Impact of Hood Steaming on Tuber Vitality of Yellow Nutsedge (Cyperus esculentus)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiments
2.1.1. Field Experiment
Experimental Sites
Steaming Equipment (Figure 2)

Experimental Set-Up
- Genetic clone
- Burial depth
- Steaming duration
2.1.2. Laboratory Experiment
Incubator
Experimental Set-Up
- Genetic clone
- Exposure duration and temperature
2.2. Measurements
2.2.1. Soil Temperature
2.2.2. Tuber Sprouting Assessment
2.3. Statistical Data Analysis
3. Results
3.1. Field Experiment
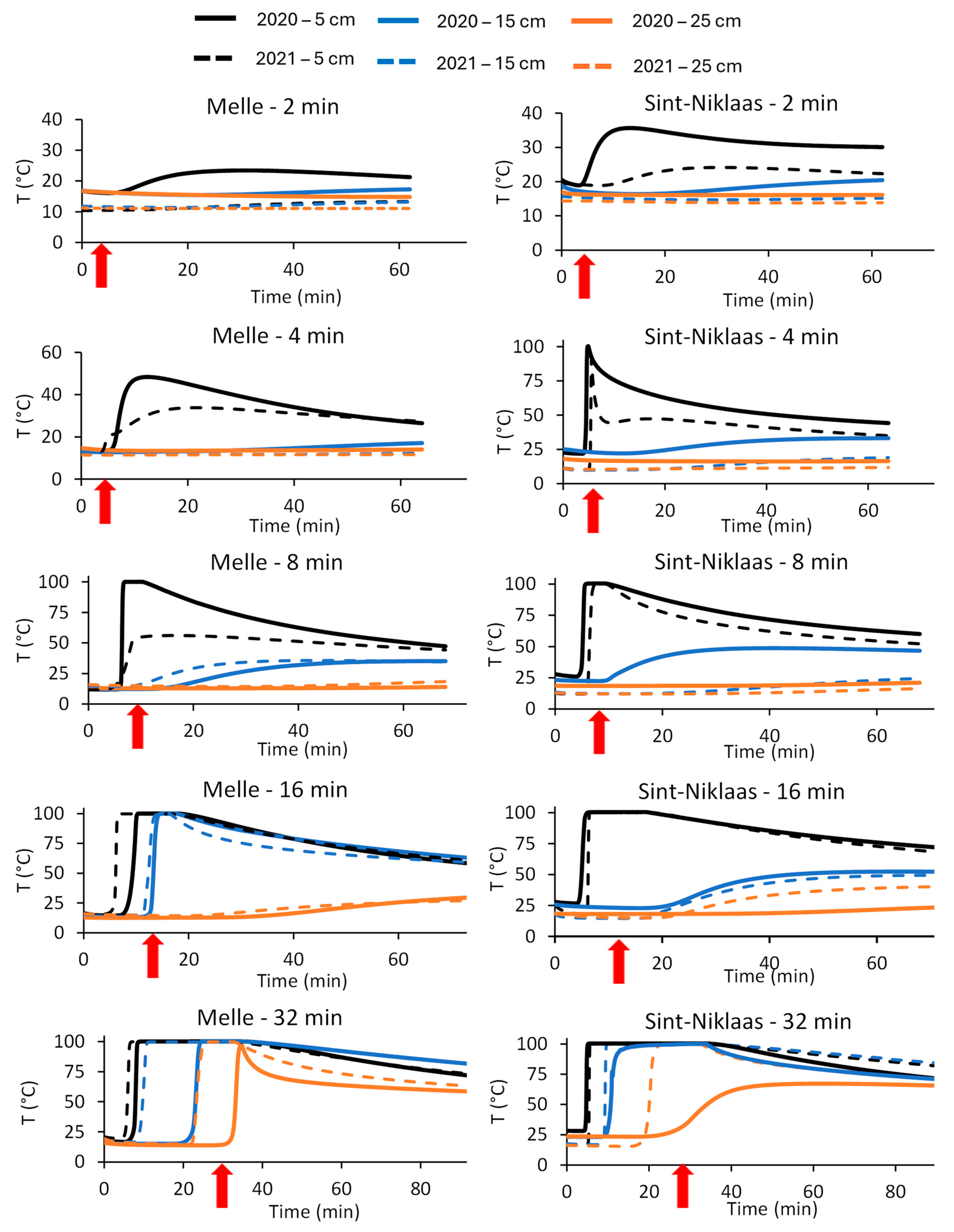
3.1.1. Monitoring of Soil Temperature
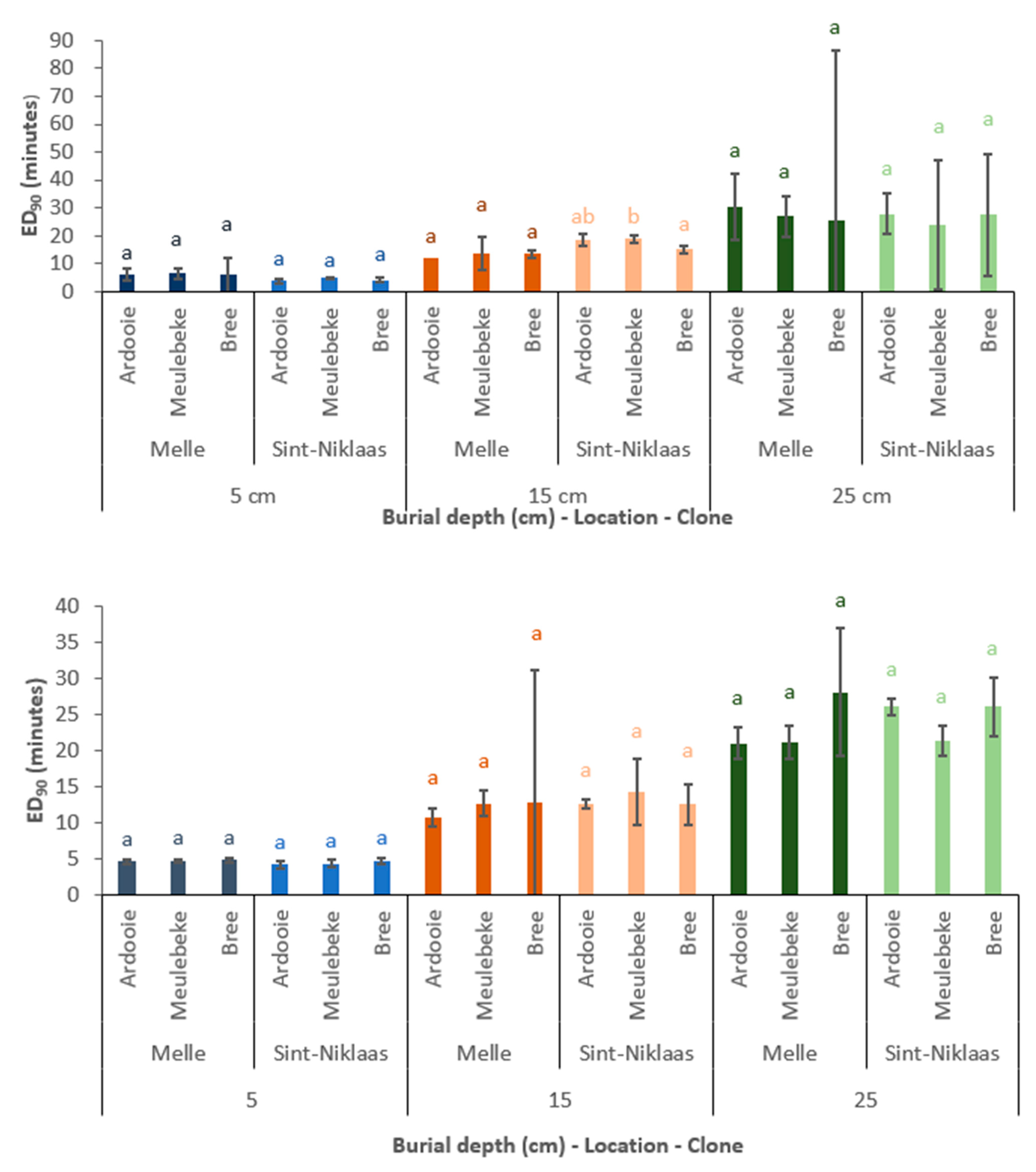
3.1.2. Tuber Vitality Measures
Effect of Location
Effect of Burial Depth
Effect of Genetic Clone
3.2. Laboratory Experiment
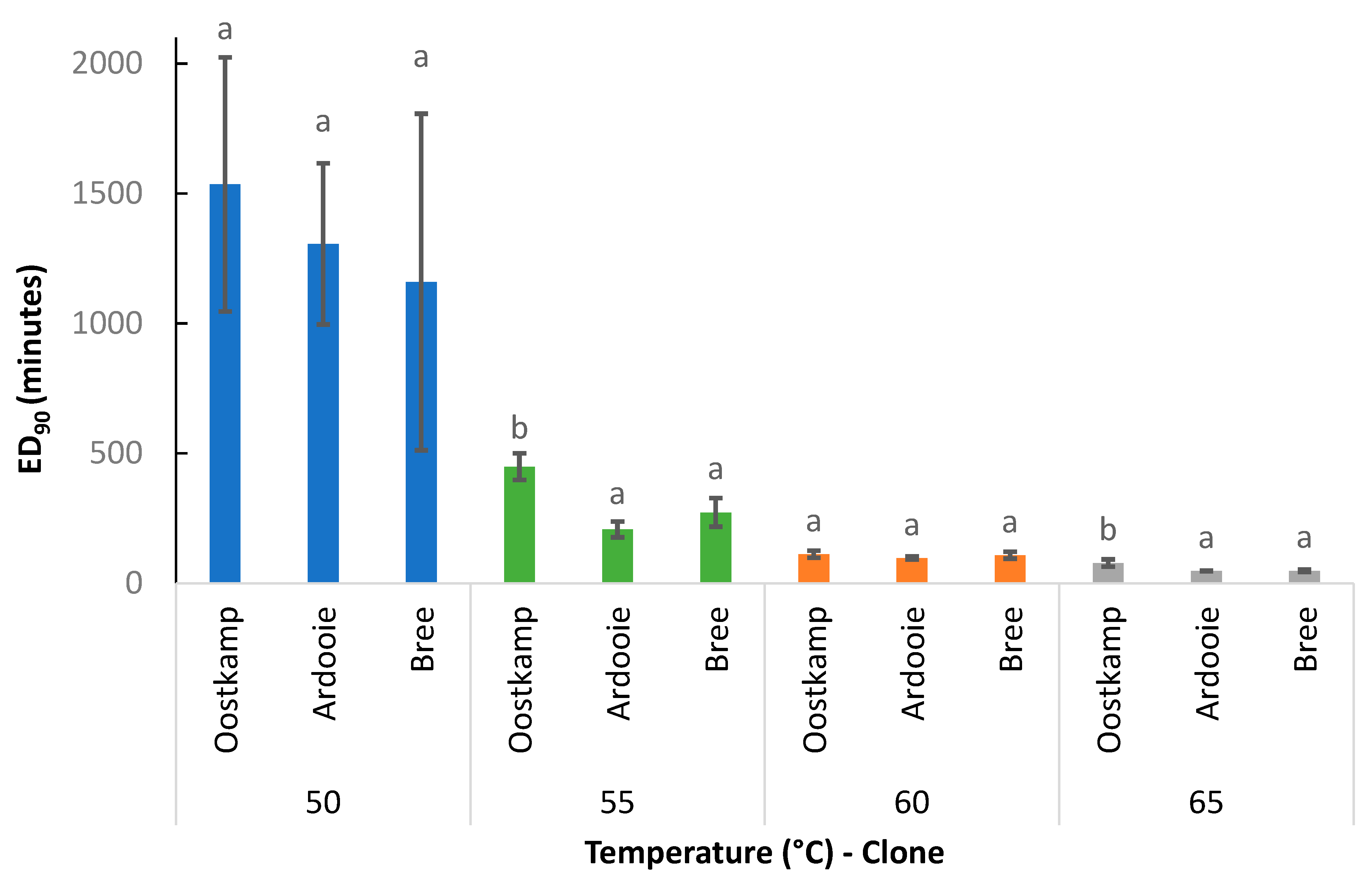
3.2.1. Effect of Temperature
3.2.2. Effect of Genetic Clone
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holm, L.G.; Plucknett, D.L.; Pancho, J.V.; Herberger, J.P. The World’s Worst Weeds; Kriegar Publishing: Malabar, FL, USA, 1991; ISBN 0894644157. [Google Scholar]
- Bohren, C.; Wirth, J. Souchet comestible (Cyperus esculentus L.): Situation actuelle en Suisse. Rech. Agron. Suisse 2016, 4, 460–467. [Google Scholar]
- Total, R.; Collet, L.; Heyer, J.; Keller, M. Yield losses in vegetable and arable crops caused by yellow nutsedge (Cyperus esculentus) in farmers fields in Switzerland. Julius-Kühn-Archiv 2018, 458, 473–477. [Google Scholar]
- Stoller, E.W.; Wax, L.M.; Slife, F.W. Yellow nutsedge (Cyperus esculentus) competition and control in corn (Zea mays). Weed Sci. 1979, 27, 32–37. [Google Scholar] [CrossRef]
