The Influence of Sodium Salt on Growth, Photosynthesis, Na+/K+ Homeostasis and Osmotic Adjustment of Atriplex canescens under Drought Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions and Stress Treatments
2.2. Determination of Physiological Indices
2.3. Measurement of Photosynthetic Parameters
2.4. Measurement of Chlorophyll Fluorescence Kinetics
2.5. Measurement of Leaf Water Potential-Related Parameters
2.6. Determination of Ion Contents
2.7. Determination of Betaine and Free Proline
2.8. Determination of the Contributions of Solutes to Leaf ψs
2.9. Statistical Analysis
3. Results
3.1. Growth Performance
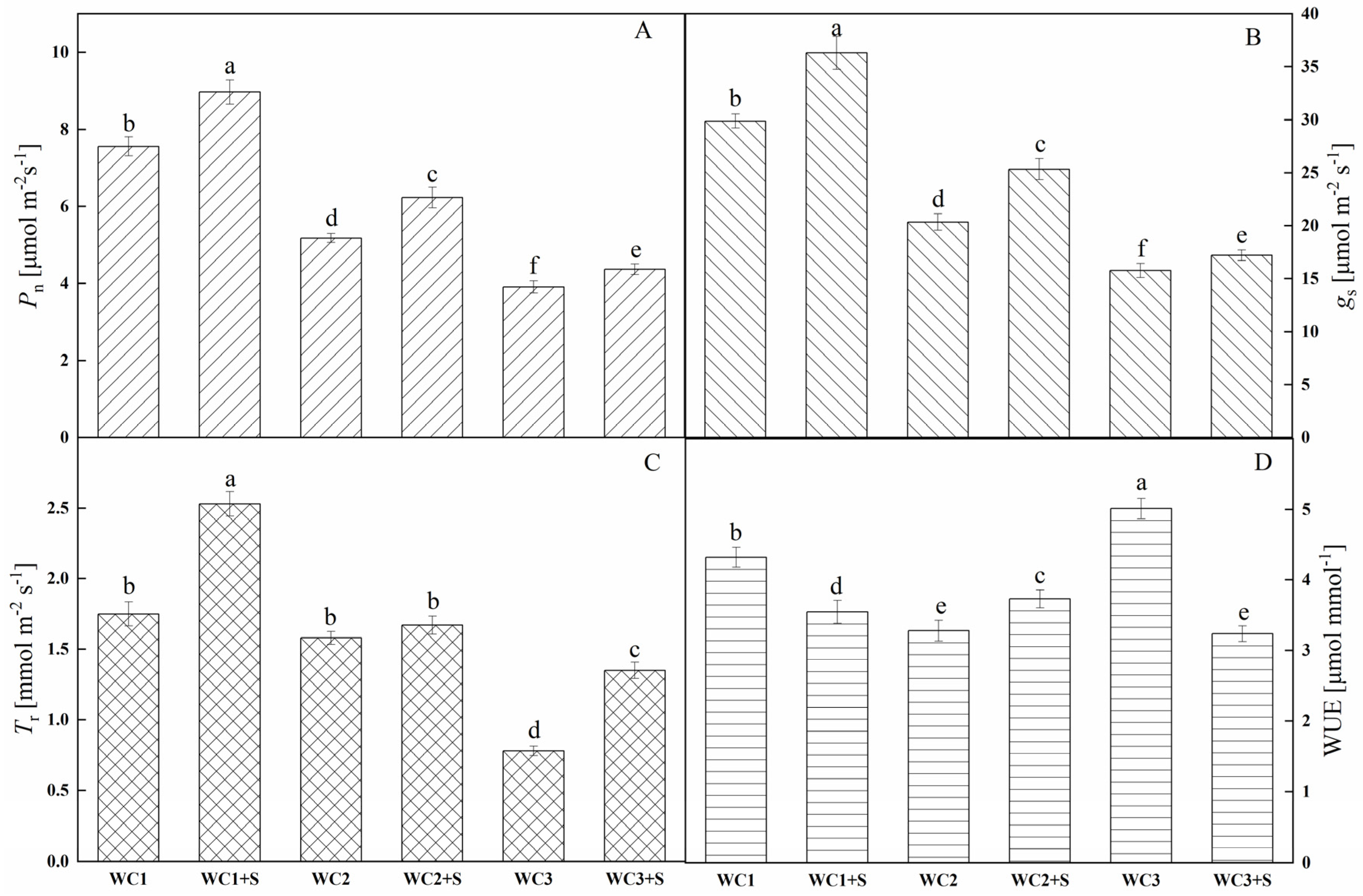
3.2. Photosynthetic Capacity and Water Use Efficiency (WUE)
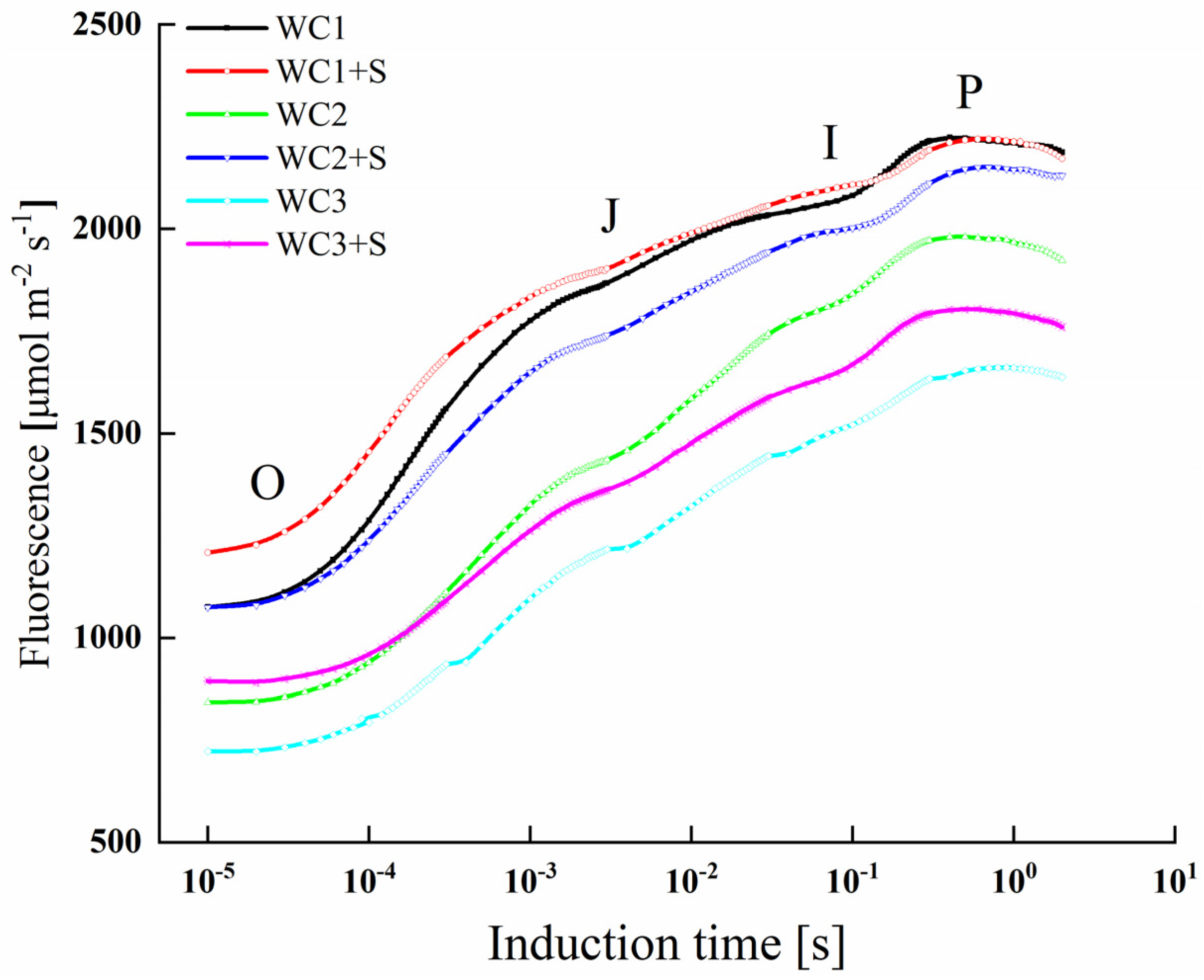
3.3. Chlorophyll Fluorescence
3.4. Leaf Water Status
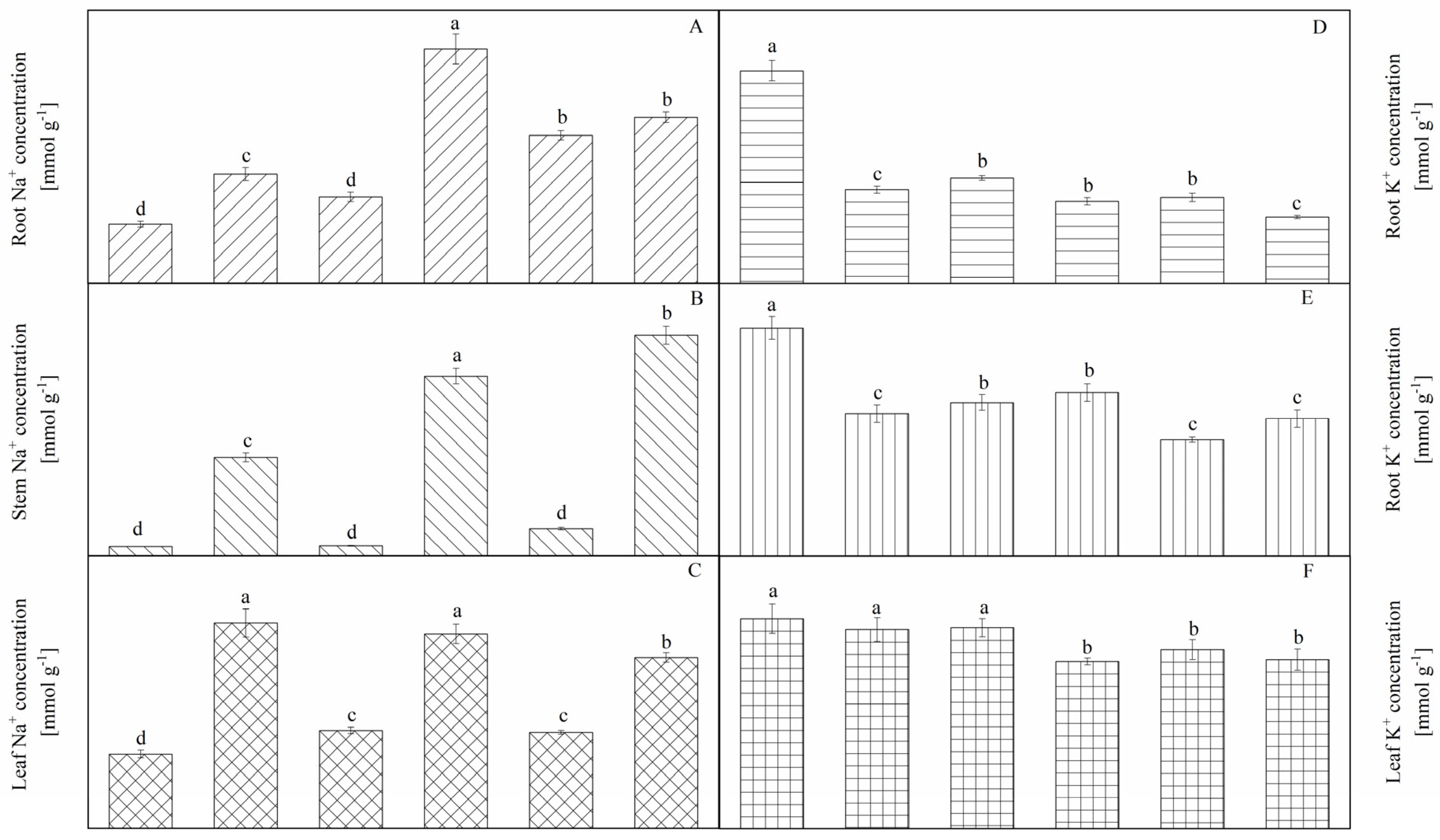
3.5. Ion Accumulation and Distribution
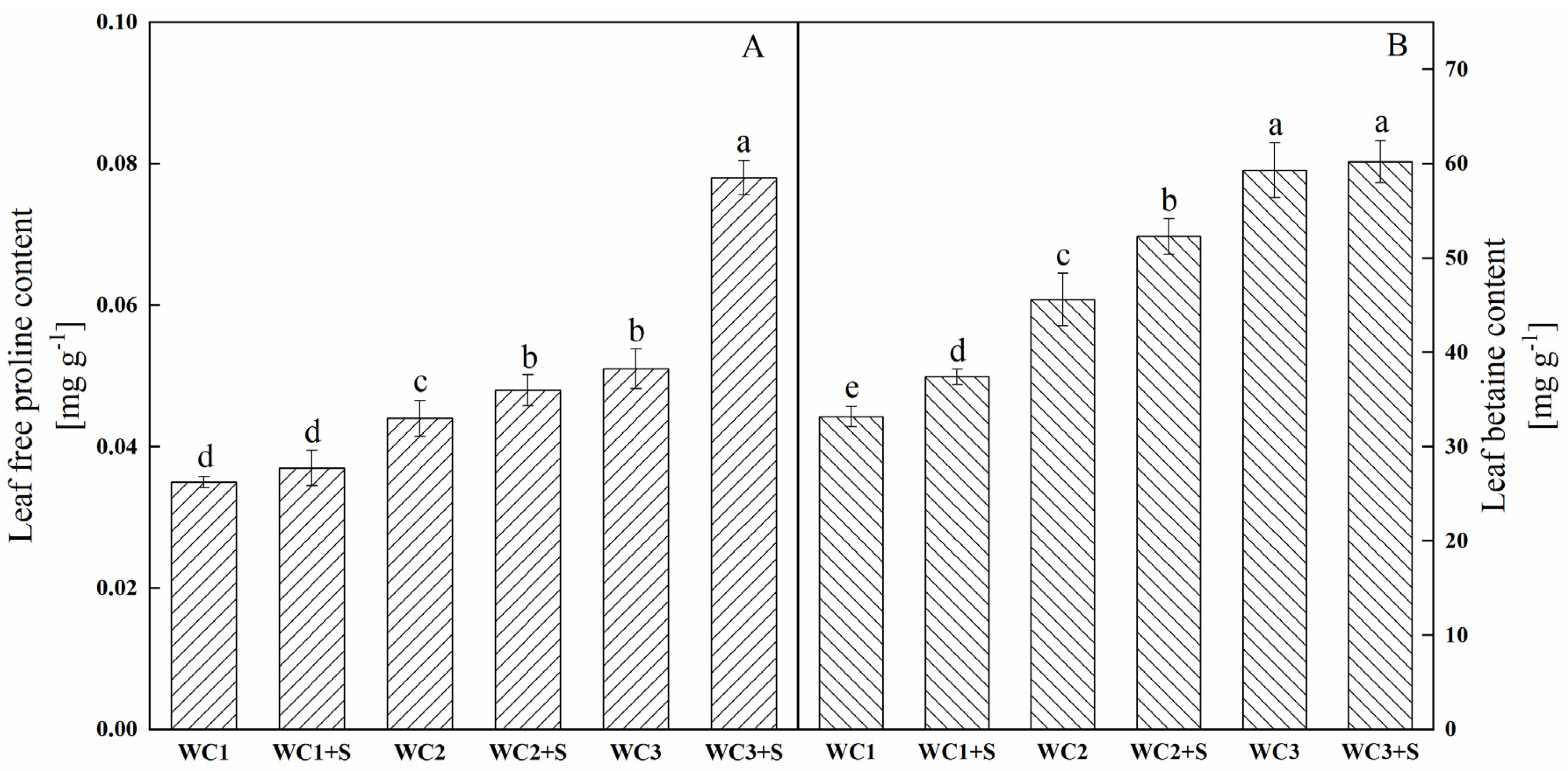
3.6. Betaine and Free Proline Contents
3.7. Contributions of the Main Solutes to ψs
4. Discussion
4.1. Moderate Sodium Salt Facilitates the Growth of A. canescens by Maintaining Good Water Status and Photosynthetic Capacity under Drought Stress
4.2. Moderate Sodium Salt Improves the Adaptability of A. canescens to Drought Stress by Mitigating the Suppression of PSII
4.3. Moderate Sodium Salt Promotes the Drought Resistance of A. canescens by Improving the Function of Compatible Solutes in OA
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OA | Osmotic adjustment |
| SRWC | Soil relative water content |
| FW | Fresh weight |
| DW | Dry weight |
| TW | Turgid weight |
| RGR | Relative growth rate |
| PAR | Photosynthetically active radiation |
| RWC | Relative water content |
| PN | Net photosynthetic rate |
| gS | Stomatal conductance |
| Tr | Transpiration rate |
| WUE | Water use efficiency |
| PSII | Photosystem II |
| RC | Reaction center |
| Fo | Initial fluorescence |
| Fm | Maximal fluorescence |
| Fv | Variable Fluorescence |
| Fv/Fm | Maximal quantum yield of PSII photochemistry |
| ΦPSII | Actual PSII efficiency |
| qP | Photochemical quenching coefficient |
| qN | Nonphotochemical quenching coefficient |
| Fv/F0 | Potential efficiency of PSII photochemistry |
| Vj | Relative variable fluorescence at the J step |
| ABS/RC | Light absorption energy flux per RC |
| DIo/RC | Relative energy flux per PSII RC |
