Towards Managing Biodiversity of European Marginal Agricultural Land for Biodiversity-Friendly Biomass Production
Abstract
:1. Introduction
1.1. The Role of Biodiversity for Biomass Production on Marginal Land
1.2. Aim of This Study
2. Methods
2.1. Literature Search
- Assessment;
- Biodiversity;
- Biodiversity assessment;
- Biomass production;
- Categorization/categorize;
- Characterization/characterize;
- Conservation;
- Ecosystem services;
- Europe;
- Evaluation/evaluate;
- Functional diversity;
- Marginal land;
- Resilience;
- Response diversity;
- Unproductive land.
2.2. Definitions
3. Results and Discussion
3.1. The Evaluation of Biodiversity in Europe
3.2. Current Biodiversity Monitoring Schemes
3.3. The Role of Genetic Diversity and Landscape Structure
3.4. Temporal Changes of Biodiversity
3.5. Cropping Systems
| Name of Farming System | Key Characteristics | References |
|---|---|---|
| Low-intensity farming systems | Farming systems that have a low level of external inputs, particularly fertilizers and agrochemicals. May involve both crop and livestock systems. These farming systems may take many different forms depending on where they are in Europe and may not fit neatly into one specific farming system. | [139] |
| Traditional agriculture landscapes | Productive, stable farm landscapes that develop slowly and may display high resilience. However, they are experiencing rapid transformation due to environmental pressures such as urban expansion, land abandonment, climate change, or agricultural intensification. | [114] |
| High nature value (HNV) farms | Combines biodiversity with the maintenance of certain land types and farming systems. | [146] |
| Livestock systems | Low-intensity livestock systems are common in mountain areas and are often characterized by transhumance; nearly all remaining HNV grasslands are associated with these systems. | [56] |
| Arable systems | Once common all over Europe but now mostly limited to the Mediterranean region, characterized by low yields and fallow periods that are important for conservation. | [56] |
| Permanent crop systems | Involve trees (olives, fruits, C4 grasses, short rotation coppice) or vines and are common, for example, in the Mediterranean region. Low intensity systems tend to be less specialized and integrate strip-intercropping or livestock grazing. | [25,56] |
| Mixed systems | Often subsistence farms in very isolated areas where farming is combined with other activities such as fishing or forestry. | [56,147] |
| Integrated farming | Aims to optimize agricultural management and inputs in a responsible way, by considering economic, ecological, and social aspects. This system seeks to minimize agrochemical inputs and include ecologically sound management practices when possible. | [128,140] |
| Precision farming | Management is targeted and spatially specific by taking advantage of modern technology. This system aims to take small-scale differences in management at the field level into account and often involves the monitoring of the nutritional status and health of crops or livestock. | [140] |
| Organic farming | Aims to sustain the health of soils, ecosystems, and people by relying on ecological processes, biodiversity, and cycles adapted to local conditions. This system combines tradition, innovation, and science and forbids the use of synthetic pesticides, fertilizers, or antibiotics. | [140,148,149,150] |
| Conservation farming | This system is characterized by three principles: minimizing mechanical soil disturbance, implementing permanent organic soil cover, and diversifying the sequence or associations of crop species. | [71,140,151] |
| Extensive small-scale, semi-subsistence farming | Characterized by low inputs or no external inputs at all and tend to be limited financially. This type represents 40% of all holdings in the EU-27, and a third of this farm type operates on less favored areas. | [152] |
