Measuring Maize Seedling Drought Response in Search of Tolerant Germplasm
Abstract
:1. Introduction
2. Results and Discussion
2.1. Seedling Germination
| Reps | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Mean | |
| Inbred | Germination % | 58 | 80 | 92 | 88 | 86 | 92 | -- | -- | 83 |
| Overall Survival % | 2 | 2 | 0 | 0 | 0 | 0 | -- | -- | 1 | |
| End Date | 4/5 | 4/5 | 5/13 | 5/13 | 5/13 | 5/13 | -- | -- | -- | |
| Hybrid | Germination % | 90 | 88 | 90 | 91 | 88 | 82 | 85 | 81 | 87 |
| Overall Survival % | 0 | 0 | 1 | 3 | 1 | 3 | 4 | 2 | 2 | |
| End Date | 2/17 | 2/17 | 2/17 | 2/17 | 3/17 | 3/17 | 3/17 | 3/17 | -- |
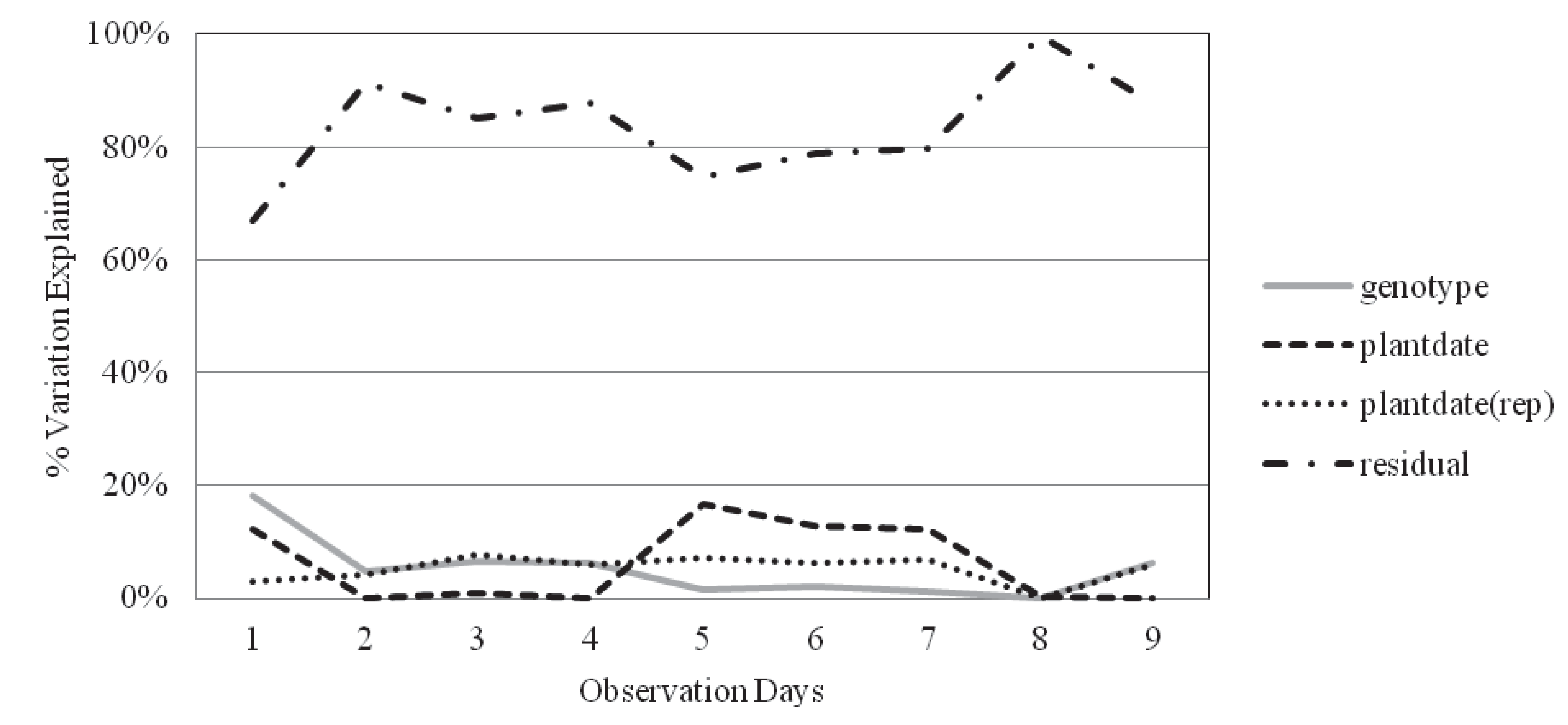
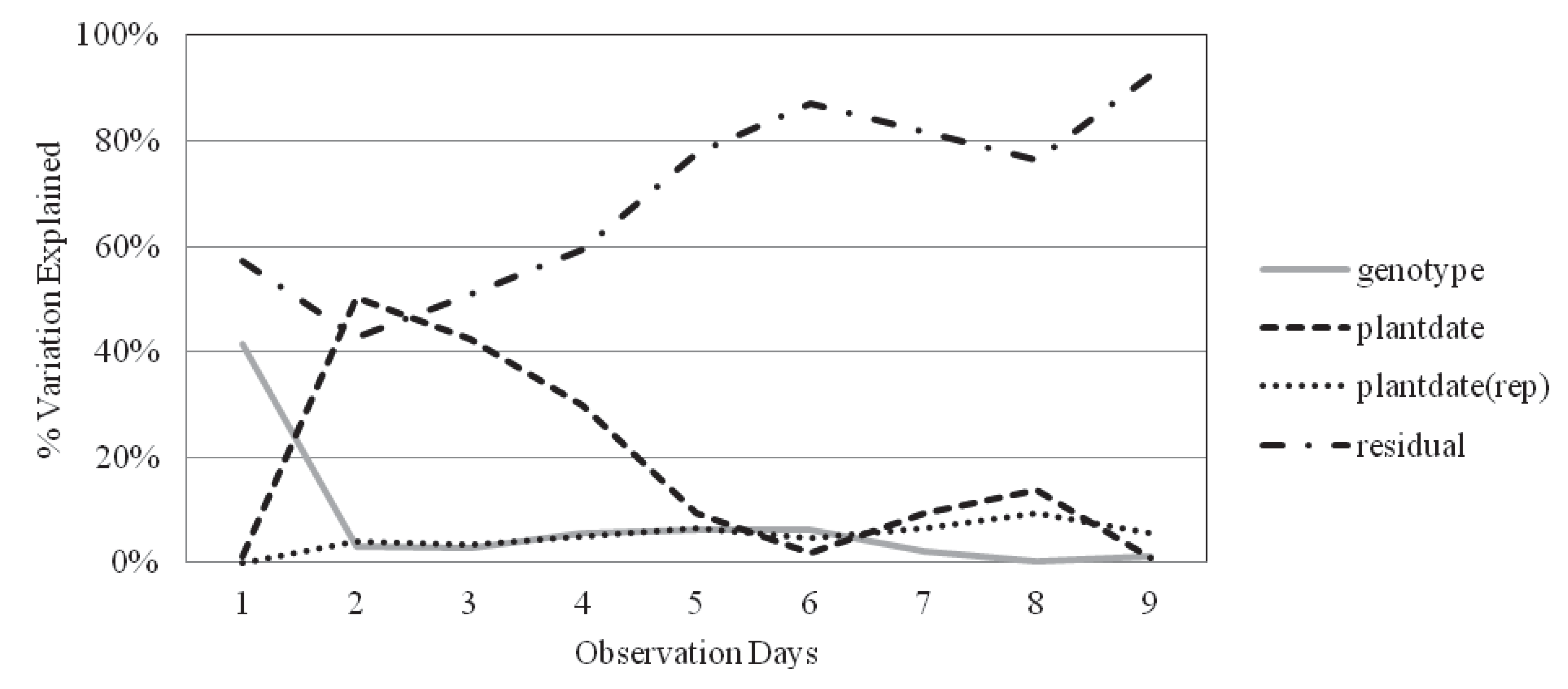
2.2. Survival and Recovery
| Obs. day | Genotype | Plantdate | Plantdate(rep) | Residual error | H2 | |
|---|---|---|---|---|---|---|
| Inbred | 3 | 6.56% | 0.80% | 7.68% | 84.96% | 0.32 |
| Hybrid | 6 | 6.25% | 1.99% | 4.79% | 86.98% | 0.37 |
| Genotype | Pedigree | % Survival |
|---|---|---|
| 1 | ((B104/CML285-B-2-B-B-B-B-B) | 53 |
| 7 | ((B104/NC300)-B-1-B-1-B-B-B-B) | 53 |
| 23 | (CML78 × CML269-B-B-B-B-B)) | 54 |
| Mean | 29 |
| Genotype | Inbred pedigree testcrossed to LH195 × LH287 | % Recovery | Significance |
|---|---|---|---|
| 2 | Tx770/CML288-B-3-B-B-B-B-B | 29 | * |
| 13 | (Tx601 × B104-B/FR2128 × Unknown)-2-2-B-B-B-B-B | 25 | * |
