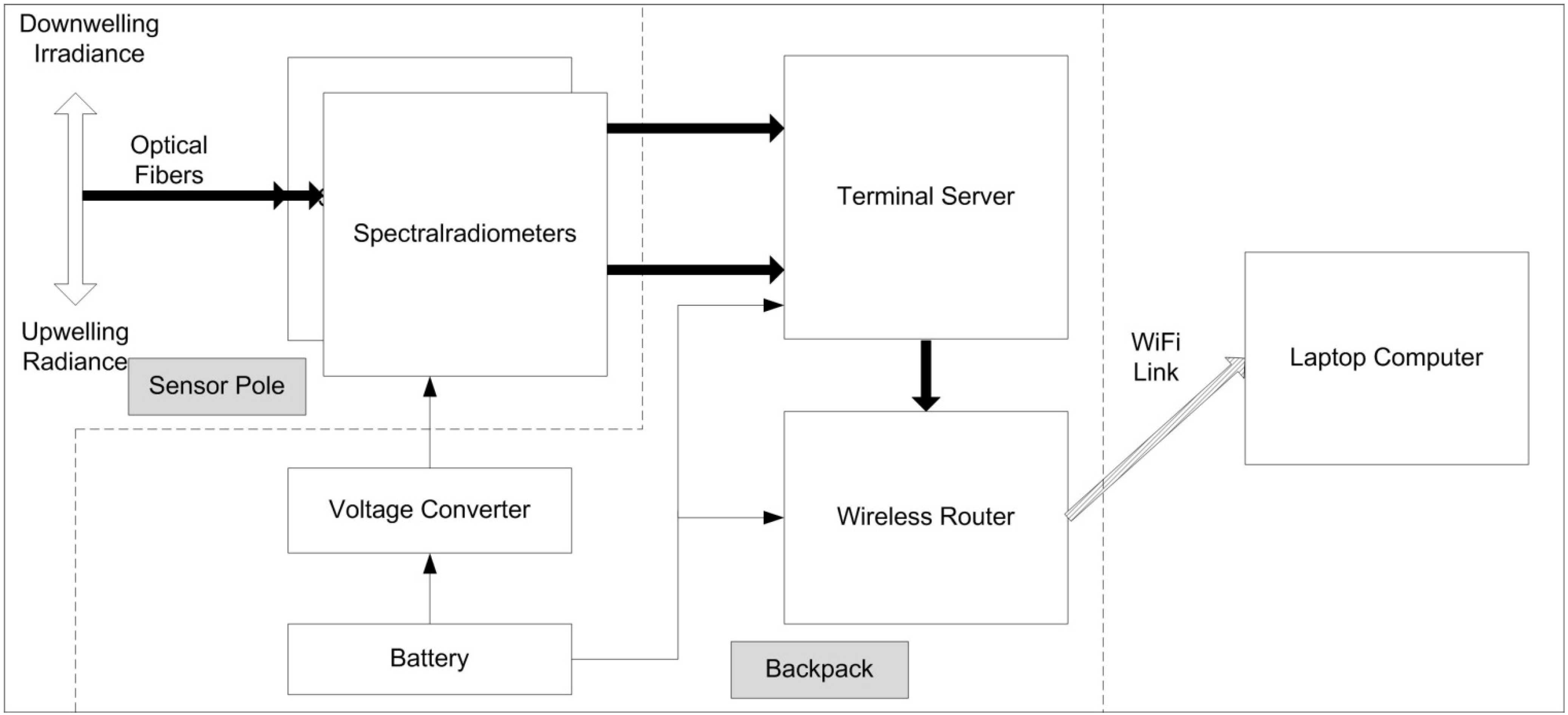

3.3. Acquiring Spectral-Reflectance Data at Canopy and Plant Community Levels: The Hercules Motorized-Vehicle System with Wireless Communications


4. The Data-Collection, Processing, and Display Software
4.1. Equipment Configuration
4.2. Operating—Data Acquisition

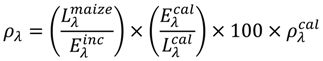
5. Algorithms and Standard Products for Inferring Crop-Biophysical Characteristics
5.1. Vegetation Fraction (VF)
5.2. Fraction of Absorbed Photosynthetically Active Radiation (fAPARgreen)

5.3. Green Leaf Area Index (LAI), Green Leaf Biomass and Chlorophyll Content
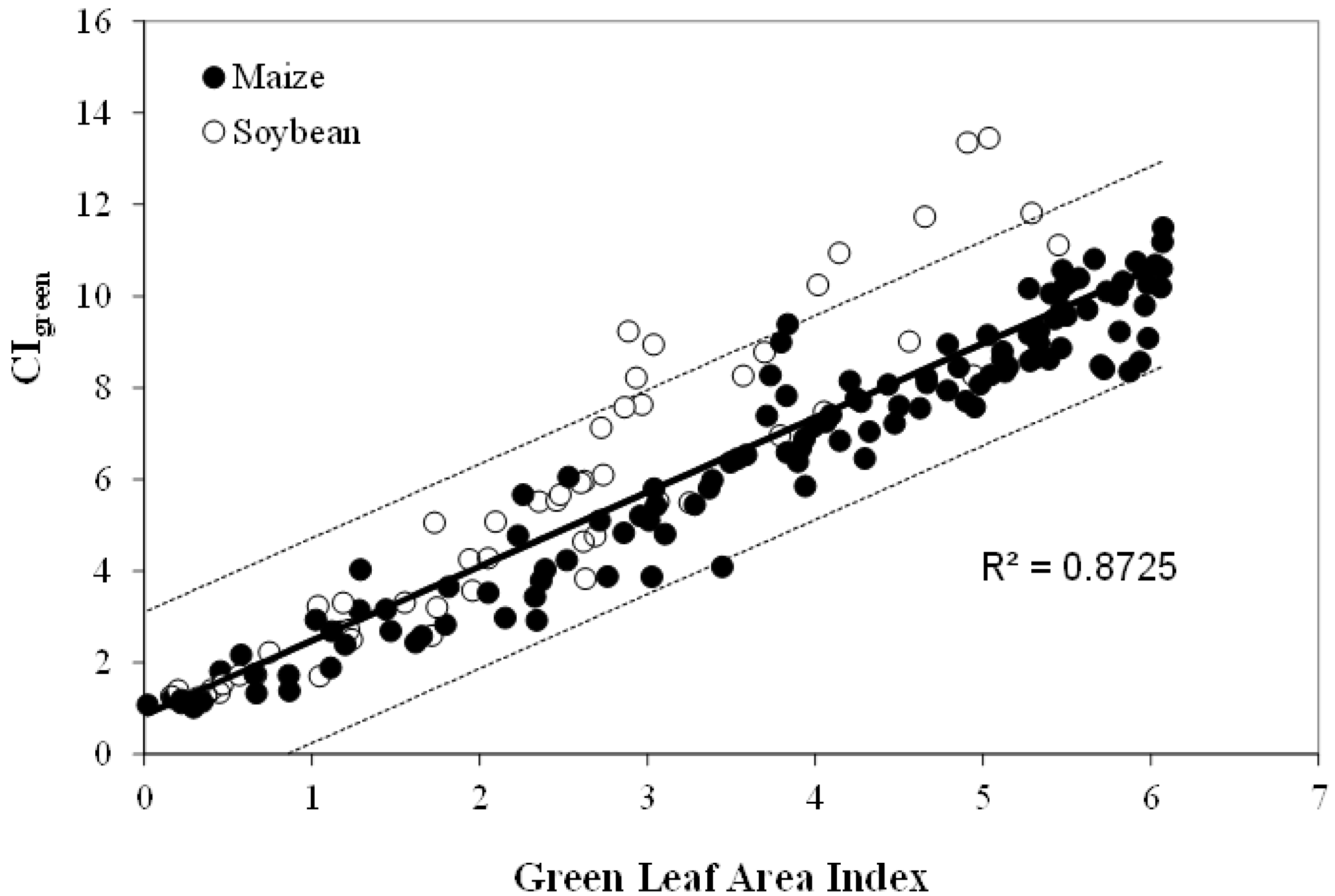
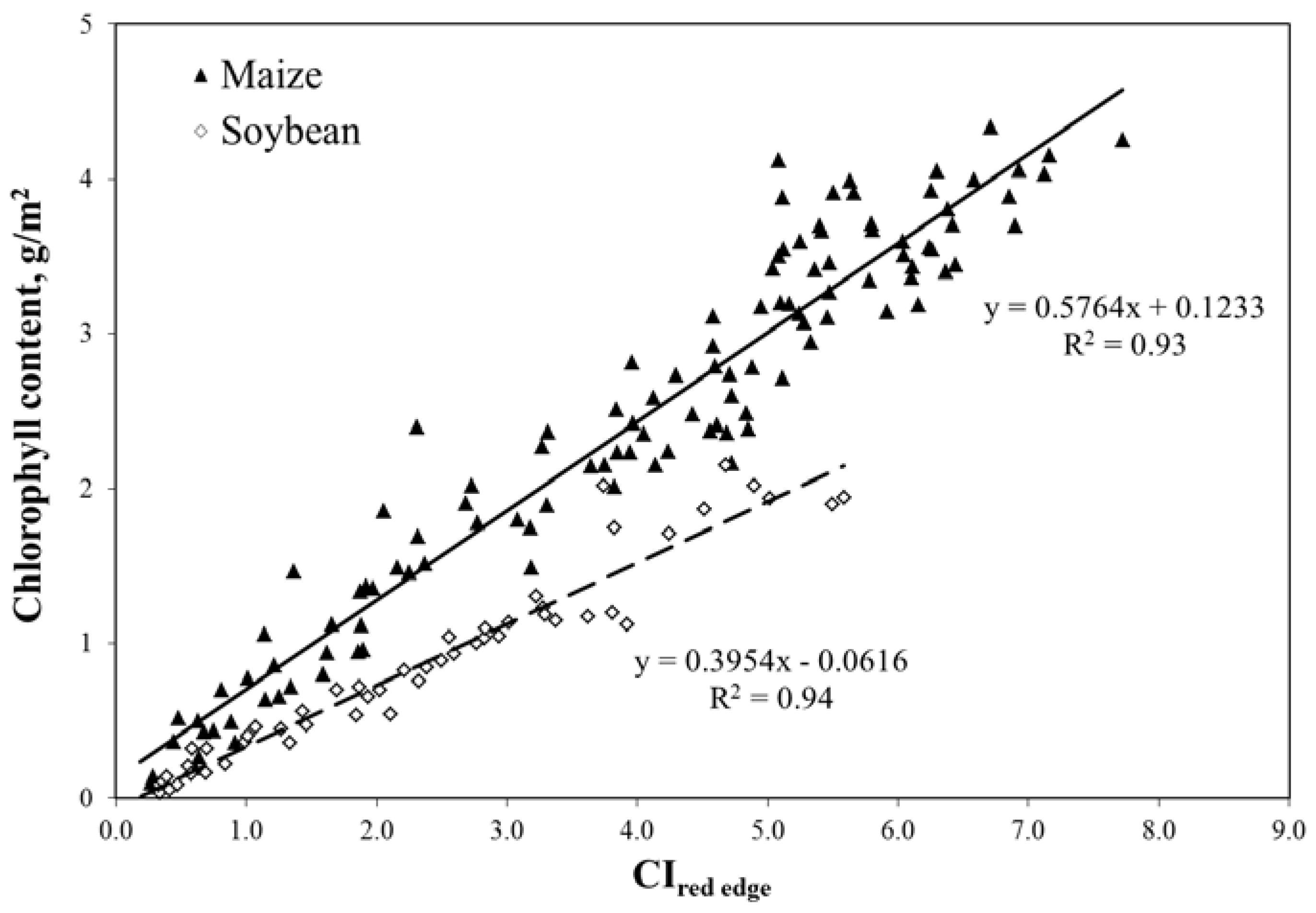
6. Conclusions
Acknowledgements
Conflicts of Interest
References
- Pinter, P., Jr.; Hatfield, J.; Schepers, J.; Barnes, E.; Moran, M.; Daughtry, C.; Upchurch, D. Remote sensing for crop management. Photogramm. Eng. Remote Sens. 2003, 69, 647–664. [Google Scholar] [CrossRef]
- Nicodemus, F.; Richmond, J.; Hsia, J.; Ginsberg, I.; Limperis, T.; Ott, W. Geometrical Considerations and Nomenclature for Reflectance; US Department of Commerce, National Bureau of Standards: Washington, DC, USA, 1977. [Google Scholar]
- Duggin, M. Simultaneous measurement of irradiance and reflected radiance in field determination of spectral reflectance. Appl. Opt. 2005, 20, 3816–3818. [Google Scholar] [CrossRef]
- Duggin, M.; Philipson, W. Field measurement of reflectance: Some major considerations. Appl. Opt. 1982, 21, 2833–2840. [Google Scholar] [CrossRef]
- Milton, E. Does the use of two radiometers correct for irradiance changes during measurements. Photogramm. Eng. Remote Sens. 1981, 47, 1223–1225. [Google Scholar]