- Pascual, B.; Maroto, J.V.; López-Galarza, S.; Sanbautista, A.; Alagarda, J. Chufa (Cyperus esculentus L. var. sativus Boeck.): An unconventional crop. Studies related to applications and cultivation. Econ. Bot. 2000, 54, 439–448. [Google Scholar] [CrossRef]
- De Cauwer, B.; De Ryck, S.; Claerhout, S.; Biesemans, N.; Reheul, D. Differences in growth and herbicide sensitivity among Cyperus esculentus clones found in Belgian maize fields. Weed Res. 2017, 57, 234–246. [Google Scholar] [CrossRef]
- Vonnez, J. Rapport Final Relatif au Projet OFAG: Stratégies de Lutte Contre le Souchet Comestible. Available online: https://www.souchet-comestible.ch/fileadmin/PDF/rapport_final_souchet/Rapport_final_projet_souchet_comestible.pdf (accessed on 13 July 2023).
- Demeyere, A. Praktijkgids Gewasbescherming. Module IPM Akkerbouw. Departement Landbouw En Visserij. Available online: https://www.vlaanderen.be/publicaties/praktijkgids-gewasbescherming-module-ipm-akkerbouw (accessed on 13 July 2023).
- Lapham, J.; Drennan, D.S.H. The fate of yellow nutsedge (Cyperus esculentus) seed and seedlings in soil. Weed Sci. 1990, 38, 125–128. [Google Scholar] [CrossRef]
- Stoller, E.W.; Sweet, R.D. Biology and life cycle of purple and yellow nutsedges (Cyperus rotundus and C. esculentus). Weed Technol. 1987, 1, 66–73. [Google Scholar] [CrossRef]
- Keller, M.; Eppler, L.; Collet, L.; Wirth, J.; Total, R. Beim Erdmandelgras auf nummer sicher gehen: Auch blütenbildung und abblühen verhindern! Gemüsebau Info 2015, 22, 7–9. [Google Scholar]
