Identification of Water Stress in Citrus Leaves Using Sensing Technologies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Water Stress Monitoring and Characterization
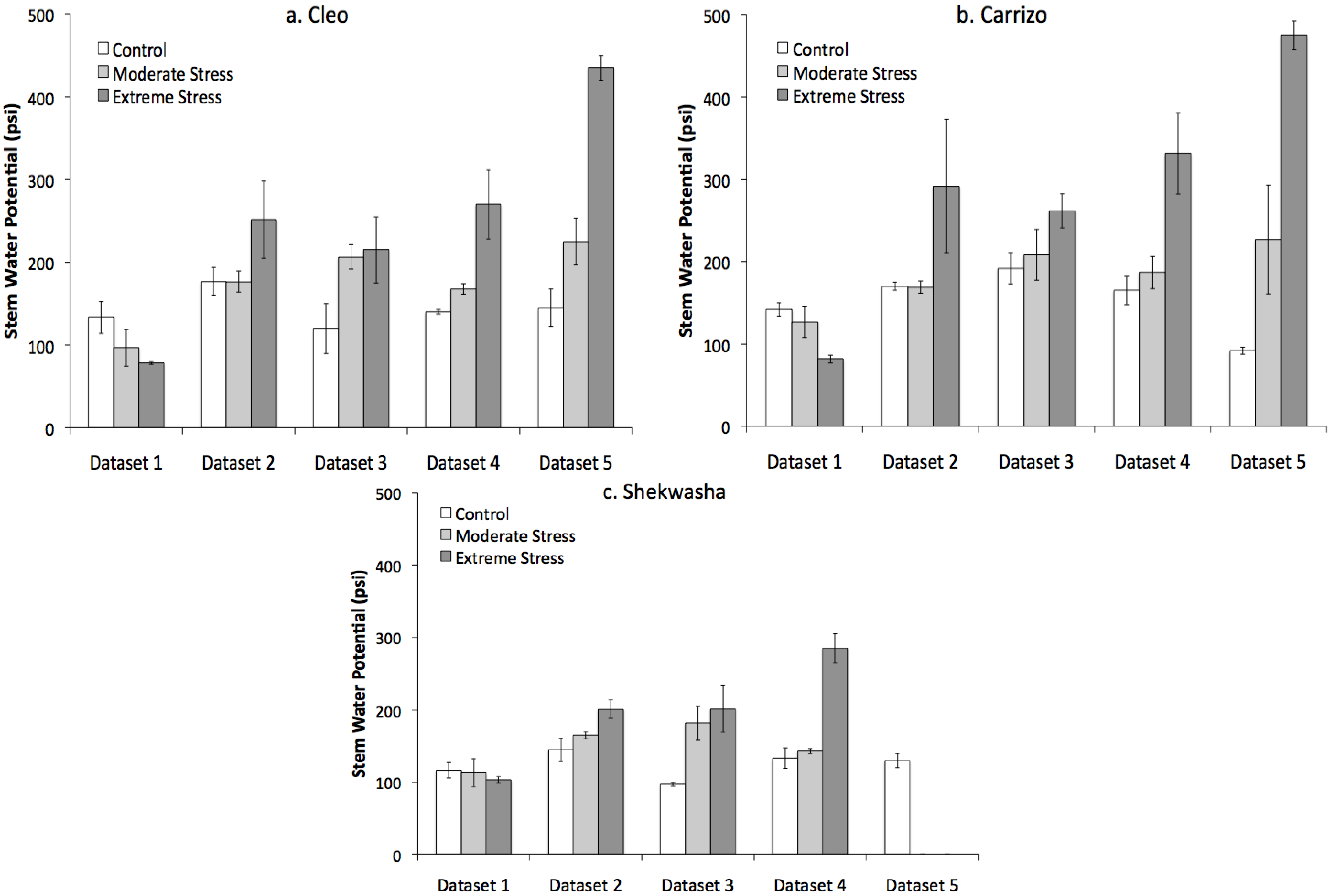

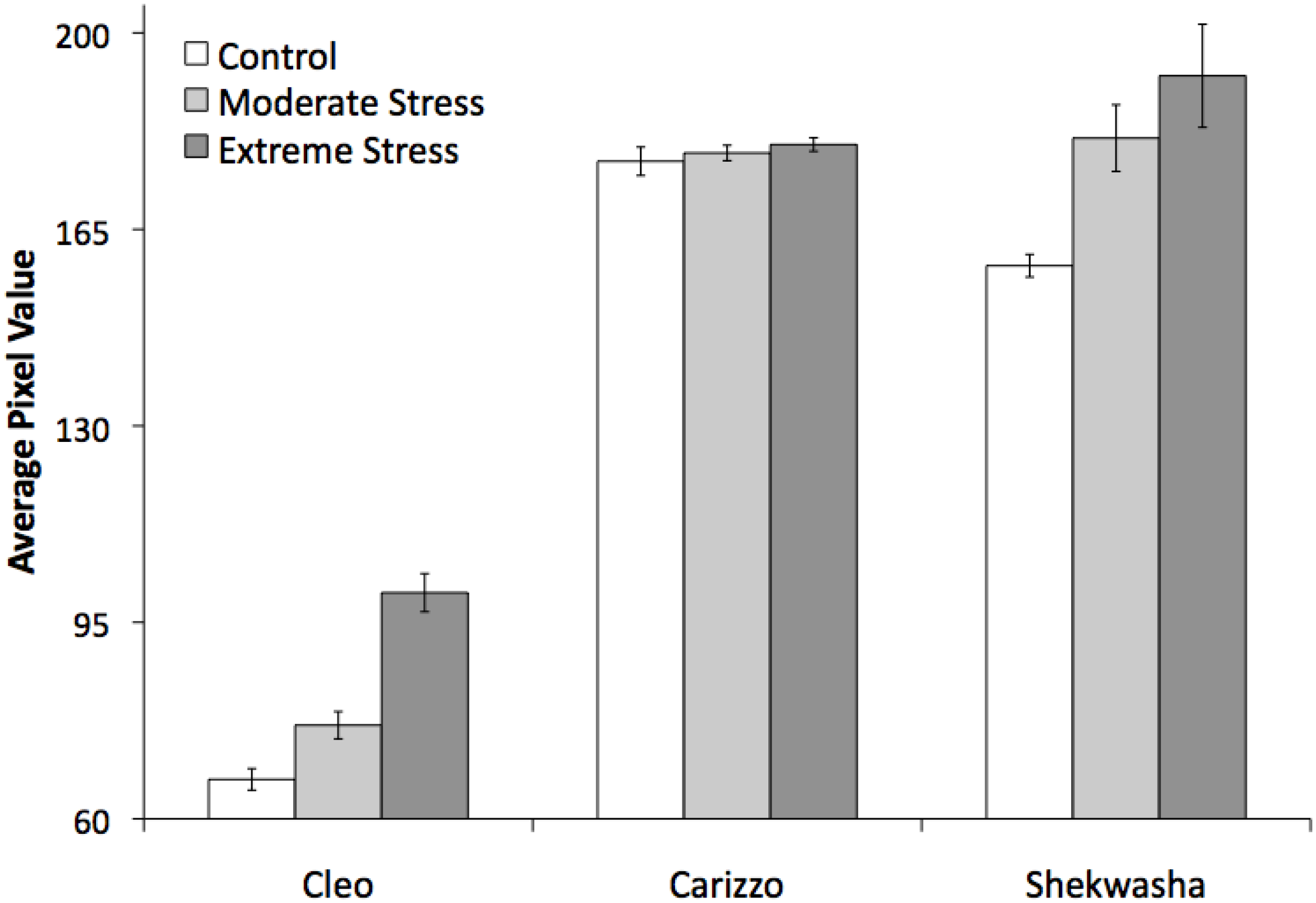
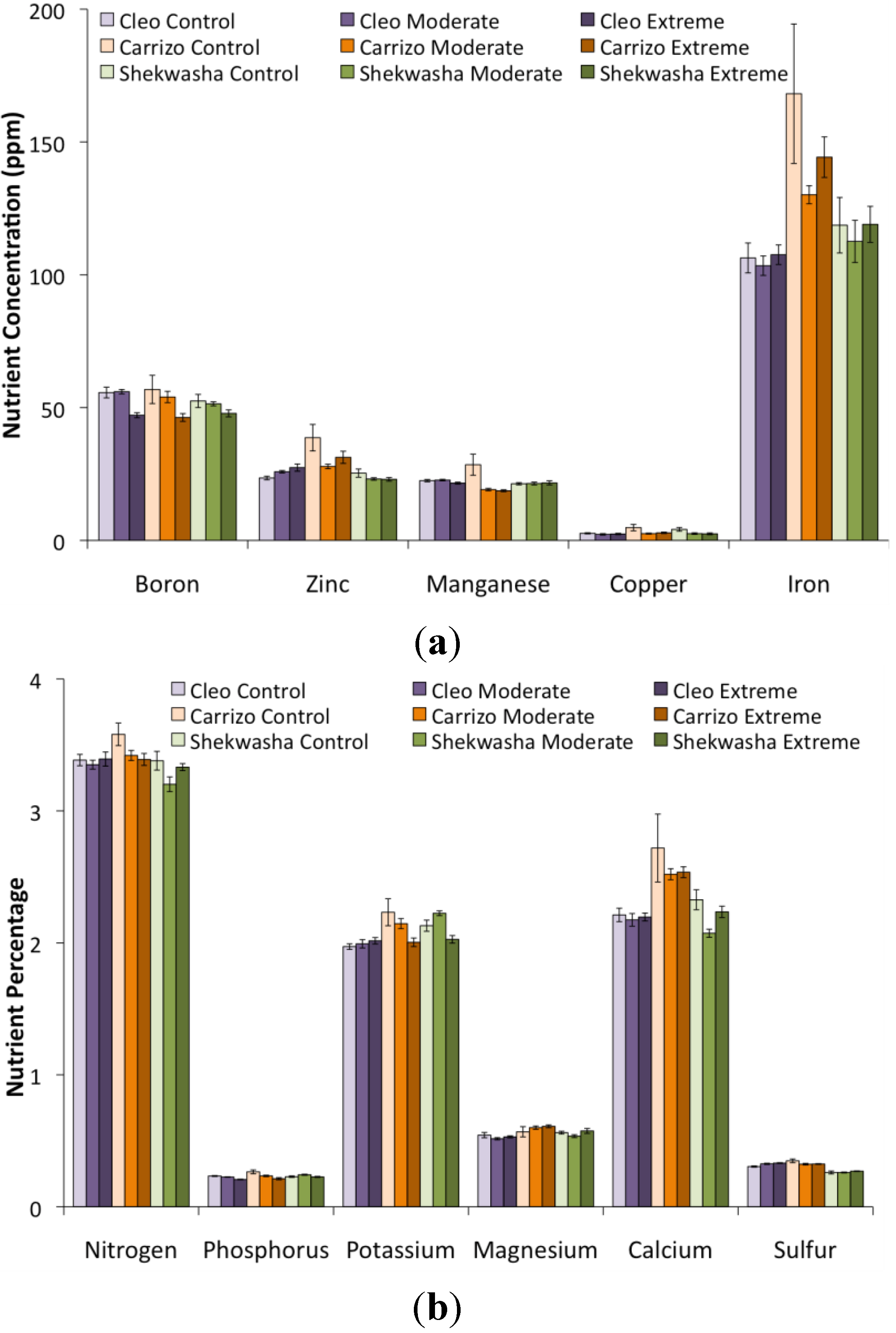

2.2. LIBS and VNIR Classification
| Dataset | Cleo | Carrizo | Shekwasha | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LDA | NB | BDT | LDA | NB | BDT | LDA | NB | BDT | |
| 1 | 67 | 58 | 67 | 20 | 0 | 10 | 36 | 27 | 36 |
| 2 | 50 | 50 | 26 | 11 | 33 | 22 | 44 | 22 | 22 |
| 3 | 60 | 60 | 70 | 22 | 22 | 44 | 56 | 56 | 78 |
| 4 | 70 | 70 | 60 | 70 | 80 | 80 | 70 | 80 | 80 |
| 5 | 45 | 30 | 50 | 60 | 50 | 60 | 35 | 35 | 30 |
| Dataset | Cleo | Carrizo | Shekwasha | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LDA | NB | BDT | LDA | NB | BDT | LDA | NB | BDT | |
| 1 | 47 | 60 | 67 | 40 | 33 | 60 | 67 | 80 | 73 |
| 2 | 40 | 60 | 67 | 40 | 60 | 67 | 64 | 86 | 86 |
| 3 | 36 | 43 | 57 | 47 | 60 | 67 | 71 | 57 | 79 |
| 4 | 67 | 47 | 73 | 33 | 73 | 67 | 86 | 64 | 71 |
| 5 | 67 | 77 | 100 | 57 | 63 | 93 | 58 | 73 | 97 |
3. Experimental Section
3.1. Citrus Rootstock Seedlings
3.2. Sensors and Data Collection
| Dataset | Sampling Date | LIBS | VNIR |
|---|---|---|---|
| 1 | Week 1 | 142 | 185 |
| 2 | Week 2 | 117 | 177 |