- Milton, E.; Schaepman, M.; Anderson, K.; Kneubühler, M.; Fox, N. Progress in field spectroscopy. Remote Sens. Environ. 2009, 113, S92–S109. [Google Scholar] [CrossRef]
- Milton, E. Principles of field spectroscopy. Int. J. Remote Sens. 1987, 8, 1807–1827. [Google Scholar] [CrossRef]
- Rundquist, D.; Perk, R.; Leavitt, B.; Keydan, G.; Gitelson, A. Collecting spectral data over cropland vegetation using machine positioning versus hand-held positioning of the sensor. Comput. Electron. Agric. 2004, 43, 173–178. [Google Scholar] [CrossRef]
- Anderson, K.; Milton, E.; Rollin, E. Calibration of dual-beam spectroradiometric data. Int. J. Remote Sens. 2006, 27, 975–986. [Google Scholar] [CrossRef]
- Dall’Olmo, G.; Gitelson, A.A. Effect of bio-optical parameter variability on the remote estimation of chlorophyll-a concentration in turbid productive waters: Experimental results. Appl. Opt. 2005, 44, 412–422. [Google Scholar] [CrossRef]
- Meyer, G.E.; Hindman, T.; Lakshmi, K. Machine Detection Parameters for Plant Species Identification. In Precision Agriculture and Biological Quality; Meyer, G.E., DeShazer, J.A., Eds.; The International Society for Optical Engineering: Boston, MA, USA, 1998; pp. 327–335. [Google Scholar]
- Vina, A.; Gitelson, A.; Rundquist, D.; Keydan, G.; Leavitt, B.; Schepers, J. Monitoring maize (Zea mays L.) phenology with remote sensing. Agron. J. 2004, 96, 1139–1147. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Verma, S.B.; Rundquist, D.C.; Arkebauer, T.J.; Keydan, G.; Leavitt, B.; Ciganda, V.; Burba, G.G.; Suyker, A.E. Relationship between gross primary production and chlorophyll content in crops: Implications for the synoptic monitoring of vegetation productivity. J. Geophys. Res. 2006, 111, D08S11. [Google Scholar] [CrossRef]