| TRo/RC | Trapped energy flux per RC |
| ETo/RC | Maximum electron transport flux per PSII RC |
| ψw | Leaf water potential |
| ψs | Leaf water osmotic potential |
| ψt | Leaf turgor potential |
| COP | Calculated osmotic potential |
| CR | Contribution rate to the leaf ψs |
References
- He, F.L.; Bao, A.K.; Wang, S.M.; Jin, H.X. NaCl stimulates growth and alleviates drought stress in the salt-secreting xerophyte Reaumuria soongorica. Environ. Exp. Bot. 2019, 162, 433–443. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 1–11. [Google Scholar] [CrossRef]
- Glenn, E.P.; Nelson, S.G.; Ambrose, B.; Martinez, R.; Soliz, D.; Pabendinskas, V.; Hultine, K. Comparison of salinity tolerance of three Atriplex spp. in well-watered and drying soils. Environ. Exp. Bot. 2012, 83, 62–72. [Google Scholar] [CrossRef]
- Daliakopoulos, I.N.; Panagea, I.S.; Tsanis, I.K.; Grillakis, M.G.; Koutroulis, A.G.; Hessel, R.; Mayor, A.G.; Ritsema, C.J. Yield Response of Mediterranean Rangelands under a Changing Climate. Land Degrad. Dev. 2017, 28, 1962–1972. [Google Scholar] [CrossRef]
- Wang, F.; Tong, W.; Zhu, H.; Kong, W.; Peng, R.; Liu, Q.; Yao, Q. A novel Cys(2)/His(2) zinc finger protein gene from sweet-potato, IbZFP1, is involved in salt and drought tolerance in transgenic Arabidopsis. Planta 2016, 243, 783–797. [Google Scholar] [CrossRef]
- Cui, Y.N.; Xia, Z.R.; Ma, Q.; Wang, W.Y.; Chai, W.W.; Wang, S.M. The synergistic effects of sodium and potassium on the xerophyte in response to drought stress. Plant Physiol. Biochem. 2019, 135, 489–498. [Google Scholar] [CrossRef]
- Kang, J.J.; Zhao, W.Z.; Zheng, Y.; Zhang, D.M.; Zhou, H.; Sun, P.C. Calcium chloride improves photosynthesis and water status in the C-4 succulent xerophyte Haloxylon ammodendron under water deficit. Plant Growth Regul. 2017, 82, 467–478. [Google Scholar] [CrossRef]
- Letey, J.; Hoffman, G.; Hopmans, J.; Grattan, S.; Suarez, D.; Corwin, D.; Oster, J.; Wu, L.; Amrhein, C. Evaluation of soil salinity leaching requirement guidelines. Agric. Water Manag. 2011, 98, 502–506. [Google Scholar] [CrossRef]
- Binks, O.; Meir, P.; Rowland, L.; da Costa, A.C.L.; Vasconcelos, S.S.; de Oliveira, A.A.R.; Ferreira, L.; Christoffersen, B.; Nardini, A.; Mencuccini, M. Plasticity in leaf-level water relations of tropical rainforest trees in response to experimental drought. New Phytol. 2016, 211, 477–488. [Google Scholar] [CrossRef]