| 18 | ((Tx772 × T246) × Tx772)-1-5-B-B-B-B-B-B | 28 | * |
| 28 | CML269/TX130-B-B-B-1-3-B-B-B-B-B | 25 | * |
| 31 | Ethiopia12-B-3-3-B-B2-B1-2-B(blue) | 44 | *** |
| 38 | CML325-B-B-B-B-B-B-B-B-B | 25 | * |
| 39 | NC280-B-B-B-B-B-B | 26 | * |
| 42 | B73-B-B-B-B-B-B-B-B | 28 | * |
| 47 | Tx760-B-B-B-B-B-B-B | 34 | ** |
| 50 | FR2128-B-B-B-B-B-B-B | 25 | * |
| 54 | S2B73BC | 25 | * |
| 57 | C3S1B73-3-3 | 29 | * |
| 58 | C3S1B73-3-1 | 36 | ** |
| 61 | Tx204 | 24 | * |
| Mean | 16 |
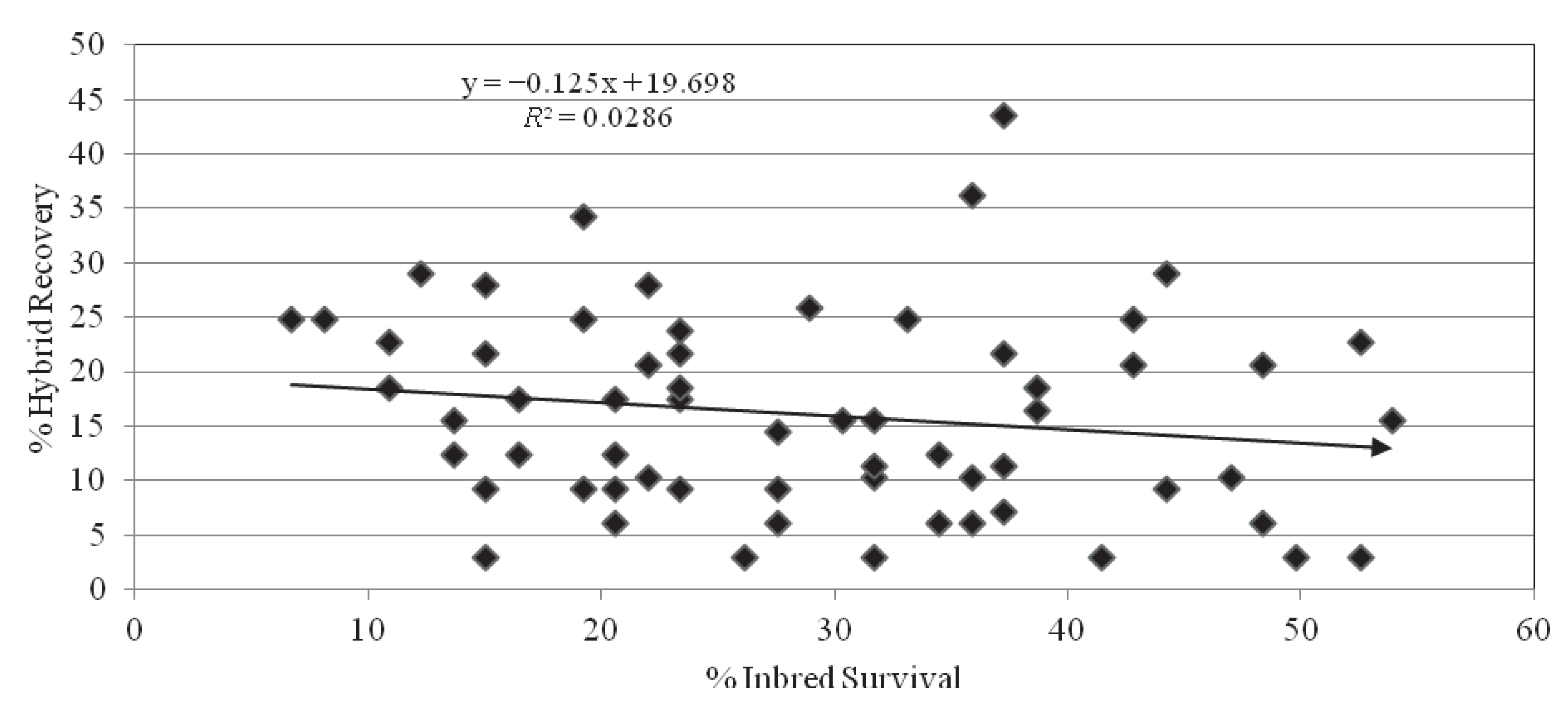
2.5. Inbred-Hybrid Correlation
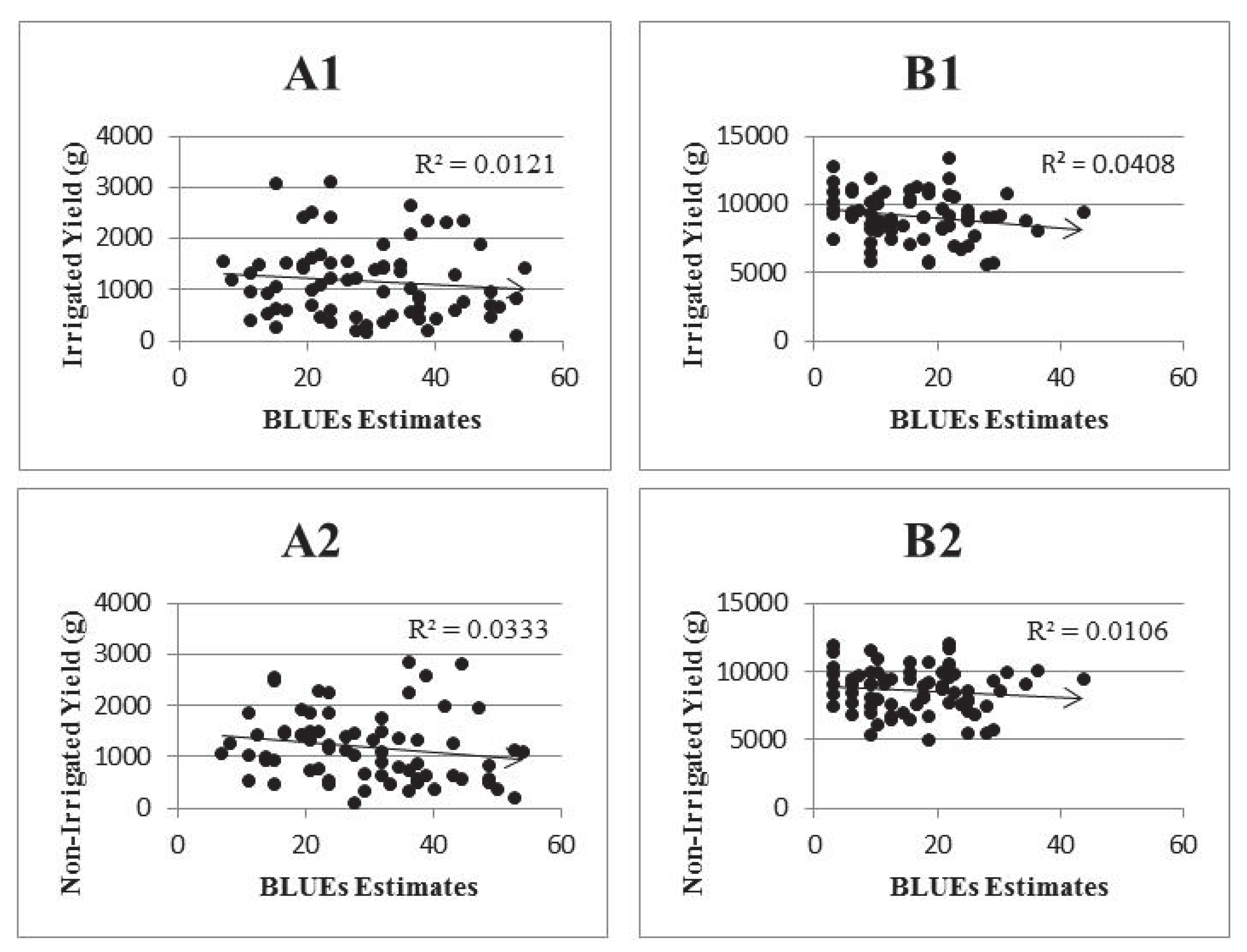
2.6. Correlation with Field Results
| r | R2 | Upper 95% | Lower 95% | P-value | |
|---|---|---|---|---|---|
| Inbred | −0.15 | 0.02 | −0.37 | 0.09 | 0.21 |
| Hybrid | −0.17 | 0.03 | −0.39 | 0.07 | 0.19 |
3. Experimental Section
3.1. Germplasm
3.2. Experimental Design
| Year (Plant Type) | Replication | Planting Date | 1st Irrigation | 2nd Irrigation |
|---|---|---|---|---|
| 2009 (Inbred) | 1 & 2 | 3/12 | 3/22 | 3/29 |
| 3, 4, 5 & 6 | 4/17 | 4/27 | 5/2 | |
| 2010 (Hybrid) | 1, 2, 3 & 4 | 1/22 | 1/30 | 2/7 |
| 5, 6, 7 & 8 | 2/19 | 2/27 | 3/5 |
3.2. Germination Percentage
3.3. Survival and Recovery Percentages
3.4. Field Study
3.5. Statistical Analysis
4. Conclusions
Conflict of Interest
Acknowledgments
References