3.6. Findings of the Evaluation of Biodiversity in Marginal Land
- Lifecycle sustainability assessments (LCSAs) of the biomass crops and their respective biobased value webs,
- Geographic information system (GIS) data and simulation models that validate LCSA results,
- Sampling procedures with methodological adaptations/innovations to reduce uncertainty,
- Connections of diversity patterns with complexity of processes in distinct biomass landscapes.

3.7. Hypothetical Case Studies on Biomass Production on Marginal Land
3.7.1. Abandoned Agricultural Land
3.7.2. Brownfield Sites
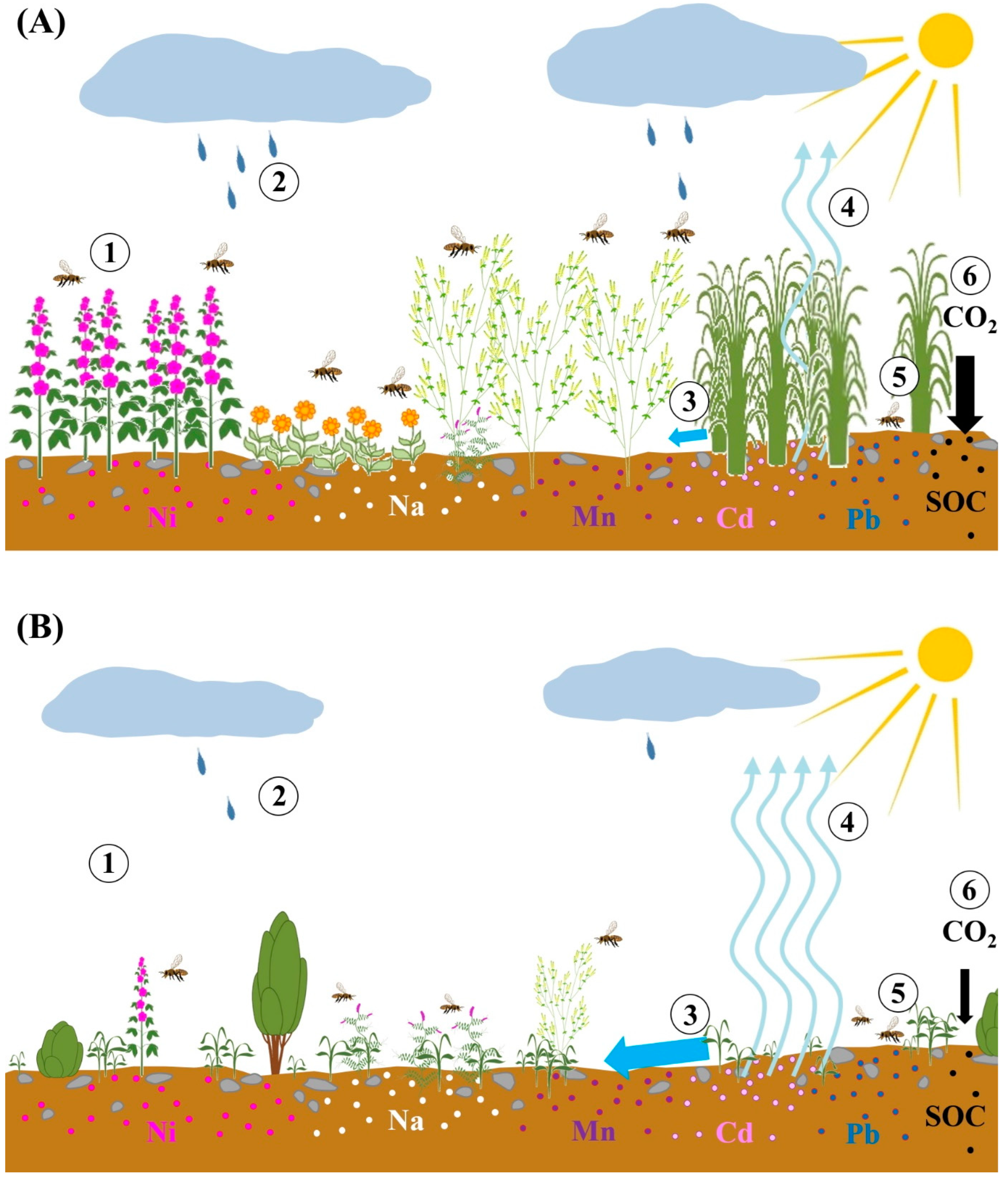
3.7.3. Buffer Strips
3.8. Towards the Development of Biodiversity Indicators for Marginal Land
3.9. Benefits and Limitations of Biodiversity Evaluation on Marginal Land
4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EU Biodiversity Strategy for 2030. Available online: https://environment.ec.europa.eu/strategy/biodiversity-strategy-2030_en (accessed on 18 May 2023).
- Weesie, P.; van Andel, J. On Biodiversity and Its Valuation; Science and Society—Faculty of Science and Engineering; Energy and Sustainability Research Insitute Groningen, University of Groningen: Groningen, The Netherlands, 2003; p. 37. [Google Scholar]
- United Nations. Convention on Biological Diversity; United Nations: New York, NY, USA, 1992; pp. 1–28. [Google Scholar]
- Ghilarov, A. What Does ‘Biodiversity’ Mean—Scientific Problem or Convenient Myth? Trends Ecol. Evol. 1996, 11, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Swingland, I.R. Biodiversity, Definition of. In Enclyclopedia of Biodviersity; Academic Press: New York, NY, USA, 2001; p. 15. ISBN 978-0-08-091782-5. [Google Scholar]
- Gamfeldt, L.; Roger, F. Revisiting the Biodiversity–Ecosystem Multifunctionality Relationship. Nat. Ecol. Evol. 2017, 1, 168. [Google Scholar] [CrossRef] [PubMed]
- DeLong, D.C. Defining Biodiversity. Wildl. Soc. Bull. 1996, 24, 738–749. [Google Scholar]
- Hasan, R.; Scholes, R.J.; Ash, N. Millennium Ecosystem Assessment Ecosystems and Human Well-Being, Current State and Trends; Island Press: Washington, DC, USA, 2005; Volume 1. [Google Scholar]
- Mayer, P. Biodiversity—The Appreciation of Different Thought Styles and Values Helps to Clarify the Term. Restor. Ecol. 2006, 14, 105–111. [Google Scholar] [CrossRef]
- The Norwegian Scientific Committee for Food and Environment; Finne, M.A.; Wendell, M. Summary Report of Joint VKM and EFSA Symposium on Risk Assessment and Risk Management Cooperation on Environmental Protection Goals. EFSA Support. Publ. 2018, 15, 1405E. [Google Scholar] [CrossRef] [Green Version]
- De Groot, R.; Fisher, B.; Christie, M.; Aronson, J.; Braat, L.; Gowdy, J.; Haines-Young, R.; Maltby, E.; Neuville, A.; Polasky, S.; et al. Integrating the Ecological and Economic Dimensions in Biodiversity and Ecosystem Service Valuation. In TEEB Foundations, the Economics of Ecosystems and Biodiversity: Ecological and Economic Foundations; Kumar, P., Ed.; Earthscan (Routledge): Oxford, UK, 2010. [Google Scholar]
- De Groot, R.; Brander, L.; van der Ploeg, S.; Costanza, R.; Bernard, F.; Braat, L.; Christie, M.; Crossman, N.; Ghermandi, A.; Hein, L.; et al. Global Estimates of the Value of Ecosystems and Their Services in Monetary Units. Ecosyst. Serv. 2012, 1, 50–61. [Google Scholar] [CrossRef]
- Fritsche, U.; Brunori, G.; Chiaramonti, D.; Galanakis, C.M.; Hellweg, S.; Matthews, R.; Panoutsou, C. Future Transitions for the Bioeconomy towards Sustainable Development and a Climate-Neutral Economy—Knowledge Synthesis Final Report; Publications Office of the European Union: Luxembourg, 2020; p. 89. [Google Scholar]
- Antar, M.; Lyu, D.; Nazari, M.; Shah, A.; Zhou, X.; Smith, D.L. Biomass for a Sustainable Bioeconomy: An Overview of World Biomass Production and Utilization. Renew. Sustain. Energy Rev. 2021, 139, 110691. [Google Scholar] [CrossRef]