- Steele, M.; Gitelson, A.A.; Rundquist, D. Non-destructive estimation of leaf chlorophyll content in grapes. Am. J. Enol. Vitic. 2008, 59, 299–305. [Google Scholar]
- Steele, M.R.; Gitelson, A.A.; Rundquist, D. Non-destructive estimation of anthocyanin content in grapevine leaves. Am. J. Enol. Vitic. 2008, 60, 87–92. [Google Scholar]
- Hall, F.G.; Huemmrich, K.F.; Goetz, S.J.; Sellers, P.J.; Nickeson, J.E. Satellite remote sensing of surface energy balance: Success, failures and unresolved issues in FIFE. J. Geophys. Res. 1992, 97, 19061–19089. [Google Scholar] [CrossRef]
- Gitelson, A.; Kaufman, Y.; Stark, R.; Rundquist, D. Novel algorithms for remote estimation of vegetation fraction. Remote Sens. Environ. 2002, 80, 76–87. [Google Scholar] [CrossRef]
- Viña, A.; Gitelson, A.A. New developments in the remote estimation of the fraction of absorbed photosynthetically active radiation in crops. Geophys. Res. Lett. 2005, 32, L17403. [Google Scholar] [CrossRef]
- Gitelson, A.; Kaufman, Y.; Merzlyak, M. Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Gitelson, A.A. Wide dynamic range vegetation index for remote quantification of crop biophysical characteristics. J. Plant Physiol. 2004, 161, 165–173. [Google Scholar] [CrossRef]
- Viña, A.; Gitelson, A.A.; Nguy-Robertson, A.L.; Peng, Y. Comparison of different vegetation indices for the remote assessment of green leaf area index of crops. Remote Sens. Environ. 2011, 115, 3468–3478. [Google Scholar] [CrossRef]
