Agronomic Biofortification of Fodder Maize (Zea mays L.) with Zn for Improving Herbage Productivity and Its Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site, Weather and Soil Characteristics
2.2. Experimental Setup and Treatment Detail
2.3. Crop Husbandry
2.4. Crop Traits Measured
2.5. Plant Analysis for Nutrient Composition and Quality
2.6. Statistical Analyses
3. Results
3.1. Growth Parameters
3.2. Green Herbage Yield (GHY) and Dry Matter Yield (DMY)
3.3. Zn Fertilization and Fodder Micronutrient Composition
3.4. Zn Fertilization and Fodder Macronutrient Composition
3.5. CP Content of Fodder
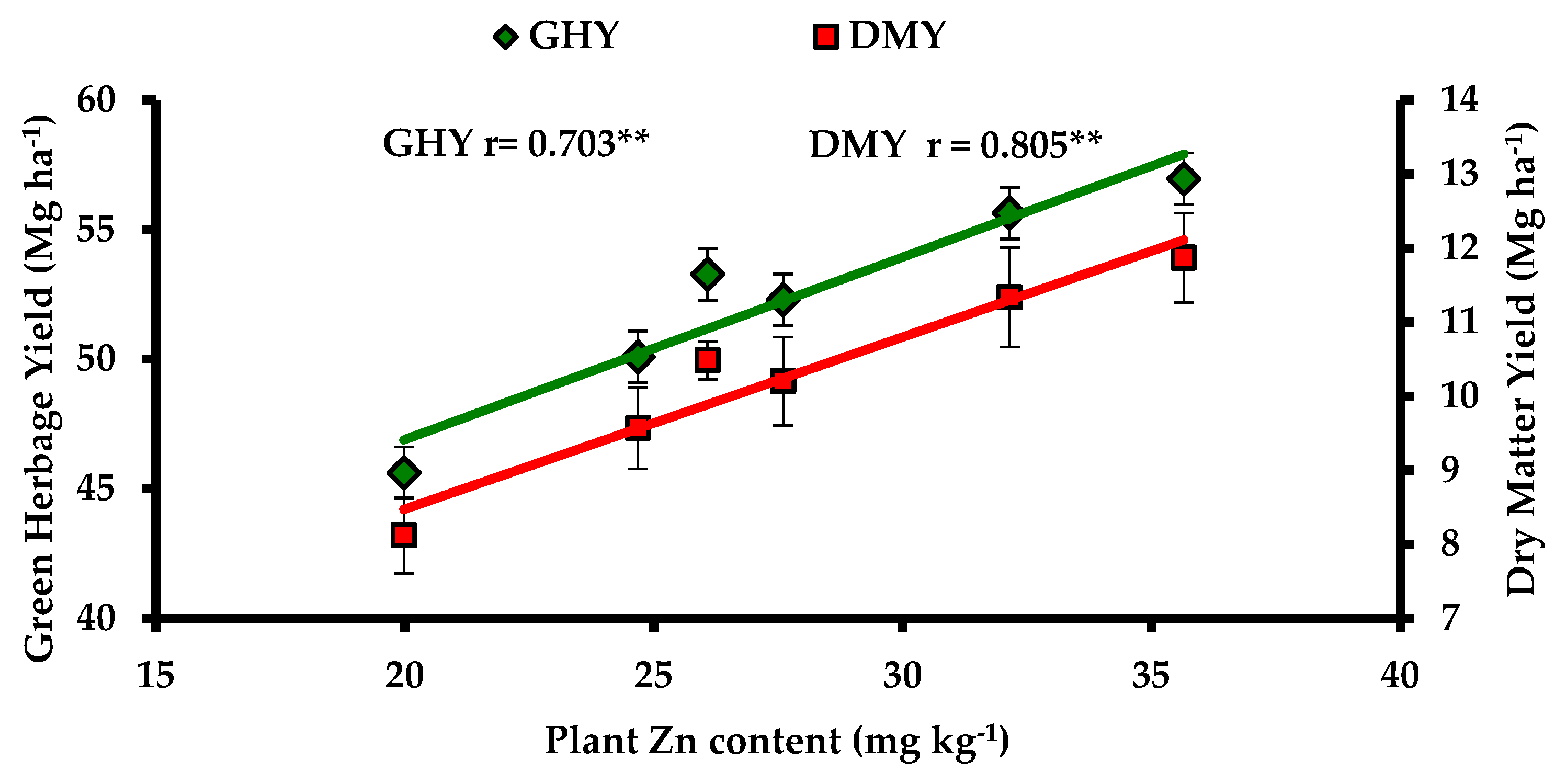
3.6. Correlation Studies
4. Discussion
4.1. Effect of Zn on Growth Parameters
4.2. Effect of Zn on Green Herbage and Dry Matter Yield
4.3. Zn Biofortification and Effect on Other Micronutrients
4.4. Zn Biofortification and Effect on Macronutrients
4.5. Zn Effect on Herbage Crude Protein
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Potarzycki, J.; Grzebisz, W. Effect of zinc foliar application on grain yield of maize and its yielding components. Plant Soil Environ. 2009, 55, 519–527. [Google Scholar] [CrossRef]
- Wang, J.; Mao, H.; Zhao, H.; Huang, D.; Wang, Z. Different increases in maize and wheat grain zinc concentrations caused by soil and foliar applications of zinc in Loess Plateau, China. Field Crops Res. 2012, 135, 89–96. [Google Scholar] [CrossRef]
- Miller, D.D.; Welch, R.M. Food system strategies for preventing micronutrient malnutrition. Food Policy 2013, 42, 115–128. [Google Scholar] [CrossRef]
- Cakmak, I.; Kutman, U.B. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef]
- Kumar, B.; Dhaliwal, S.S.; Singh, S.T.; Lamba, J.S.; Ram, H. Herbage production, nutritional composition and quality of Teosinte under Fe fertilization. Int. J. Agric. Biol. 2016, 18, 319–329. [Google Scholar] [CrossRef]
- Gupta, N.; Ram, H.; Kumar, B. Mechanism of Zinc absorption in plants: Uptake, transport, translocation and accumulation. Rev. Environ. Sci. Bio./Technol. 2016, 15, 89–109. [Google Scholar] [CrossRef]