- Lewandowski, I. (Ed.) Bioeconomy: Shaping the Transition to a Sustainable, Biobased Economy; Springer Nature Switzerland AG: Cham, Switzerland, 2018; ISBN 978-3-319-68152-8. [Google Scholar]
- Wagner, M.; Winkler, B.; Lask, J.; Weik, J.; Kiesel, A.; Koch, M.; Clifton-Brown, J.; Von Cossel, M. The True Costs and Benefits of Miscanthus Cultivation. Agronomy 2022, 12, 3071. [Google Scholar] [CrossRef]
- Von Cossel, M.; Winkler, B.; Mangold, A.; Lask, J.; Wagner, M.; Lewandowski, I.; Elbersen, B.; van Eupen, M.; Mantel, S.; Kiesel, A. Bridging the gap between biofuels and biodiversity through monetizing environmental services of miscanthus cultivation. Earth’s Future 2020, 8, e2020EF001478. [Google Scholar] [CrossRef]
- Csikós, N.; Tóth, G. Concepts of Agricultural Marginal Lands and Their Utilisation: A Review. Agric. Syst. 2023, 204, 103560. [Google Scholar] [CrossRef]
- Arshad, M.N.; Donnison, I.; Rowe, R. Marginal Lands: Concept, Classification Criteria and Management; Supergen Bioenergy Hub; Aston University: Birmingham, UK, 2022. [Google Scholar]
- Ciria, C.S.; Sanz, M.; Carrasco, J.; Ciria, P. Identification of Arable Marginal Lands under Rainfed Conditions for Bioenergy Purposes in Spain. Sustainability 2019, 11, 1833. [Google Scholar] [CrossRef] [Green Version]
- Galatsidas, S.; Gounaris, N.; Vlachaki, D.; Dimitriadis, E.; Kiourtsis, F.; Keramitzis, D.; Gerwin, W.; Repmann, F.; Rettenmaier, N.; Reinhardt, G. Revealing Bioenergy Potentials: Mapping Marginal Lands in Europe—The SEEMLA Approach. In Proceedings of the Papers of the 26th European Biomass Conference Setting the Course for a biobased Economy, Copenhagen, Denmark, 14–17 May 2018; Forschungszentrum Landschaftsentwicklung und Bergbaulandschaften (FZLB). pp. 31–37. [Google Scholar]
- Gerwin, W.; Repmann, F.; Galatsidas, S.; Vlachaki, D.; Gounaris, N.; Baumgarten, W.; Volkmann, C.; Keramitzis, D.; Kiourtsis, F.; Freese, D. Assessment and Quantification of Marginal Lands for Biomass Production in Europe Using Soil-Quality Indicators. Soil 2018, 4, 267–290. [Google Scholar] [CrossRef] [Green Version]
- Mellor, P.; Lord, R.A.; João, E.; Thomas, R.; Hursthouse, A. Identifying Non-Agricultural Marginal Lands as a Route to Sustainable Bioenergy Provision—A Review and Holistic Definition. Renew. Sustain. Energy Rev. 2021, 135, 110220. [Google Scholar] [CrossRef]
- Scordia, D.; Cosentino, S.L. Perennial Energy Grasses: Resilient Crops in a Changing European Agriculture. Agriculture 2019, 9, 169. [Google Scholar] [CrossRef] [Green Version]
- Von Cossel, M.; Lewandowski, I.; Elbersen, B.; Staritsky, I.; Van Eupen, M.; Iqbal, Y.; Mantel, S.; Scordia, D.; Testa, G.; Cosentino, S.L.; et al. Marginal Agricultural Land Low-Input Systems for Biomass Production. Energies 2019, 12, 3123. [Google Scholar] [CrossRef] [Green Version]
- Rossiter, D.; Schulte, R.; van Velthuizen, H.; Le-Bas, C.; Nachtergaele, F.; Jones, R. Updated Common Bio-Physical Criteria to Define Natural Constraints for Agriculture in Europe; Terres, J., van Orshoven, J., Toth, T., Eds.; EUR, Scientific and technical research series; Publications Office: Luxembourg, 2014; Volume 26638, ISBN 92-79-38190-3. [Google Scholar]
- Confalonieri, R.; Jones, R.; van Diepen, K.; Van Orshoven, J. Scientific Contribution on Combining Biophysical Criteria Underpinning the Delineation of Agricultural Areas Affected by Specific Constraints; Methodology and Factsheets for Plausible Criteria Combinations; European Commission: Brussels, Belgium, 2014. [Google Scholar]
- Hou, W.; Xu, Y.; Xue, S.; Li, J.; Yang, Y.; Yi, Z.; Fu, T. Effects of Soil Physics, Chemistry, and Microbiology on Soil Carbon Sequestration in Infertile Red Soils after Long-Term Cultivation of Perennial Grasses. GCB Bioenergy 2023, 15, 239–253. [Google Scholar] [CrossRef]