- Wu, G.Q.; Feng, R.J.; Liang, N.; Yuan, H.J.; Sun, W.B. Sodium chloride stimulates growth and alleviates sorbitol-induced osmotic stress in sugar beet seedlings. Plant Growth Regul. 2015, 75, 307–316. [Google Scholar] [CrossRef]
- Hatami, M.; Hadian, J.; Ghorbanpour, M. Mechanisms underlying toxicity and stimulatory role of single-walled carbon nanotubes in Hyoscyamus niger during drought stress simulated by polyethylene glycol. J. Hazard. Mater. 2017, 324, 306–320. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Agarwal, R.M. Potassium up-regulates antioxidant metabolism and alleviates growth inhibition under water and osmotic stress in wheat (Triticum aestivum L). Protoplasma 2017, 254, 1471–1486. [Google Scholar] [CrossRef]
- Zahoor, R.; Zhao, W.Q.; Abid, M.; Dong, H.R.; Zhou, Z.G. Title: Potassium application regulates nitrogen metabolism and osmotic adjustment in cotton (Gossypium hirsutum L.) functional leaf under drought stress. J. Plant Physiol. 2017, 215, 30–38. [Google Scholar] [CrossRef]
- Jupa, R.; Plichta, R.; Paschova, Z.; Nadezhdina, N.; Gebauer, R. Mechanisms underlying the long-term survival of the monocot Dracaena marginata under drought conditions. Tree Physiol. 2017, 37, 1182–1197. [Google Scholar] [CrossRef]
- Dudley, L.M.; Ben-Gal, A.; Shani, U. Influence of plant, soil, and water on the leaching fraction. Vadose Zone J. 2008, 7, 420–425. [Google Scholar] [CrossRef]
- Slama, I.; Ghnaya, T.; Messedi, D.; Hessini, K.; Labidi, N.; Savoure, A.; Abdelly, C. Effect of sodium chloride on the response of the halophyte species Sesuvium portulacastrum grown in mannitol-induced water stress. J. Plant Res. 2007, 120, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Slama, I.; Ghnaya, T.; Savoure, A.; Abdelly, C. Combined effects of long-term salinity and soil drying on growth, water relations, nutrient status and proline accumulation of Sesuvium portulacastrum. Comptes Rendus Biol. 2008, 331, 442–451. [Google Scholar] [CrossRef]
- Brown, C.E.; Pezeshki, S.R.; DeLaune, R.D. The effects of salinity and soil drying on nutrient uptake and growth of Spartina alternilora in a simulated tidal system. Environ. Exp. Bot. 2006, 58, 140–148. [Google Scholar] [CrossRef]