- Follak, S.; Belz, R.; Bohren, C.; De Castro, O.; Del Guacchio, E.; Pascual-Seva, N.; Schwarz, M.; Verloove, F.; Essl, F. Biological flora of Central Europe: Cyperus esculentus L. Perspect. Plant Ecol. Evol. Syst. 2016, 23, 33–51. [Google Scholar] [CrossRef]
- De Ryck, S.; Reheul, D.; De Cauwer, B. Impacts of herbicide sequences and vertical tuber distribution on the chemical control of yellow nutsedge (Cyperus esculentus L.). Weed Res. 2021, 61, 454–464. [Google Scholar] [CrossRef]
- Webster, T.M. High temperatures and durations of exposure reduce nutsedge (Cyperus spp.) tuber viability. Weed Sci. 2003, 51, 1010–1015. [Google Scholar] [CrossRef]
- Feys, J.; De Cauwer, B.; Reheul, D.; Sciffer, C.; Clercx, S.; Palmans, S. Impact of electrocution on shoot and tuber vitality of yellow nutsedge (Cyperus esculentus). Agriculture 2023, 13, 696. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Al-Hammadi, F. Soil amendments enhance soil solarisation efficiency in controlling weeds under the environment of the United Arab Emerites. In Proceedings of the 16th Australian Weeds Conference, Cairns Convention Centre, North Queensland, Australia, 18–22 May 2008. [Google Scholar]
- Tumbleson, M.E.; Kommedahl, T. Reproductive potential of Cyperus esculentus by tubers. Weeds 1961, 9, 646–653. [Google Scholar] [CrossRef]
- Gay, P.; Piccarolo, P.; Aimonino, D.R.; Tortia, C. A high efficiency steam soil disinfestation system, part I: Physical background and steam supply optimisation. Biosyst. Eng. 2010, 107, 74–85. [Google Scholar] [CrossRef]