| 3 | Week 4 | 116 | 180 |
| 4 | Week 5 | 118 | 177 |
| 5 | Week 8 | 120 | 180 |
3.3. Data Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ballester, C.; Castel, J.; Intrigliolo, D.D.; Castel, J.R. Response of clementina de nules citrus trees to summer deficit irrigation yield components and fruit composition. Agric. Water Manag. 2011, 98, 1027–1032. [Google Scholar] [CrossRef]
- Radziemski, L.; Cremers, D. A brief history of laser-induced breakdown spectroscopy: From the concept of atoms to LIBS 2012. Spectrochim. Acta Part B 2013, 87, 3–10. [Google Scholar] [CrossRef]
- Trevizan, L.C.; Santos, D., Jr.; Samad, R.E.; Vieira, N.D., Jr.; Nomura, C.S.; Nunes, L.C.; Rufini, I.A.; Krug, F.J. Evaluation of laser induced spectroscopy for the determination of macronutrients in plant materials. Spectrochim. Acta Part B 2008, 63, 1151–1158. [Google Scholar] [CrossRef]
- Trevizan, L.C.; Santos, D., Jr.; Samad, R.E.; Nunes, L.C.; Rufini, I.A.; Krug, F.J. Evaluation of laser induced spectroscopy for the determination of micronutrients in plant materials. Spectrochim. Acta Part B 2009, 64, 369–377. [Google Scholar] [CrossRef]
- Kim, G.; Kwak, J.; Choi, J.; Park, K. Detection of nutrient elements and contamination by pesticides in spinach and rice samples using Laser-Induced Breakdown Spectroscopy (LIBS). J. Agric. Food Chem. 2012, 60, 718–724. [Google Scholar] [CrossRef]