- Gitelson, A.; Vina, A.; Arkebauer, T.; Rundquist, D.; Keydan, G.; Leavitt, B. Remote estimation of leaf area index and green leaf biomass in maize canopies. Geophys. Res. Lett. 2003, 30, 1148. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Rundquist, D.C.; Ciganda, V.; Arkebauer, T.J. Remote estimation of canopy chlorophyll content in crops. Geophys. Res. Lett. 2005, 32, L08403. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Keydan, G.P.; Merzlyak, M.N. Three-band model for noninvasive estimation of chlorophyll carotenoids and anthocyanin contents in higher plant leaves. Geophys. Res. Lett. 2006, 33, L11402. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rundquist, D.; Gitelson, A.; Leavitt, B.; Zygielbaum, A.; Perk, R.; Keydan, G. Elements of an Integrated Phenotyping System for Monitoring Crop Status at Canopy Level. Agronomy 2014, 4, 108-123. https://doi.org/10.3390/agronomy4010108
Rundquist D, Gitelson A, Leavitt B, Zygielbaum A, Perk R, Keydan G. Elements of an Integrated Phenotyping System for Monitoring Crop Status at Canopy Level. Agronomy. 2014; 4(1):108-123. https://doi.org/10.3390/agronomy4010108
Chicago/Turabian StyleRundquist, Donald, Anatoly Gitelson, Bryan Leavitt, Arthur Zygielbaum, Richard Perk, and Galina Keydan. 2014. "Elements of an Integrated Phenotyping System for Monitoring Crop Status at Canopy Level" Agronomy 4, no. 1: 108-123. https://doi.org/10.3390/agronomy4010108
APA StyleRundquist, D., Gitelson, A., Leavitt, B., Zygielbaum, A., Perk, R., & Keydan, G. (2014). Elements of an Integrated Phenotyping System for Monitoring Crop Status at Canopy Level. Agronomy, 4(1), 108-123. https://doi.org/10.3390/agronomy4010108