- Gay, P.; Piccarolo, P.; Aimonino, D.R.; Tortia, C. Soil parameters effects on steam disinfestation efficiency. In Agricultural and biosystems engineering for a sustainable world. In Proceedings of the International Conference on Agricultural Engineering, Hersonissos, Crete, Greece, 23–25 June 2008. [Google Scholar]
- Sauer, T.J.; Horton, R. Soil heat flux. In Micrometeorology in Agricultural Systems; Hatfield, J.L., Baker, J.M., Viney, M.K., Eds.; American Society of Agronomy: Madison, WI, USA, 2005; ISBN 089118158X. [Google Scholar]
- Melander, B.; Kristensen, J.K. Soil steaming effects on weed seedling emergence under the influence of soil type, soil moisture, soil structure and heat duration. Ann. Appl. Biol. 2011, 158, 194–203. [Google Scholar] [CrossRef]
- Keller, M.; Collet, L.; Total, R. Using steam to eradicate Cyperus esculentus infestations in vegetable fields in Switzerland. In Proceedings of the Joint EWRS Workshop of the Working Groups Physical and Cultural Weed Control and Crop-Weed Interactions, Nyon, Switzerland, 2–5 April 2017. [Google Scholar]
- Total, R.; Collet, L.; Keller, M. Souchet comestible: Élimination des foyers primaires par traitement à la vapeur. Agrocope Transf. 2016, 137, 1–6. [Google Scholar]
- Samtani, J.B.; Gilbert, C.; Weber, J.B.; Subbarao, K.V.; Goodhue, R.E.; Fennimore, S.A. Effect of steam and solarization treatments on pest control, strawberry yield, and economic returns relative to methyl bromide fumigation. HortScience 2012, 47, 64–70. [Google Scholar] [CrossRef]