- Battisti, D.S.; Naylor, R.L. Historical warnings of future food insecurity with unprecedented seasonal heat. Science 2009, 323, 240–244. [Google Scholar] [CrossRef]
- Lobell, D.B.; Schlenker, W.; Costa-Roberts, J. Climate trends and global crop production since 1980. Science 2011, 333, 616. [Google Scholar] [CrossRef]
- Mishra, V.; Cherkauer, K.A. Retrospective droughts in the crop growing season: Implications to corn and soybean yield in the midwestern united states. Agric. For. Meteorol. 2010, 150, 1030–1045. [Google Scholar] [CrossRef]
- Campos, H.; Cooper, M.; Habben, J.; Edmeades, G.; Schussler, J. Improving drought tolerance in maize: A view from industry. Field Crops Res. 2004, 90, 19–34. [Google Scholar] [CrossRef]
- Ribaut, J.M.; Betran, J.; Monneveux, P.; Setter, T. Drought tolerance in maize. In Handbook of Maize: Its Biology; Bennetzen, J.L., Hake, S.C., Eds.; Springer: New York, NY, USA, 2009; pp. 311–344. [Google Scholar]
- Richards, R. Defining selection criteria to improve yield under drought. Plant Growth Regul. 1996, 20, 157–166. [Google Scholar] [CrossRef]
- Obeng-Bio, E.; Bonsu, M.; Obeng-Antwi, K.; Akromah, R. Establishing the basis for drought tolerance in maize (Zea mays l.) using some secondary traits in the field. Afr. J. Plant Sci. 2011, 5, 702–709. [Google Scholar]
- Bruce, W.B.; Edmeades, G.O.; Barker, T.C. Molecular and physiological approaches to maize improvement for drought tolerance. J. Exp. Bot. 2002, 53, 13–25. [Google Scholar] [CrossRef]