- Martani, E.; Ferrarini, A.; Hastings, A.; Amaducci, S. Soil Organic Carbon Significantly Increases When Perennial Biomass Plantations Are Reverted Back to Annual Arable Crops. Agronomy 2023, 13, 447. [Google Scholar] [CrossRef]
- Von Cossel, M.; Wagner, M.; Lask, J.; Magenau, E.; Bauerle, A.; Von Cossel, V.; Warrach-Sagi, K.; Elbersen, B.; Staritsky, I.; Van Eupen, M.; et al. Prospects of Bioenergy Cropping Systems for a More Social-Ecologically Sound Bioeconomy. Agronomy 2019, 9, 605. [Google Scholar] [CrossRef] [Green Version]
- Cervelli, E.; Perta, E.S.; Pindozzi, S. Identification of Marginal Landscapes as Support for Sustainable Development: GIS-Based Analysis and Landscape Metrics Assessment in Southern Italy Areas. Sustainability 2020, 12, 5400. [Google Scholar] [CrossRef]
- Englund, O.; Börjesson, P.; Berndes, G.; Scarlat, N.; Dallemand, J.-F.; Grizzetti, B.; Dimitriou, I.; Mola-Yudego, B.; Fahl, F. Beneficial Land Use Change: Strategic Expansion of New Biomass Plantations Can Reduce Environmental Impacts from EU Agriculture. Glob. Environ. Chang. 2020, 60, 101990. [Google Scholar] [CrossRef]
- Kang, S.; Post, W.M.; Nichols, J.A.; Wang, D.; West, T.O.; Bandaru, V.; Izaurralde, R.C. Marginal Lands: Concept, Assessment and Management. J. Agric. Sci. 2013, 5, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Strijker, D. Marginal Lands in Europe—Causes of Decline. Basic Appl. Ecol. 2005, 6, 99–106. [Google Scholar] [CrossRef]
- Elbersen, B.; van Eupen, E.; Mantel, S.; Verzandvoort, S.; Boogaard, H.; Macher, S.; Cicareli, T.; Elbersen, W.; Bai, Z.; Iqbal, Y.; et al. Deliverable 2.6 Methodological Approaches to Identify and Map Marginal Land Suitable for Industrial Crops in Europe; Wageningen University & Research: Wageningen, The Netherlands, 2018. [Google Scholar]
- Eurostat Glossary: Fallow Land. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Glossary:Fallow_land (accessed on 10 May 2021).
- European Environmental Agency Regional Policy Glossary. Available online: https://ec.europa.eu/regional_policy/en/policy/what/glossary/ (accessed on 10 June 2023).
- Wiegmann, K.; Hennenberg, K.J.; Fritsche, U.R. Degraded Land and Sustainable Bioenergy Feedstock Production. In Proceedings of the Degraded Land and Sustainable Bioenergy Feedstock Production, Öko-Institut, Darmstadt Office, Paris, France, 30 June–1 July 2008; pp. 1–10. [Google Scholar]
- Oldemann, L.R.; Hakkeling, R.T.A.; Sombroek, W.G. World Map of the Status of Human-Induced Soil Degradation: An Explanatory Note; International Soil Reference and Information Centre & United Nations Environment Programme: Wageningen, The Netherlands; Nairobi, Kenya, 1991; pp. 1–22. [Google Scholar]
- Gopalakrishnan, G.; Cristina Negri, M.; Snyder, S.W. A Novel Framework to Classify Marginal Land for Sustainable Biomass Feedstock Production. J. Environ. Qual. 2011, 40, 1593–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferdini, S.; Von Cossel, M.; Wulfmeyer, V.; Warrach-Sagi, K. Climate-Based Identification of Suitable Cropping Areas for Giant Reed and Reed Canary Grass on Marginal Land in Central and Southern Europe under Climate Change. GCB Bioenergy 2023, 15, 424–443. [Google Scholar] [CrossRef]
- Pörtner, H.-O.; Roberts, D.C.; Adams, H.; Adler, C.; Aldunce, P.; Ali, E.; Begum, R.A.; Betts, R.; Kerr, R.B.; Biesbroek, R. Climate Change 2022: Impacts, Adaptation, and Vulnerability; Contribution of Working Group Ii to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar]
- Liu, T.T.; McConkey, B.G.; Ma, Z.Y.; Li, X.; Cheng, L.L. Strengths, Weaknessness, Opportunities and Threats Analysis of Bioenergy Production on Marginal Land|Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/S1876610211013452?token=DDA7B27BFBDEDE9AE7C423FDA9913137AE50A2192710EB65C6B27DCA85BCB13849ECEA50725F8A4739BEA4AAA7FC98F2 (accessed on 10 September 2020).