- Ma, Q.; Yue, L.J.; Zhang, J.L.; Wu, G.Q.; Bao, A.K.; Wang, S.M. Sodium chloride improves photosynthesis and water status in the succulent xerophyte Zygophyllum xanthoxylum. Tree Physiol. 2012, 32, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Li, S.; Ma, Q.; Zhou, X.; Wu, G.; Bao, A.; Zhang, J.; Wang, S. NaCl stimulates growth and alleviates water stress in the xerophyte Zygophyllum xanthoxylum. J. Arid. Environ. 2012, 87, 153–160. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Yu, H.Y.; Kong, D.S.; Yan, F.; Zhang, Y.J. Effects of drought stress on growth and chlorophyll fluorescence of Lycium ruthenicum Murr. seedlings. Photosynthetica 2016, 54, 524–531. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Si, J.H.; Feng, Q.; Deo, R.C.; Yu, T.F.; Li, P.D. Physiological response to salinity stress and tolerance mechanics of Populus euphratica. Environ. Monit. Assess. 2017, 189, 533. [Google Scholar] [CrossRef]
- Schansker, G.; Toth, S.Z.; Strasser, R.J. Dark recovery of the Chl a fluorescence transient (OJIP) after light adaptation: The qT-component of non-photochemical quenching is related to an activated photosystem I acceptor side. Biochim. Et Biophys. Acta-Bioenerg. 2006, 1757, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.Y.; Lucero, M.E.; Sanderson, S.C.; Zacharias, E.H.; Holbrook, N.M. Polyploidy enhances the occupation of heterogeneous environments through hydraulic related trade-offs in Atriplex canescens (Chenopodiaceae). New Phytol. 2013, 197, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Benzarti, M.; Ben Rejeb, K.; Debez, A.; Abdelly, C. Environmental and Economical Opportunities for the Valorisation of the Genus Atriplex: New Insights. In Crop Improvement: New Approaches and Modern Techniques; Hakeem, K.R., Ahmad, P., Ozturk, M., Eds.; Springer: Boston, MA, USA, 2013; pp. 441–457. [Google Scholar]
- Pan, Y.Q.; Guo, H.; Wang, S.M.; Zhao, B.; Zhang, J.L.; Ma, Q.; Yin, H.J.; Bao, A.K. The Photosynthesis, Na+/K+ Homeostasis and Osmotic Adjustment of Atriplex canescens in Response to Salinity. Front. Plant Sci. 2016, 7, 848. [Google Scholar] [CrossRef] [PubMed]
- Glenn, E.P.; Olsen, M.; Frye, R.; Moore, D.; Miyamoto, S. How much sodium accumulation is necessary for salt tolerance in subspecies of the halophyte Atriplex canescens? Plant Cell Environ. 2010, 17, 711–719. [Google Scholar] [CrossRef]
- Habibi, G. Silicon supplementation improves drought tolerance in canola plants. Russ. J. Plant Physiol. 2014, 61, 784–791. [Google Scholar] [CrossRef]