- Betran, F.; Beck, D.; Bänziger, M.; Edmeades, G. Secondary traits in parental inbreds and hybrids under stress and non-stress environments in tropical maize. Field Crops Res. 2003, 83, 51–65. [Google Scholar] [CrossRef]
- Bolanos, J.; Edmeades, G. The importance of the anthesis-silking interval in breeding for drought tolerance in tropical maize. Field Crops Res. 1996, 48, 65–80. [Google Scholar] [CrossRef]
- Ludlow, M.; Muchow, R. A critical evaluation of traits for improving crop yields in water-limited environments. Adv. Agron. 1990, 43, 107–153. [Google Scholar] [CrossRef]
- Wan, C.; Xu, W.; Sosebee, R.E.; Machado, S.; Archer, T. Hydraulic lift in drought-tolerant and-susceptible maize hybrids. Plant Soil 2000, 219, 117–126. [Google Scholar] [CrossRef]
- Muenchrath, D.A. Morphological and physiological characterization of a desert adapted traditional native american maize (zea mays l.) cultivar. Ph.D. thesis, Iowa State University, Ames, IA, USA, 1995. [Google Scholar]
- Bänziger, M.; Setimela, P.; Hodson, D.; Vivek, B. Breeding for improved drought tolerance in maize adapted to southern africa. Agric. Water Manage. 2006, 80, 212–214. [Google Scholar] [CrossRef]
- Singh, B.; Mai-Kodomi, Y.; Terao, T. A simple screening method for drought tolerance in cowpea. Indian J. Genet. Plant Breed. 1999, 59, 211–220. [Google Scholar]