- Pinel, M.P.C.; Bond, W.; de Coursey Williams, M.; Johnston, E.; White, J.G. Field Vegetables: Assessment of the Potential for Mobile Soil Steaming Machinery to Control Diseases, Weeds and Mites of Field Salad and Related Crops; Final Report, Horticultural Development Council Project; Horticultural Development Council: East Malling, UK, 1999. [Google Scholar]
- Roux-Michollet, D.; Dudal, Y.; Jocteur-Monrozier, L.; Czarnes, S. Steam treatment of surface soil: How does it affect water-soluble organic matter, C mineralization, and bacterial community composition? Biol. Fert. Soils 2010, 46, 607–616. [Google Scholar] [CrossRef]
- De Ryck, S.; Reheul, D.; De Riek, J.; De Keyser, E.; De Cauwer, B. Genetic and morphological variation of Belgian Cyperus esculentus L. clonal populations and their significance for integrated management. Agronomy 2023, 13, 572. [Google Scholar] [CrossRef]
- Simox. Polyvap—Onkruid Verdelgen Met Respect Voor Milieu en Gezondheid. Available online: https://www.bluesphere.be/images/sites/51/article/117/1/Folder_Stoomgeneratoren.pdf (accessed on 14 August 2023).
- Feredec Bretagne. Guide des Alternatives au Désherbage Chimique dans les Communes. Available online: https://www.sagebaiededouarnenez.org/telechargement/Phytos_non_agricoles/Guide-des-alternatives-au-d%C3%A9sherbage-chimique-dans-les-communes.pdf (accessed on 14 August 2023).
- Jaichandar, S.; Annamalai, K. The status of biodiesel as an alternative fuel for diesel engine—An overview. J. Sustain. Energy Environ. 2011, 2, 71–75. [Google Scholar]
- Artisan Technology Group. General Purpose Incubators. Available online: https://www.artisantg.com/info/VWR_1500_Series_Datasheet.pdf (accessed on 11 December 2023).
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 17 August 2023).