- Kumar, B.; Dhaliwal, S.S. Zinc biofortification of dual-purpose cowpea [Vigna unguiculate (L.) Walp.] for enhancing the productivity and nutritional quality in a semi-arid regions of India. Arch. Agron. Soil Sci. 2022, 68, 1034–1048. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets—Iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Singh, S.T.; Dua, K.; Kumar, B.; Randhawa, C.S. Mineral status of fodders offered to dairy animals in Mansa and Fazilka district of Punjab. Range Manag. Agrofor. 2014, 35, 240–244. [Google Scholar]
- Anees, M.A.; Ali, A.; Shakoor, U.; Ahmed, F.; Hasnain, Z.; Hussain, A. Foliar Applied Potassium and Zinc Enhances Growth and Yield Performance of Maize under Rainfed Conditions. Int. J. Agric. Biol. 2016, 18, 1025–1032. [Google Scholar] [CrossRef]
- Wadhwa, M.; Kaur, K.; Kumar, B.; Bakshi MP, S. Comparative evaluation of non leguminous forages as livestock feed. Indian J. Anim. Nutr. 2010, 27, 44–49. [Google Scholar]
- Ahmad, W.; Watts, M.J.; Imtiaz, M.; Ahmed, I.; Zia, M.H. Zinc deficiency in soils, crops and humans: A review. Agrochimica 2012, 56, 75–89. [Google Scholar]
- Greene, L. Designing mineral supplementation of forage programs for beef cattle. J. Anim. Sci. 2000, 77, 1–9. [Google Scholar] [CrossRef]
- Hosnedlova, B.; Travnicek, J.; Soch, M. Current view of the significance of zinc for ruminants: A review. Agric. Trop. Subtrop. 2007, 40, 57–64. [Google Scholar]
- Capstaff, N.M.; Miller, A.J. Improving the Yield and Nutritional Quality of Forage Crops. Front. Plant Sci. 2018, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemist. Official Methods of Analysis; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Radford, P.J. Growth Analysis Formulae—Their Use and Abuse. Crop Sci. 1967, 7, 171–175. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis. Part 2, 2nd ed.; American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice-Hall of India Pvt. Ltd.: New Delhi, India, 1973; pp. 38–204. [Google Scholar]
- International Rice Research Institute. IRRISTAT Version 92; Department of Statistics, International Rice Research Institute: Los Baños, CA, USA, 1992. [Google Scholar]