- Ristic, Z.; Jenks, M.A. Leaf cuticle and water loss in maize lines differing in dehydration avoidance. J. Plant Physiol. 2002, 159, 645–651. [Google Scholar] [CrossRef]
- Meeks, M.; Murray, S.; Hague, S.; Hays, D.; Ibrahim, A. Genetic variation for maize epicuticular wax response to drought stress at flowering. J. Agron. Crop Sci. 2012, 198, 161–172. [Google Scholar] [CrossRef]
- Collins, N.C.; Tardieu, F.; Tuberosa, R. Quantitative trait loci and crop performance under abiotic stress: Where do we stand? Plant Physiol. 2008, 147, 469–486. [Google Scholar] [CrossRef]
- Bänziger, M.; Araus, J.L. Recent advances in breeding maize for drought and salinity stress tolerance. In Advances in Molecular Breeding toward Drought and Salt Tolerant Crops; Jenks, M.A., Hasegawa, P.M., Jain, S.M., Eds.; Springer: Dordrecht, Netherlands, 2007; pp. 587–601. [Google Scholar]
- Singh, B.B.; Matsui, T. Cowpea varieties for drought tolerance. In Challenges and Opportunities for Enhancing Sustainable Cowpea Production, Proceedings of the World Cowpea Conference III Held at the International Institute of Tropical Agriculture (IITA), Ibadan, Nigeria, 4–8 September 2000; Fatokun, C.A., Tarawali, S.A., Singh, B.B., Kormawa, P.M., Tamò, M., Eds.; IITA: Ibadan, Nigeria, 2002; pp. 287–300. [Google Scholar]