- Qu, C.; Liu, C.; Gong, X.; Li, C.; Hong, M.; Wang, L.; Hong, F. Impairment of maize seedling photosynthesis caused by a combination of potassium deficiency and salt stress. Environ. Exp. Bot. 2012, 75, 134–141. [Google Scholar] [CrossRef]
- Guerrier, G. Fluxes of Na+, K+ and Cl-, and osmotic adjustment in Lycopersicon pimpinellifolium and L. esculentum during short- and long-term exposures to NaCl. Physiol. Plant. 2010, 97, 583–591. [Google Scholar] [CrossRef]
- van Heerden, P.D.R.; Swanepoel, J.W.; Kruer, G.H.J. Modulation of photosynthesis by drought in two desert scrub species exhibiting C3-mode CO2 assimilation. Environ. Exp. Bot. 2007, 61, 124–136. [Google Scholar]
- Yuan, F.; Leng, B.Y.; Wang, B.S. Progress in Studying Salt Secretion from the Salt Glands in Recreto-halophytes: How Do Plants Secrete Salt? Front. Plant Sci. 2016, 7, 977. [Google Scholar] [PubMed]
- Guo, H.; Cui, Y.N.; Pan, Y.Q.; Wang, S.M.; Bao, A.K. Sodium chloride facilitates the secretohalophyte Atriplex canescens adaptation to drought stress. Plant Physiol. Biochem. 2020, 150, 99–108. [Google Scholar] [PubMed]
- Kiani-Pouya, A.; Roessner, U.; Jayasinghe, N.S.; Lutz, A.; Rupasinghe, T.; Bazihizina, N.; Bohm, J.; Alharbi, S.; Hedrich, R.; Shabala, S. Epidermal bladder cells confer salinity stress tolerance in the halophyte Quinoa and Atriplex species. Plant Cell Environ. 2017, 40, 1900–1915. [Google Scholar] [PubMed]
- Hu, L.; Wang, Z.; Huang, B. Diffusion limitations and metabolic factors associated with inhibition and recovery of photosynthesis from drought stress in a C perennial grass species. Physiol. Plant. 2010, 139, 93–106. [Google Scholar]
- Ashraf, M. Some important physiological selection criteria for salt tolerance in plants. Flora 2004, 199, 361–376. [Google Scholar]
- Franks, P.J.; Farquhar, G.D. The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiol. 2007, 143, 78–87. [Google Scholar]
- Hedrich, R.; Shabala, S. Stomata in a saline world. Curr. Opin. Plant Biol. 2018, 46, 87–95. [Google Scholar]
- Tahjib-Ul-Arif, M.; Sohag, A.A.M.; Afrin, S.; Bashar, K.K.; Afrin, T.; Mahamud, A.G.M.S.U.; Polash, M.A.S.; Hossain, M.T.; Sohel, M.A.T.; Brestic, M.; et al. Differential Response of Sugar Beet to Long-Term Mild to Severe Salinity in a Soil-Pot Culture. Agriculture 2019, 9, 223. [Google Scholar]