- Palmgren, M.G.; Clemens, S.; Williams, L.E.; Krämer, U.; Borg, S.; Schjørring, J.K.; Sanders, D. Zinc biofortification of cereals: Problems and solutions. Trends Plant Sci. 2008, 13, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.D.; Narwal, R.P.; Malik, R.S.; Saha, B.N.; Kumar, S. Impact of zinc application methods on green gram (Vigna radiata L.) productivity and grain zinc fortification. J. Environ. Biol. 2014, 35, 851–854. [Google Scholar] [PubMed]
- Castagnara, D.D.; Krutzmann, A.; Zoz, T.; Steiner, F.; e Castro, A.M.C.; Neres, M.A.; de Oliveira, P.S.R. Effect of boron and zinc fertilization on white oats grown in soil with average content of these nutrients. Rev. Bras. Zootec. 2012, 41, 1598–1607. [Google Scholar] [CrossRef]
- Adiloglu, S. The effect of increasing nitrogen and zinc doses on the iron, copper and manganese contents of maize plant in calcareous and zinc deficient soils. Asian J. Plant Sci. 2006, 5, 504–507. [Google Scholar] [CrossRef]
- Aref, F. Influence of zinc and boron nutrition on copper, manganese and iron concentrations in maize leaf. Aust. J. Basic Appl. Sci. 2011, 5, 52–62. [Google Scholar]
- Weiss, W.; Socha, M. Dietary Manganese for Dry and Lactating Holstein Cows. J. Dairy Sci. 2005, 88, 2517–2523. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.; Spears, J.; Lloyd, K.; Whisnant, C. Feeding a low manganese diet to heifers during gestation impairs fetal growth and development. J. Dairy Sci. 2006, 89, 4305–4311. [Google Scholar] [CrossRef] [PubMed]
- Aref, F. Manganese, iron and copper contents in leaves of maize plants (Zea mays L.) grown with different boron and zinc micronutrients. Afr. J. Biotechnol. 2012, 11, 896–903. [Google Scholar] [CrossRef]
- Alloway, B.J. Soil factors associated with zinc deficiency in crops and humans. Environ. Geochem. Health 2009, 31, 537–548. [Google Scholar] [CrossRef]
- Olsen’s Agricultural Laboratory. Plant Tissue Interpretative Guidelines; Olsen’s Agricultural Laboratory: McCook, NE, USA, 2011; pp. 1–64. Available online: https://www.olsenlab.com (accessed on 12 December 2023).