- Longenberger, P.S.; Smith, C.; Thaxton, P.; McMichael, B. Development of a screening method for drought tolerance in cotton seedlings. Crop Sci. 2006, 46, 2104–2110. [Google Scholar] [CrossRef]
- Tomar, S.; Kumar, G. Seedling survivability as a selection criterion for drought tolerance in wheat. Plant Breed. 2004, 123, 392–394. [Google Scholar] [CrossRef]
- Banziger, M.; Edmeades, G.; Quarrie, S. Drought stress at seedling stage-are there genetic solutions? In Proceedings of a Symposium, El Batan, Mexico, 25–29 March 1996; CIMMYT: El Batan, Mexico, 1997. [Google Scholar]
- Xu, W.; Odvody, G.; Williams, W.P. Tx204 and Tx205 inbred lines. Germplasm Enhancement of Maize (GEM); Annual Report. USDA-ARS: Lubbock, TX, USA, 2004. Available online: http://www.public.iastate.edu/~usda-gem/GEM_Annual_Reports/GEM_AR_04.htm#SCA (accessed on 6 February 2013).
- Singh, B.; Matsui, T. Cowpea varieties for drought tolerance. In Challenges and Opportunities for Enhancing Sustainable Cowpea Production; IITA: Ibadan, Nigeria, 2002; pp. 287–300. [Google Scholar]
- Betrán, F.; Fojt, A.; Mayfield, F.; K Pietsch, D. Registration of Tx714 maize germplasm line. Crop Sci. 2004, 44, 1028. [Google Scholar] [CrossRef]
- Betrán, F.J.; Bockholt, A.; Fojt, F.; Mayfield, K. Registration of Tx732 maize germplasm line. Crop Sci. 2004, 44, 1028–1029. [Google Scholar] [CrossRef]
- Betrán, F.J.; Bockholt, A.; Fojt, F.; Mayfield, K. Registration of Tx770 maize germplasm line. Crop Sci. 2004, 44, 1029–1030. [Google Scholar] [CrossRef]