- Ache, P.; Bauer, H.; Kollist, H.; Al-Rasheid, K.A.; Lautner, S.; Hartung, W.; Hedrich, R. Stomatal action directly feeds back on leaf turgor: New insights into the regulation of the plant water status from non-invasive pressure probe measurements. Plant J. 2010, 62, 1072–1082. [Google Scholar]
- Swoczyna, T.; Kalaji, H.M.; Pietkiewicz, S.; Borowski, J.; Zaras-Januszkiewicz, E. Photosynthetic apparatus efficiency of eight tree taxa as an indicator of their tolerance to urban environments. Dendrobiology 2010, 63, 65–75. [Google Scholar]
- Oukarroum, A.; Bussotti, F.; Goltsev, V.; Kalaji, H.M.; Botany, E. Correlation between reactive oxygen species production and photochemistry of photosystems I and II in Lemna gibba L. plants under salt stress. Environ. Exp. Bot. 2015, 109, 80–88. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhang, T.; Zhang, Z.Z.; He, K.N. The physiological and biochemical photosynthetic properties of Lycium ruthenicum Murr in response to salinity and drought. Sci. Hortic. 2019, 256, 108530. [Google Scholar] [CrossRef]
- de Melo, H.F.; de Souza, E.R.; Cunha, J.C. Fluorescence of chlorophyll a and photosynthetic pigments in Atriplex nummularia under abiotic stresses. Rev. Bras. De Eng. Agric. E Ambient. 2017, 21, 232–237. [Google Scholar] [CrossRef]
- Bjrkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Kraslavsky, V.; Bharti, S.; Allakhverdiev, S.I.; Jajoo, A. Analysis of salt stress induced changes in Photosystem II heterogeneity by prompt fluorescence and delayed fluorescence in wheat (Triticum aestivum) leaves. J. Photochem. Photobiol. B-Biol. 2011, 104, 308–313. [Google Scholar] [CrossRef]
- Shu, S.; Yuan, L.Y.; Guo, S.R.; Sun, J.; Yuan, Y.H. Effects of exogenous spermine on chlorophyll fluorescence, antioxidant system and ultrastructure of chloroplasts in Cucumis sativus L. under salt stress. Plant Physiol. Biochem. 2013, 63, 209–216. [Google Scholar] [CrossRef]
- Pereira, W.E.; Siqueira, D.L.D.; Martínez, C.A.; Puiatti, M. Gas exchange and chlorophyll fluorescence in four citrus rootstocks under aluminium stress. J. Plant Physiology. 2000, 157, 513–520. [Google Scholar] [CrossRef]