- Siggia, D.; Lasorella, M.V.; Kolte, A.; Rossi, M.; Pawar, A. Performance Orientation Towords Biodiversity Impacts of Energy Crop Production on Agricultural Land Use and Farmland Habitats in Europe. J. Crit. Rev. 2020, 7, 1–6. [Google Scholar] [CrossRef]
- Carlsson, G.; Mårtensson, L.-M.; Prade, T.; Svensson, S.-E.; Jensen, E.S. Perennial Species Mixtures for Multifunctional Production of Biomass on Marginal Land. GCB Bioenergy 2017, 9, 191–201. [Google Scholar] [CrossRef]
- Castro, H.; Castro, P. Mediterranean Marginal Lands in Face of Climate Change: Biodiversity and Ecosystem Services. In Climate Change-Resilient Agriculture and Agroforestry; Climate Change Management; Springer: Berlin/Heidelberg, Germany, 2019; pp. 175–187. [Google Scholar] [CrossRef]
- Biała, K.; Condé, S.; Delbaere, B.; Jones-Walters, L.; Torre-Marín, A. Streamlining European Biodiversity Indicators 2020: Building a Future on Lessons Learnt from the SEBI 2010 Process; European Environment Agency: Copenhagen, Denmark, 2012; pp. 1–45. [Google Scholar]
- EASAC. A User’s Guide to Biodiversity Indicators; European Academies Science Advisory Council: Halle, Germany, 2005. [Google Scholar]
- Holling, C.S. Resilience and Stability of Ecological Systems. Annu. Rev. Ecol. Syst. 1973, 4, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Mori, A.S.; Furukawa, T.; Sasaki, T. Response Diversity Determines the Resilience of Ecosystems to Environmental Change. Biol. Rev. 2013, 88, 349–364. [Google Scholar] [CrossRef]
- Sato, C.F.; Westgate, M.J.; Barton, P.S.; Foster, C.N.; O’Loughlin, L.S.; Pierson, J.C.; Balmer, J.; Chapman, J.; Catt, G.; Detto, T.; et al. The Use and Utility of Surrogates in Biodiversity Monitoring Programmes. J. Appl. Ecol. 2019, 56, 1304–1310. [Google Scholar] [CrossRef] [Green Version]
- Whittaker, R.H. Evolution and Measurement of Species Diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef] [Green Version]
- Veron, S.; Davies, T.J.; Cadotte, M.W.; Clergeau, P.; Pavoine, S. Predicting Loss of Evolutionary History: Where Are We? Biol. Rev. 2017, 92, 271–291. [Google Scholar] [CrossRef] [PubMed]
- Kark, S.; van Rensburg, B.J. Ecotones: Marginal or Central Areas of Transition? Isr. J. Ecol. Evol. 2006, 52, 29–53. [Google Scholar] [CrossRef] [Green Version]
- Shimadzu, H.; Dornelas, M.; Magurran, A.E. Measuring Temporal Turnover in Ecological Communities. Methods Ecol. Evol. 2015, 6, 1384–1394. [Google Scholar] [CrossRef] [Green Version]
- Bignal, E.M.; McCracken, D.I. The Nature Conservation Value of European Traditional Farming Systems. Environ. Rev. 2000, 8, 149–171. [Google Scholar] [CrossRef]
- Maes, J.; Teller, A.; Erhard, M.; Condé, S.; Vallecillo Rodrigeuz, S.; Barredo Cano, J.I.; Paracchini, M.L.; Abdul Malak, D.; Trombetti, M.; Vigiak, O.; et al. Mapping and Assessment of Ecosystems and Their Services (MAES)—Biodiversity Information System for Europe. Available online: https://biodiversity.europa.eu/maes (accessed on 1 October 2020).
- Maes, J.; Teller, A.; Erhard, M.; Liquete, C.; Braat, L.; Berry, P.M.; Egoh, B.N.; Puydarrieux, P.; Fiorina, C.; Santos-Martin, F.; et al. Mapping and Assessment of Ecosystems and Their Services: An Analytical Framework for Ecosystem Assessments under Action 5 of the EU Biodiversity Strategy to 2020; European Commission: Brussels, Belgium, 2013; pp. 1–53. [Google Scholar]
- Lengyel, S.; Kobler, A.; Kutnar, L.; Framstad, E.; Henry, P.Y.; Babij, V.; Gruber, B.; Schmeller, D.; Henle, K. A Review and a Framework for the Integration of Biodiversity Monitoring at the Habitat Level. Biodivers. Conserv. 2008, 17, 3341–3356. [Google Scholar] [CrossRef]
- European Commission Biodiversity Strategy—Environment—European Commission. Available online: https://ec.europa.eu/environment/nature/biodiversity/strategy/index_en.htm (accessed on 1 October 2020).
- Magurran, A.E.; Baillie, S.R.; Buckland, S.T.; Dick, J.M.P.; Elston, D.A.; Scott, E.M.; Smith, R.I.; Somerfield, P.J.; Watt, A.D. Long-Term Datasets in Biodiversity Research and Monitoring: Assessing Change in Ecological Communities through Time. Trends Ecol. Evol. 2010, 25, 574–582. [Google Scholar] [CrossRef]
- Julliard, R.; Henry, P.Y.; Clobert, J.; Dziock, F.; Henle, K.; Nowicki, P.; Sammul, M. Deliverable 2: Recommendations for Survey Design and Data Analysis; EuMon Project No. 006463. 2005. Available online: https://www.google.com/url?sa=i&rct=j&q=&esrc=s&source=web&cd=&ved=0CAIQw7AJahcKEwiIzJWkscD_AhUAAAAAHQAAAAAQAg&url=https%3A%2F%2Fec.europa.eu%2Fresearch%2Fparticipants%2Fdocuments%2FdownloadPublic%3FdocumentIds%3D080166e5ac7b6471%26appId%3DPPGMS&psig=AOvVaw1KNtGWXyc0lk88nbbRJU4P&ust=1686750077870728 (accessed on 10 June 2023).