- Ritz, C.; Streibig, J.C. Bioassay analysis using R. J. Stat. Softw. 2005, 12, 1–22. [Google Scholar] [CrossRef]
- Streibig, J.C.; Rudemo, M.; Jensen, J.E. Dose-response curves and statistical models. In Herbicide Bioassays; Streibig, J.C., Kudsk, P., Eds.; CRC Press: Boca Raton, FL, USA, 1993; ISBN 0849366038. [Google Scholar]
- Van Der Vaart, A.W. Asymptotic Statistics; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Thullen, R.J.; Keeley, P.E. Yellow nutsedge sprouting and resprouting potential. Weed Sci. 1975, 23, 333–337. [Google Scholar] [CrossRef]
- Kehler, R. Investigations on Yellow Nutsedge. Master’s Thesis, University of Manitoba, Winnipeg, Canada, 1991. [Google Scholar]
- Matthiesen, R.L.; Stoller, E.W. Tuber composition in yellow nutsedge (Cyperus esculentus (L.)) variants. Weed Res. 1978, 18, 373–377. [Google Scholar] [CrossRef]
- Dabbene, F.; Gay, P.; Tortia, C. Modeling and control of steam soil disinfestation processes. Biosyst. Eng. 2003, 84, 247–256. [Google Scholar] [CrossRef]
- Abu-Hamdeh, N.H. Thermal properties of soils as affected by density and water content. Biosyst. Eng. 2003, 86, 97–102. [Google Scholar] [CrossRef]
- Hamdhan, I.N.; Clarke, B.G. Determination of thermal conductivity of coarse and fine sand soils. In Proceedings of the World Geothermal Congress, Bali, Indonesia, 25–29 April 2010. [Google Scholar]
- Zhu, X.; Gao, Z.; Chen, T.; Wang, W.; Lu, C.; Zhang, Q. Study on the thermophysical properties and influencing factors of regional surface shallow rock and soil in China. Front. Earth Sci. 2022, 10, 864548. [Google Scholar] [CrossRef]
- Waples, D.W.; Waples, J.S. A review and evaluation of specific heat capacities of rocks, minerals, and subsurface fluids. Part 1: Minerals and nonporous rocks. Nat. Resour. Res. 2004, 13, 97–122. [Google Scholar] [CrossRef]
- Ratna, D. Thermal properties of thermosets. In Thermosets: Structure, Properties and Applications; Guo, Q., Ed.; Woodhead Publishing: Sawston, UK, 2012; ISBN 978-0-85709-086-7. [Google Scholar]
- Malek, K.; Malek, K.; Khanmohammadi, F. Response of soil thermal conductivity to various soil properties. Int. Commun. Heat Mass 2021, 127, 105516. [Google Scholar] [CrossRef]
- Huber, M.L.; Perkins, R.A.; Friend, D.G.; Sengers, J.V.; Assael, M.J.; Metaxa, I.N.; Miyagawa, K.; Hellmann, R.; Vogel, E. New international formulation for the thermal conductivity of H2O. J. Phys. Chem. Ref. Data 2012, 41, 033102. [Google Scholar] [CrossRef]
- Runia, W.T. A recent development in steam sterilisation. Acta Hortic. 1983, 152, 195–200. [Google Scholar] [CrossRef]
- Minuto, G.; Gilardi, G.; Kejji, S.; Gullino, M.L.; Garibaldi, A. Effect of physical nature of soil and humidity on stream disinfestation. Acta Hortic. 2005, 698, 257–262. [Google Scholar] [CrossRef]
- Melander, B.; Jørgensen, M.H. Soil steaming to reduce intra-row weed seedling emergence. Weed Res. 2005, 45, 202–211. [Google Scholar] [CrossRef]
- The Engineering Toolbox. Air–Thermal Conductivity vs. Temperature and Pressure. Available online: https://www.engineeringtoolbox.com/air-properties-viscosity-conductivity-heat-capacity-d_1509.html (accessed on 15 April 2024).