- Tang, X.L.; Mu, X.M.; Shao, H.B.; Wang, H.Y.; Brestic, M. Global plant-responding mechanisms to salt stress: Physiological and molecular levels and implications in biotechnology. Crit. Rev. Biotechnol. 2015, 35, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.A.; Hajirezaei, M.R.; Seiler, C.; Sreenivasulu, N.; von Wiren, N. A Potential Role of Flag Leaf Potassium in Conferring Tolerance to Drought-Induced Leaf Senescence in Barley. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Erel, R.; Ben-Gal, A.; Dag, A.; Schwartz, A.; Yermiyahu, U. Sodium replacement of potassium in physiological processes of olive trees (var. Barnea) as affected by drought. Tree Physiol. 2014, 34, 1102–1117. [Google Scholar] [PubMed]
- Wakeel, A.; Farooq, M.; Qadir, M.; Schubert, S. Potassium Substitution by Sodium in Plants. Crit. Rev. Plant Sci. 2011, 30, 401–413. [Google Scholar] [CrossRef]
- Baoshan, W.; Ulrich, L.; Rafael, R. Effects of salt treatment and osmotic stress on V-ATPase and V-PPase in leaves of the halophyte Suaeda salsa. J. Exp. Bot. 2001, 52, 2355–2365. [Google Scholar]
- Maggio, A.; Reddy, M.P.; Joly, R.J.; Botany, E. Leaf gas exchange and solute accumulation in the halophyte Salvadora persica grown at moderate salinity. Environ. Exp. Bot. 2000, 44, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, K.; Yamada, N.; Cha-um, S.; Tanaka, Y.; Takabe, T. Differential accumulation of glycine betaine and choline monooxygenase in bladder hairs and lamina leaves of Atriplex gmelini under high salinity. J. Plant Physiol. 2015, 176, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chen, J.; Chen, L.; Wang, X.; Wang, R.; Ma, L.; Peng, S.; Luo, J.; Chen, Y. Combined drought and heat stress in Camellia oleifera cultivars: Leaf characteristics, soluble sugar and protein contents, and Rubisco gene expression. Trees-Struct. Funct. 2015, 29, 1483–1492. [Google Scholar] [CrossRef]
- Ming, D.F.; Pei, Z.F.; Naeem, M.S.; Gong, H.J.; Zhou, W.J. Silicon Alleviates PEG-Induced Water-Deficit Stress in Upland Rice Seedlings by Enhancing Osmotic Adjustment. J. Agron. Crop Sci. 2012, 198, 14–26. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Coskun, D.; Schulze, L.M.; Wong, J.R.; Britto, D.T. Sodium as nutrient and toxicant. Plant Soil 2013, 369, 1–23. [Google Scholar] [CrossRef]
- Amoroso, M.M.; Daniels, L.D.; Villalba, R.; Cherubini, P. Does drought incite tree decline and death in Austrocedrus chilensis forests? J. Veg. Sci. 2015, 26, 1171–1183. [Google Scholar] [CrossRef]



| Water Soluble Ion Concentration (μmol g−1) | Changeable Ion Concentration (μmol g−1) | Available P (μmol g−1) | Available N (μmol g−1) | pH | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Ca2+ | Mg2+ | Na+ | K+ | Ca2+ | Mg2+ | |||