- Kandil, E.E.; Lamlom, S.F.; Gheith, E.-S.M.; Javed, T.; Ghareeb, R.Y.; Abdelsalam, N.R.; Hussain, S. Biofortification of maize growth, productivity and quality using nano-silver, silicon and zinc particles with different irrigation intervals. J. Agric. Sci. 2023, 161, 339–355. [Google Scholar] [CrossRef]
| Treatment | Plant Height (cm) | Leaves Plant−1 | Stem Diameter (cm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Year-I | Year-II | Mean | Year-I | Year-II | Mean | Year-I | Year-II | Mean | |
| Control | 177.5 b | 186.3 b | 181.9 c | 11.6 c | 12.6 b | 12.1 c | 1.69 c | 2.05 c | 1.87 c |
| F1 | 188.9 a | 199.1 a | 194.0 b | 12.5 b | 13.2 b | 12.8 b | 1.80 b | 2.22 b | 2.01 b |
| F2 | 192.3 a | 202.5 a | 197.4 ab | 12.9 ab | 13.6 ab | 13.2 b | 1.85 b | 2.29 b | 2.07 b |
| S16 | 192.0 a | 206.3 a | 199.1 ab | 12.8 ab | 13.6 ab | 13.3 ab | 1.86 b | 2.30 b | 2.08 b |
| S16 + F1 | 193.6 a | 208.3 a | 200.1 ab | 13.1 ab | 13.8 ab | 13.5 ab | 1.93 ab | 2.35 b | 2.14 ab |
| S16 + F2 | 195.8 a | 211.0 a | 203.4 a | 13.4 ab | 14.0 a | 13.7 a | 1.98 a | 2.37 a | 2.17 a |
| SEm± | 1.70 | 2.38 | 1.88 | 0.17 a | 0.12 | 0.12 | 0.02 | 0.03 | 0.03 |
| LSD (p ≤ 0.05) | 8.2 | 11.6 | 6.7 | 0.80 | 0.60 | 0.5 | 0.10 | 0.14 | 0.08 |
| Treatment | LAI | FW Plant−1 (g) | DW Plant−1 (g) | LSR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year-I | Year-II | Mean | Year-I | Year-II | Mean | Year-I | Year-II | Mean | Year-I | Year-II | Mean | |
| Control | 5.54 c | 6.27 b | 5.91 c | 351.6 b | 386.7 b | 369.2 c | 91.5 d | 102.0 c | 96.8 d | 0.578 b | 0.674 c | 0.626 d |
| F1 | 6.03 bc | 6.91 b | 6.47 b | 386.7 ab | 421.7 ab | 404.2 b | 106.3 c | 127.7 b | 117.0 c | 0.635 b | 0.747 b | 0.691 c |
| F2 | 6.29 b | 7.21 ab | 6.75 b | 403.3 a | 436.6 a | 420.0 b | 114.5 c | 136.3 b | 125.4 c | 0.661 b | 0.789 b | 0.725 c |
| S16 | 6.40 b | 7.31 ab | 6.85 b | 411.7 a | 438.3 a | 425.0 a | 115.3 b | 138.7 b | 127.0 c | 0.665 b | 0.790 b | 0.728 bc |
| S16 + F1 | 6.94 a | 7.65 ab | 7.30 a | 420.0 a | 447.3 a | 433.7 a | 127.6 a | 149.4 ab | 138.5 b | 0.709 ab | 0.852 ab | 0.780 b |
| S16 + F2 | 7.35 a | 7.98 a | 7.66 a | 430.0 a | 441.6 a | 445.8 a | 135.0 a | 159.8 a | 147.4 a | 0.775 a | 0.889 a | 0.832 a |
| SEm± | 0.15 | 0.15 | 0.14 | 7.24 | 7.18 | 6.74 | 3.64 | 4.60 | 4.00 | 0.02 | 0.01 | 0.02 |
| LSD (p ≤ 0.05) | 0.50 | 0.77 | 0.42 | 36.4 | 35.6 | 23.8 | 12.4 | 13.0 | 8.4 | 0.091 | 0.067 | 0.053 |