| Location | Year | Day | Depth (cm) | Gravimetric Soil Moisture Content (%) |
|---|---|---|---|---|
| Melle | 2020 | 13 May | 5 | 16.12 |
| 15 | 14.95 | |||
| 25 | 18.58 | |||
| 2021 | 28 April | 5 | 12.99 | |
| 15 | 15.35 | |||
| 25 | 17.64 | |||
| Sint-Niklaas | 2020 | 19 May | 5 | 8.56 |
| 15 | 11.12 | |||
| 25 | 12.57 | |||
| 2021 | 12 May | 5 | 10.14 | |
| 15 | 10.65 | |||
| 25 | 11.27 |
| Temperature (°C) | ||||
|---|---|---|---|---|
| 50 | 55 | 60 | 65 | |
| Exposure durations (min) | 0 | 0 | 0 | 0 |
| 25 | 13 | 7 | 7 | |
| 48 | 24 | 12 | 12 | |
| 96 | 48 | 24 | 24 | |
| 192 | 96 | 48 | 48 | |
| 384 | 192 | 96 | 96 | |
| 768 | 384 | 192 | 192 | |
| Location | Steaming Duration (min) | Temperature Threshold (°C) | Residence Time (s) above an Indicated Temperature Threshold at a Particular Burial Depth | |||||
|---|---|---|---|---|---|---|---|---|
| 2020 | 2021 | |||||||
| 5 cm | 15 cm | 25 cm | 5 cm | 15 cm | 25 cm | |||
| Melle | 2 | 50 | 0 | 0 | 0 | 0 | 0 | 0 |
| 65 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 80 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 95 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 4 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 65 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 80 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 95 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 8 | 50 | 3310 | 0 | 0 | 2185 | 0 | 0 | |
| 65 | 1831 | 0 | 0 | 0 | 0 | 0 | ||
| 80 | 984 | 0 | 0 | 0 | 0 | 0 | ||
| 95 | 409 | 0 | 0 | 0 | 0 | 0 | ||
| 16 | 50 | 4001 | 3764 | 0 | 4196 | 3820 | 0 | |
| 65 | 3005 | 3266 | 0 | 3432 | 2313 | 0 | ||
| 80 | 1737 | 1496 | 0 | 1918 | 752 | 0 | ||
| 95 | 855 | 451 | 0 | 954 | 281 | 0 | ||
| 32 | 50 | 5047 | 4136 | 3528 | 5170 | 4939 | 4121 | |
| 65 | 5040 | 4121 | 1314 | 5160 | 4921 | 3627 | ||
| 80 | 3895 | 4106 | 243 | 4083 | 4904 | 1493 | ||
| 95 | 2331 | 1692 | 47 | 2220 | 2511 | 670 | ||
| Sint-Niklaas | 2 | 50 | 0 | 0 | 0 | 0 | 0 | 0 |
| 65 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 80 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 95 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 4 | 50 | 2260 | 0 | 0 | 102 | 0 | 0 | |
| 65 | 780 | 0 | 0 | 40 | 0 | 0 | ||
| 80 | 213 | 0 | 0 | 14 | 0 | 0 | ||
| 95 | 38 | 0 | 0 | 0 | 0 | 0 | ||
| 8 | 50 | 3773 | 0 | 0 | 3696 | 0 | 0 | |
| 65 | 2898 | 0 | 0 | 1695 | 0 | 0 | ||
| 80 | 1369 | 0 | 0 | 700 | 0 | 0 | ||
| 95 | 523 | 0 | 0 | 267 | 0 | 0 | ||
| 16 | 50 | 4261 | 1915 | 0 | 4188 | 0 | 0 | |
| 65 | 4252 | 0 | 0 | 4186 | 0 | 0 | ||
| 80 | 2711 | 0 | 0 | 2386 | 0 | 0 | ||
| 95 | 1169 | 0 | 0 | 1143 | 0 | 0 | ||
| 32 | 50 | 5238 | 4880 | 3478 | 5196 | 4963 | 4316 | |
| 65 | 5235 | 4867 | 2803 | 5194 | 4960 | 4299 | ||
| 80 | 3879 | 2869 | 0 | 5192 | 4956 | 2234 | ||
| 95 | 2381 | 1418 | 0 | 2698 | 2699 | 840 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feys, J.; De Ryck, S.; Sciffer, C.; Reheul, D.; Latré, J.; Callens, D.; De Cauwer, B. Impact of Hood Steaming on Tuber Vitality of Yellow Nutsedge (Cyperus esculentus). Agronomy 2024, 14, 918. https://doi.org/10.3390/agronomy14050918
Feys J, De Ryck S, Sciffer C, Reheul D, Latré J, Callens D, De Cauwer B. Impact of Hood Steaming on Tuber Vitality of Yellow Nutsedge (Cyperus esculentus). Agronomy. 2024; 14(5):918. https://doi.org/10.3390/agronomy14050918
Chicago/Turabian StyleFeys, Jeroen, Sander De Ryck, Clara Sciffer, Dirk Reheul, Joos Latré, Danny Callens, and Benny De Cauwer. 2024. "Impact of Hood Steaming on Tuber Vitality of Yellow Nutsedge (Cyperus esculentus)" Agronomy 14, no. 5: 918. https://doi.org/10.3390/agronomy14050918