- Llorente, C.; Fojt, F.; Bockholt, A.; Betrán, F. Registration of Tx772 maize. Crop Sci. 2004, 44, 1036–1037. [Google Scholar] [CrossRef]
- Hallauer, A.R.; Lamkey, K.R.; White, P.R. Registration of five inbred lines of maize: B102, B103, B104, B105, and B106. Crop Sci. 1997, 37, 1405–1406. [Google Scholar] [CrossRef]
- Russell, W. Registration of B70 and B73 parental lines of maize (reg. Nos. Pl16 and pl17). Crop Sci. 1972, 12, 721–721. [Google Scholar] [CrossRef]
- Foley, T.J. Inbred corn line LH195. U.S. Patent No. 5,059,745, 22 October 1991. [Google Scholar]
- Foley, T.J. Inbred corn line LH287. U.S. Patent No. 6,281,414, 28 August 2001. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Meeks, M.; Murray, S.C.; Hague, S.; Hays, D. Measuring Maize Seedling Drought Response in Search of Tolerant Germplasm. Agronomy 2013, 3, 135-147. https://doi.org/10.3390/agronomy3010135
Meeks M, Murray SC, Hague S, Hays D. Measuring Maize Seedling Drought Response in Search of Tolerant Germplasm. Agronomy. 2013; 3(1):135-147. https://doi.org/10.3390/agronomy3010135
Chicago/Turabian StyleMeeks, Meghyn, Seth C. Murray, Steve Hague, and Dirk Hays. 2013. "Measuring Maize Seedling Drought Response in Search of Tolerant Germplasm" Agronomy 3, no. 1: 135-147. https://doi.org/10.3390/agronomy3010135