| Treatment | GHY (Mg ha−1) | DMY (Mg ha−1) | ||||
|---|---|---|---|---|---|---|
| Year-I | Year-II | Mean | Year-I | Year-II | Mean | |
| Control | 43.7 b | 47.5 b | 45.6 c | 8.0 c | 8.2 c | 8.1 c |
| F1 | 48.6 b | 51.6 b | 50.1 b | 9.4 b | 9.8 b | 9.6 c |
| F2 | 50.5 ab | 54.1 ab | 52.3 b | 9.9 b | 10.5 b | 10.2 bc |
| S16 | 51.6 ab | 54.9 ab | 53.2 ab | 10.3 ab | 10.7 b | 10.5 b |
| S16 + F1 | 53.6 ab | 57.7 ab | 55.6 ab | 10.8 ab | 11.9 a | 11.3 a |
| S16 + F2 | 54.8 a | 59.1 a | 57.0 a | 11.4 a | 12.3 a | 11.9 a |
| SEm± | 1.08 | 1.25 | 0.99 | 0.30 | 0.34 | 0.30 |
| LSD (p ≤ 0.05) | 5.9 | 6.5 | 4.2 | 1.3 | 0.90 | 0.8 |
| Treatment | Zn | Fe | Mn | Cu | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg kg−1 on DM Basis) | ||||||||||||
| Year-I | Year-II | Mean | Year-I | Year-II | Mean | Year-I | Year-II | Mean | Year-I | Year-II | Mean | |
| Control | 18.6 c | 21.3 c | 19.9 e | 105 | 111 | 107.8 | 25.0 | 27.2 a | 26.1 a | 2.1 b | 2.5 b | 2.3 c |
| F1 | 23.5 b | 25.8 b | 24.7 d | 106 | 114 | 109.7 | 24.8 | 26.8 ab | 25.7 a | 2.5 b | 3.0 b | 2.8 bc |
| F2 | 26.2 b | 29.0 b | 27.6 c | 107 | 114 | 110.7 | 23.9 | 25.7 b | 24.7 ab | 3.3 a | 3.5 ab | 3.4 b |
| S16 | 25.2 b | 27.0 b | 26.1 c | 101 | 110 | 105.1 | 24.7 | 26.3 b | 25.5 b | 3.3 a | 3.5 ab | 3.4 b |
| S16 + F1 | 30.6 a | 33.7 a | 32.2 b | 106 | 115 | 110.5 | 24.1 | 25.6 bc | 24.8 b | 3.5 a | 4.0 ab | 3.8 ab |
| S16 + F2 | 34.0 a | 37.3 a | 35.6 a | 106 | 115 | 110.7 | 23.7 | 25.3 c | 24.5 b | 3.8 a | 4.5 a | 4.1 a |
| SEm± | 1.26 | 1.32 | 1.26 | 1.17 | 0.96 | 0.18 | 0.18 | 0.18 | 0.17 | 0.15 | 0.17 | 0.15 |
| LSD (p ≤ 0.05) | 4.0 | 3.7 | 2.5 | NS | NS | NS | NS | 0.8 | 0.7 | 0.7 | 1.0 | 0.6 |
| Treatment | N | P | K | CP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | ||||||||||||
| Year-I | Year-II | Mean | Year-I | Year-II | Mean | Year-I | Year-II | Mean | Year-I | Year-II | Mean | |
| Control | 1.28 c | 1.32 b | 1.30 c | 0.93 a | 1.03 | 0.98 | 1.96 b | 2.09 | 2.02 b | 8.02 c | 8.25 b | 8.13 c |
| F1 | 1.40 b | 1.41 b | 1.40 b | 0.91 ab | 0.99 | 0.96 | 2.06 b | 2.14 | 2.10 b | 8.75 b | 8.81 b | 8.78 b |
| F2 | 1.42 b | 1.46 ab | 1.44 b | 0.89 b | 0.99 | 0.94 | 2.09 b | 2.22 | 2.15 ab | 8.91 b | 9.13 ab | 9.02 b |
| S16 | 1.46 ab | 1.49 ab | 1.47 b | 0.86 bc | 0.97 | 0.91 | 2.16 ab | 2.23 | 2.19 ab | 9.14 ab | 9.31 ab | 9.23 b |