- Yao, M.; Huang, L.; Zheng, J.; Fan, S.; Liu, M. Assessment of feasibility in determining of Cr in gannan navel orange treated in controlled conditions by Laser Induced Breakdown Spectroscopy. Opt. Laser Technol. 2013, 52, 70–74. [Google Scholar] [CrossRef]
- Chapin, F.S., III. Integrated response of plants to stress. Bioscience 1991, 41, 29–36. [Google Scholar] [CrossRef]
- Carter, G.A. Primary and secondary effects of water content on the spectral reflectance of leaves. Am. J. Bot. 1991, 78, 916–924. [Google Scholar] [CrossRef]
- Kriston-Vizi, J.; Umeda, M.; Miyamoto, K. Assessment of water status of mandarin and peach canopies using visible multispectral imagery. Precis. Agric. 2008, 100, 338–345. [Google Scholar]
- Pinter, P.J., Jr.; Hatfield, J.L.; Schepers, J.S.; Barnes, E.M.; Moran, M.S.; Daughtry, C.S.T.; Upchurch, D.R. Remote sensing for crop management. Photogramm. Eng. Rem. Sens. 2003, 69, 647–664. [Google Scholar]
- Ballester, C.; Castel, J.; Jimenez-Bello, M.A.; Castel, J.R.; Intrigliolo, D.S. Thermographic measurement of canopy temperature is a useful tool for predicting water deficit effects on fruit weight in citrus trees. Agric. Water Manag. 2013, 122, 1–6. [Google Scholar] [CrossRef]