| 7.4 ± 0.5 | 2.5 ± 0.2 | 1.6 ± 0.1 | 1.3 ± 0.1 | 3.4 ± 0.3 | 3.6 ± 0.3 | 0.9 ± 0.0 | 0.7 ± 0.0 | 0.6 ± 0.0 | 0.07 ± 0.0 | 8.7 ± 0.7 |
| Treatments | Plant Height (cm) | Ground Diameter (cm) | Dry Mass (g) | Fresh Mass (g) | RGR × 103 (g kg−1 d−1) |
|---|---|---|---|---|---|
| WC1 | 18.3 ± 0.96 b | 0.62 ± 0.08 c | 36.1 ± 2.23 b | 365.5 ± 12.11 b | 16.35 ± 0.79 b |
| WC1+S | 21.1 ± 0.67 a | 0.69 ± 0.07 a | 41.2 ± 1.39 a | 412.2 ± 10.35 a | 18.65 ± 0.89 a |
| WC2 | 13.8 ± 0.88 d | 0.53 ± 0.08 d | 28.7 ± 1.18 d | 283.2 ± 13.15 d | 12.84 ± 0.82 d |
| WC2+S | 15.9 ± 0.52 c | 0.63 ± 0.06 b | 32.6 ± 2.61 c | 313.6 ± 12.39 c | 14.38 ± 0.69 c |
| WC3 | 10.1 ± 0.31 f | 0.39 ± 0.05 f | 19.4 ± 1.33 f | 191.6 ± 10.31 f | 10.29 ± 0.81 f |
| WC3+S | 11.6 ± 0.12 e | 0.46 ± 0.06 c | 22.3 ± 1.98 e | 229.1 ± 16.11 e | 11.86 ± 0.59 e |
| Treatments | Contribution of Na+ to ψs (%) | Contribution of K+ to ψs (%) | Contribution of Leaf-Free Proline to ψs (%) | Contribution of Leaf Betaine to ψs (%) |
|---|---|---|---|---|
| WC1 | 12.29 ± 0.61 e | 70.16 ± 4.84 a | 0.38 ± 0.01 a | 8.51 ± 0.28 c |
| WC1+S | 17.74 ± 1.21 c | 34.39 ± 2.03 d | 0.36 ± 0.01 b | 5.02 ± 0.11 d |
| WC2 | 15.06 ± 0.48 d | 61.62 ± 2.71 b | 0.22 ± 0.01 d | 10.87 ± 0.66 b |
| WC2+S | 23.85 ± 1.17 a | 41.02 ± 0.86 c | 0.21 ± 0.01 d | 9.97 ± 0.36 b |
| WC3 | 16.39 ± 0.31 c | 60.91 ± 3.41 b | 0.15 ± 0.01 e | 15.67 ± 0.77 a |
| WC3+S | 21.19 ± 0.59 b | 41.93 ± 2.64 c | 0.25 ± 0.01 c | 11.60 ± 0.42 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zhang, T.; Yin, B.; Wang, Z.; Li, R.; Li, S. The Influence of Sodium Salt on Growth, Photosynthesis, Na+/K+ Homeostasis and Osmotic Adjustment of Atriplex canescens under Drought Stress. Agronomy 2023, 13, 2434. https://doi.org/10.3390/agronomy13092434
Zhang Z, Zhang T, Yin B, Wang Z, Li R, Li S. The Influence of Sodium Salt on Growth, Photosynthesis, Na+/K+ Homeostasis and Osmotic Adjustment of Atriplex canescens under Drought Stress. Agronomy. 2023; 13(9):2434. https://doi.org/10.3390/agronomy13092434
Chicago/Turabian StyleZhang, Zhenzhong, Tan Zhang, Baosi Yin, Zhongjing Wang, Runjie Li, and Shen Li. 2023. "The Influence of Sodium Salt on Growth, Photosynthesis, Na+/K+ Homeostasis and Osmotic Adjustment of Atriplex canescens under Drought Stress" Agronomy 13, no. 9: 2434. https://doi.org/10.3390/agronomy13092434