- Weber, D.; Hintermann, U.; Zangger, A. Scale and Trends in Species Richness: Considerations for Monitoring Biological Diversity for Political Purposes. Glob. Ecol. Biogeogr. 2004, 13, 97–104. [Google Scholar] [CrossRef]
- OECD. The Post-2020 Biodiversity Framework: Targets, Indicators and Measurability Implications at the Global and National Level (November Version); OECD: Paris, France, 2019. [Google Scholar]
- Geijzendorffer, I.R.; Regan, E.C.; Pereira, H.M.; Brotons, L.; Brummitt, N.; Gavish, Y.; Haase, P.; Martin, C.S.; Mihoub, J.-B.; Secades, C.; et al. Bridging the Gap between Biodiversity Data and Policy Reporting Needs: An Essential Biodiversity Variables Perspective. J. Appl. Ecol. 2016, 53, 1341–1350. [Google Scholar] [CrossRef]
- Hristov, J.; Clough, Y.; Sahlin, U.; Smith, H.G.; Stjernman, M.; Olsson, O.; Sahrbacher, A.; Brady, M.V. Impacts of the EU’s Common Agricultural Policy “Greening” Reform on Agricultural Development, Biodiversity, and Ecosystem Services. Appl. Econ. Perspect. Policy 2020, 42, 716–738. [Google Scholar] [CrossRef] [Green Version]
- Gotelli, N.J.; Colwell, R.K. Estimating Species Richness. In Biological Diversity: Frontiers in Measurement and Assessment; Oxford University Press: Oxford, UK, 2011; pp. 39–54. [Google Scholar]
- Lecoq, L.; Ernoult, A.; Mony, C. Past Landscape Structure Drives the Functional Assemblages of Plants and Birds. Sci. Rep. 2021, 11, 3443. [Google Scholar] [CrossRef]
- Convention on Biological Diversity 2010 Biodiversity Target. Available online: https://www.cbd.int/2010-target/ (accessed on 12 April 2023).
- Liu, Y.; Zhang, X.; Miao, J.; Huang, L.; Frazier, T.; Zhao, B. Evaluation of Salinity Tolerance and Genetic Diversity of Thirty-Three Switchgrass (Panicum virgatum) Populations. BioEnergy Res. 2014, 7, 1329–1342. [Google Scholar] [CrossRef]
- Butsic, V.; Kuemmerle, T.; Pallud, L.; Helmstedt, K.J.; Macchi, L.; Potts, M.D. Aligning Biodiversity Conservation and Agricultural Production in Heterogeneous Landscapes. Ecol. Appl. 2020, 30, e02057. [Google Scholar] [CrossRef] [PubMed]
- Pakeman, R.J.; Le Duc, M.G.; Marrs, R.H. Bracken Distribution in Great Britain: Strategies for Its Control and the Sustainable Management of Marginal Land. Ann. Bot. 2000, 85, 37–46. [Google Scholar] [CrossRef]
- Shinohara, N.; Uchida, K.; Yoshida, T. Contrasting Effects of Land-Use Changes on Herbivory and Pollination Networks. Ecol. Evol. 2019, 9, 13585–13595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donázar, J.A.; Cortés-Avizanda, A.; Fargallo, J.A.; Margalida, A.; Moleón, M.; Morales-Reyes, Z.; Moreno-Opo, R.; Pérez-García, J.M.; Sánchez-Zapata, J.A.; Zuberogoitia, I.; et al. Roles of Raptors in a Changing World: From Flagships to Providers of Key Ecosystem Services. Ardeola 2016, 63, 181–234. [Google Scholar] [CrossRef] [Green Version]
- Araújo, M.B. Biodiversity Hotspots and Zones of Ecological Transition. Conserv. Biol. 2002, 16, 1662–1663. [Google Scholar] [CrossRef] [Green Version]
- Vos., C.C.; Goedhart, P.W.; Lammertsma, D.R.; Spitzen-Van der Sluijs, A.M. Matrix Permeability of Agricultural Landscapes: An Analysis of Movements of the Common Frog (Rana temporaria). Herpetol. J. 2007, 17, 174–182. [Google Scholar]
- Fahrig, L. Relative Effects of Habitat Loss and Fragmentation on Population Extinction. J. Wildl. Manag. 1997, 61, 603–610. [Google Scholar] [CrossRef]
- Fahrig, L. Ecological Responses to Habitat Fragmentation Per Se. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 1–23. [Google Scholar] [CrossRef]
- Morelli, F.; Benedetti, Y.; Callaghan, C.T. Ecological Specialization and Population Trends in European Breeding Birds. Glob. Ecol. Conserv. 2020, 22, e00996. [Google Scholar] [CrossRef]
- Heilpern, S.A.; Wootton, J.T. Process Catalyzers in Amazonian Rivers: Large Woody Debris Modifies Ecosystem Processes across Freshwater Habitats. Ecosphere 2018, 9, e02030. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, A.; Chaneton, E.J. Heterotroph Species Extinction, Abundance and Biomass Dynamics in an Experimentally Fragmented Microecosystem. J. Anim. Ecol. 2002, 71, 594–602. [Google Scholar] [CrossRef] [Green Version]
- Manning, A.D.; Fischer, J.; Lindenmayer, D.B. Scattered Trees Are Keystone Structures—Implications for Conservation. Biol. Conserv. 2006, 132, 311–321. [Google Scholar] [CrossRef]
- Tiang, D.C.F.; Morris, A.; Bell, M.; Gibbins, C.N.; Azhar, B.; Lechner, A.M. Ecological Connectivity in Fragmented Agricultural Landscapes and the Importance of Scattered Trees and Small Patches. Ecol. Process 2021, 10, 20. [Google Scholar] [CrossRef]