- Garcia-Sanchez, F.; Syvertsen, J.P.; Gimeno, V.; Botia, P.; Perez-Perez, J.G. Responses to flooding and drought stress by two citrus rootstock seedlings with different water-use efficiency. Physiol. Plant. 2007, 130, 532–542. [Google Scholar] [CrossRef]
- Slaton, M.R.; Hunt, E.R., Jr.; Smith, W.K. Estimating near-infrared leaf reflectance from leaf structural characteristics. Am. J. Bot. 2001, 88, 278–284. [Google Scholar] [CrossRef]
- Usha, K.; Singh, B. Potential applications of remote sensing in horticulture. Sci. Hortic.-Amst. 2013, 153, 71–83. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Johnson, K.; Sankaran, S.; Ehsani, R. Identification of Water Stress in Citrus Leaves Using Sensing Technologies. Agronomy 2013, 3, 747-756. https://doi.org/10.3390/agronomy3040747
Johnson K, Sankaran S, Ehsani R. Identification of Water Stress in Citrus Leaves Using Sensing Technologies. Agronomy. 2013; 3(4):747-756. https://doi.org/10.3390/agronomy3040747
Chicago/Turabian StyleJohnson, Kaitlin, Sindhuja Sankaran, and Reza Ehsani. 2013. "Identification of Water Stress in Citrus Leaves Using Sensing Technologies" Agronomy 3, no. 4: 747-756. https://doi.org/10.3390/agronomy3040747
APA StyleJohnson, K., Sankaran, S., & Ehsani, R. (2013). Identification of Water Stress in Citrus Leaves Using Sensing Technologies. Agronomy, 3(4), 747-756. https://doi.org/10.3390/agronomy3040747