| S16 + F1 | 1.51 ab | 1.53 ab | 1.52 ab | 0.88 b | 0.97 | 0.92 | 2.20 ab | 2.26 | 2.23 a | 9.43 ab | 9.58 ab | 9.51 ab |
| S16 + F2 | 1.55 a | 1.57 a | 1.56 a | 0.84 c | 0.97 | 0.90 | 2.26 a | 2.27 | 2.26 a | 9.72 a | 9.79 a | 9.76 a |
| SEm± | 0.02 | 0.03 | 0.02 | 0.01 | 0.01 | 0.01 | 0.03 | 0.04 | 0.03 | 0.15 | 0.16 | 0.15 |
| LSD (p ≤ 0.05) | 0.11 | 0.12 | 0.07 | 0.03 | NS | NS | 0.16 | NS | 0.11 | 0.70 | 0.80 | 0.5 |
| PH | LN | SD | LAI | FW | DW | LSR | Zn | Fe | Mn | Cu | DMY | GHY | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PH | - | ||||||||||||
| LN | 0.684 ** | - | |||||||||||
| SD | 0.776 ** | 0.731 ** | - | ||||||||||
| LAI | 0.769 ** | 0.786 ** | 0.769 ** | - | |||||||||
| FW | 0.740 ** | 0.747 ** | 0.688 ** | 0.771 ** | - | ||||||||
| DW | 0.832 ** | 0.769 ** | 0.795 ** | 0.846 ** | 0.796 ** | - | |||||||
| LSR | 0.849 ** | 0.723 ** | 0.823 ** | 0.830 ** | 0.741 ** | 0.874 ** | - | ||||||
| Zn | 0.706 ** | 0.711 ** | 0.556 ** | 0.819 ** | 0.729 ** | 0.845 ** | 0.798 ** | - | |||||
| Fe | 0.470 ** | 0.482 ** | 0.687 ** | 0.304 NS | 0.460 ** | 0.487 ** | 0.622 ** | 0.289 NS | - | ||||
| Mn | 0.165 NS | 0.116 NS | 0.498 ** | 0.070 NS | 0.001 NS | 0.017 NS | 0.197 NS | −0.252 NS | 0.496 ** | - | |||
| Cu | 0.772 ** | 0.772 ** | 0.830 ** | 0.758 ** | 0.696 ** | 0.791 ** | 0.799 ** | 0.632 ** | 0.536 ** | 0.325 NS | - | ||
| DMY | 0.734 ** | 0.745 ** | 0.545 ** | 0.784 ** | 0.702 ** | 0.838 ** | 0.735 ** | 0.805 ** | 0.276 NS | −0.227 NS | 0.649 ** | - | |
| GHY | 0.645 ** | 0.686 ** | 0.572 ** | 0.809 ** | 0.741 ** | 0.776 ** | 0.721 ** | 0.703 ** | 0.268 NS | −0.046 NS | 0.534 ** | 0.818 ** | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, B.; Ram, H.; Schoenau, J. Agronomic Biofortification of Fodder Maize (Zea mays L.) with Zn for Improving Herbage Productivity and Its Quality. Agronomy 2024, 14, 912. https://doi.org/10.3390/agronomy14050912
Kumar B, Ram H, Schoenau J. Agronomic Biofortification of Fodder Maize (Zea mays L.) with Zn for Improving Herbage Productivity and Its Quality. Agronomy. 2024; 14(5):912. https://doi.org/10.3390/agronomy14050912
Chicago/Turabian StyleKumar, Balwinder, Hari Ram, and Jeff Schoenau. 2024. "Agronomic Biofortification of Fodder Maize (Zea mays L.) with Zn for Improving Herbage Productivity and Its Quality" Agronomy 14, no. 5: 912. https://doi.org/10.3390/agronomy14050912