- Mehrabi, Z.; Slade, E.M.; Solis, A.; Mann, D.J. The Importance of Microhabitat for Biodiversity Sampling. PLoS ONE 2014, 9, e114015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, L.M.; Proença, V.; Kaplan, J.O.; Pereira, H.M. Maintaining Disturbance-Dependent Habitats. In Rewilding European Landscapes; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Greenwell, M.P.; Brereton, T.; Day, J.C.; Roy, D.B.; Oliver, T.H. Predicting Resilience of Ecosystem Functioning from Co-Varying Species’ Responses to Environmental Change. Ecol. Evol. 2019, 9, 11775–11790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver, T.H.; Heard, M.S.; Isaac, N.J.B.; Roy, D.B.; Procter, D.; Eigenbrod, F.; Freckleton, R.; Hector, A.; Orme, C.D.L.; Petchey, O.L.; et al. Biodiversity and Resilience of Ecosystem Functions. Trends Ecol. Evol. 2015, 30, 673–684. [Google Scholar] [CrossRef] [Green Version]
- Norberg, J.; Urban, M.C.; Vellend, M.; Klausmeier, C.A.; Loeuille, N. Eco-Evolutionary Responses of Biodiversity to Climate Change. Nat. Clim. Chang. 2012, 2, 747–751. [Google Scholar] [CrossRef]
- Le Viol, I.; Jiguet, F.; Brotons, L.; Herrando, S.; Lindström, Å.; Pearce-Higgins, J.W.; Reif, J.; Van Turnhout, C.; Devictor, V. More and More Generalists: Two Decades of Changes in the European Avifauna. Biol. Lett. 2012, 8, 780–782. [Google Scholar] [CrossRef]
- Larsen, F.W.; Bladt, J.; Balmford, A.; Rahbek, C. Birds as Biodiversity Surrogates: Will Supplementing Birds with Other Taxa Improve Effectiveness? J. Appl. Ecol. 2012, 49, 349–356. [Google Scholar] [CrossRef]
- Pereira, H.M.; Navarro, L.M.; Martins, I.N. Global Biodiversity Change: The Bad, the Good, and the Unknown. Annu. Rev. Environ. Resour. 2012, 37, 25–50. [Google Scholar] [CrossRef] [Green Version]
- Plaza, P.I.; Martínez-López, E.; Lambertucci, S.A. The Perfect Threat: Pesticides and Vultures. Sci. Total Environ. 2019, 687, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Salvatori, V.; Linnell, J.D.C. Report on the Conservation Status and Threats for Wolf (Canis lupus) in Europe; Council of Europe & the Large Carnivore Initiative for Europe: Cuneo, Italy, 2005; p. 28. [Google Scholar]
- Linnell, J.D.C.; Boitani, L. Building Biological Realism into Wolf Management Policy: The Development of the Population Approach in Europe. Hystrix Ital. J. Mammal. 2011, 23, 80. [Google Scholar] [CrossRef]
- Vogt, K.; von Arx, M.; Manz, R.; Zimmermann, F.; Kunz, F.; Breitenmoser, U.; Mettler, D.; Hilfiker, D.; Luethi, R.; Hahn, F. 25 Jahre Wolf in der Schweiz; Raubtierökologie und Wildtiermanagement; KORA: Ittigen, Switzerland, 2020; pp. 1–79. [Google Scholar]
- Wagg, C.; Bender, S.F.; Widmer, F.; van der Heijden, M.G.A. Soil Biodiversity and Soil Community Composition Determine Ecosystem Multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamfeldt, L.; Hillebrand, H.; Jonsson, P.R. Multiple Functions Increase the Importance of Biodiversity for Overall Ecosystem Functioning. Ecology 2008, 89, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Verdade, L.M.; Piña, C.I.; Rosalino, L.M. Biofuels and Biodiversity: Challenges and Opportunities. Environ. Dev. 2015, 15, 64–78. [Google Scholar] [CrossRef]
- Smith, P.; Ashmore, M.R.; Black, H.I.J.; Burgess, P.J.; Evans, C.D.; Quine, T.A.; Thomson, A.M.; Hicks, K.; Orr, H.G. REVIEW: The Role of Ecosystems and Their Management in Regulating Climate, and Soil, Water and Air Quality. J. Appl. Ecol. 2013, 50, 812–829. [Google Scholar] [CrossRef]
- Leimer, S.; Kreutziger, Y.; Rosenkranz, S.; Beßler, H.; Engels, C.; Hildebrandt, A.; Oelmann, Y.; Weisser, W.W.; Wirth, C.; Wilcke, W. Plant Diversity Effects on the Water Balance of an Experimental Grassland. Ecohydrology 2014, 7, 1378–1391. [Google Scholar] [CrossRef]
- Hill, M.J.; Biggs, J.; Thornhill, I.; Briers, R.A.; Gledhill, D.G.; White, J.C.; Wood, P.J.; Hassall, C. Urban Ponds as an Aquatic Biodiversity Resource in Modified Landscapes. Glob. Chang. Biol. 2017, 23, 986–999. [Google Scholar] [CrossRef] [Green Version]
- Deharveng, L.; Stoch, F.; Gibert, J.; Bedos, A.; Galassi, D.; Zagmajster, M.; Brancelj, A.; Camacho, A.; Fiers, F.; Martin, P.; et al. Groundwater Biodiversity in Europe. Freshw. Biol. 2009, 54, 709–726. [Google Scholar] [CrossRef]
- Wang, B.; Shuman, J.; Shugart, H.H.; Lerdau, M.T. Biodiversity Matters in Feedbacks between Climate Change and Air Quality: A Study Using an Individual-Based Model. Ecol. Appl. 2018, 28, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Balmford, A.; Green, R.E.; Jenkins, M. Measuring the Changing State of Nature. Trends Ecol. Evol. 2003, 18, 326–330. [Google Scholar] [CrossRef]
- Elmqvist, T.; Folke, C.; Nyström, M.; Peterson, G.; Bengtsson, J.; Walker, B.; Norberg, J. Response Diversity, Ecosystem Change, and Resilience. Front. Ecol. Environ. 2003, 1, 488–494. [Google Scholar] [CrossRef]
- Bender, S.F.; Wagg, C.; van der Heijden, M.G.A. An Underground Revolution: Biodiversity and Soil Ecological Engineering for Agricultural Sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Philip Robertson, G.; Hamilton, S.K.; Del Grosso, S.J.; Parton, W.J. The Biogeochemistry of Bioenergy Landscapes: Carbon, Nitrogen, and Water Considerations. Ecol. Appl. 2011, 21, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.D. Causes and Consequences of Biological Diversity in Soil1. Zoology 2002, 105, 367–375. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G. Microbial Diversity and Soil Functions. Eur. J. Soil Sci. 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Tsiafouli, M.A.; Thébault, E.; Sgardelis, S.P.; de Ruiter, P.C.; van der Putten, W.H.; Birkhofer, K.; Hemerik, L.; de Vries, F.T.; Bardgett, R.D.; Brady, M.V.; et al. Intensive Agriculture Reduces Soil Biodiversity across Europe. Glob. Chang. Biol. 2015, 21, 973–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morizet-Davis, J.; Marting Vidaurre, N.A.; Reinmuth, E.; Rezaei-Chiyaneh, E.; Schlecht, V.; Schmidt, S.; Singh, K.; Vargas-Carpintero, R.; Wagner, M.; Von Cossel, M. Ecosystem Services at the Farm Level—Overview, Synergies, Trade-Offs and Stakeholder Analysis. Glob. Chall. 2023, in press. [CrossRef]
- Brunori, E.; Maesano, M.; Moresi, F.V.; Matteucci, G.; Biasi, R.; Scarascia Mugnozza, G. The Hidden Land Conservation Benefits of Olive-Based (Olea europaea L.) Landscapes: An Agroforestry Investigation in the Southern Mediterranean (Calabria Region, Italy). Land Degrad. Dev. 2020, 31, 801–815. [Google Scholar] [CrossRef]
- Panoutsou, C.; Von Cossel, M.; Ciria, P.; Ciria, C.S.; Baraniecki, P.; Monti, A.; Zanetti, F.; Dubois, J.-L. Social Considerations for the Cultivation of Industrial Crops on Marginal Agricultural Land as Feedstock for Bioeconomy. Biofuels Bioprod. Biorefin. 2022, 16, 1319–1341. [Google Scholar] [CrossRef]
- Biasi, R.; Brunori, E.; Smiraglia, D.; Salvati, L. Linking Traditional Tree-Crop Landscapes and Agro-Biodiversity in Central Italy Using a Database of Typical and Traditional Products: A Multiple Risk Assessment through a Data Mining Analysis. Biodivers. Conserv. 2015, 24, 3009–3031. [Google Scholar] [CrossRef]
- Clifton-Brown, J.; Hastings, A.; Von Cossel, M.; Murphy-Bokern, D.; McCalmont, J.; Whitaker, J.; Alexopoulou, E.; Amaducci, S.; Andronic, L.; Ashman, C.; et al. Perennial Biomass Cropping and Use: Shaping the Policy Ecosystem in European Countries. GCB Bioenergy 2023, 15, 538–558. [Google Scholar] [CrossRef]
- Norgaard, R.B. Traditional Agricultural Knowledge: Past Performance, Future Prospects, and Institutional Implications. Am. J. Agric. Econ. 1984, 66, 874–878. [Google Scholar] [CrossRef]
- Fuller, R.J.; Williamson, T.; Barnes, G.; Dolman, P.M. Human Activities and Biodiversity Opportunities in Pre-Industrial Cultural Landscapes: Relevance to Conservation. J. Appl. Ecol. 2017, 54, 459–469. [Google Scholar] [CrossRef] [Green Version]
- Barney, J.N.; DiTomaso, J.M. Global Climate Niche Estimates for Bioenergy Crops and Invasive Species of Agronomic Origin: Potential Problems and Opportunities. PLoS ONE 2011, 6, e17222. [Google Scholar] [CrossRef]
- Cramer, V.A.; Hobbs, R.J.; Standish, R.J. What’s New about Old Fields? Land Abandonment and Ecosystem Assembly. Trends Ecol. Evol. 2008, 23, 104–112. [Google Scholar] [CrossRef]
- Altieri, M.A. The Ecological Role of Biodiversity in Agroecosystems. Agric. Ecosyst. Environ. 1999, 74, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Hector, A.; Bagchi, R. Biodiversity and Ecosystem Multifunctionality. Nature 2007, 448, 188–190. [Google Scholar] [CrossRef]
- Petrovan, S.O.; Dixie, J.; Yapp, E.; Wheeler, P.M. Bioenergy Crops and Farmland Biodiversity: Benefits and Limitations Are Scale-Dependant for a Declining Mammal, the Brown Hare. Eur. J. Wildl. Res. 2017, 63, 49. [Google Scholar] [CrossRef] [Green Version]
- Van Cauwenbergh, N.; Biala, K.; Bielders, C.; Brouckaert, V.; Franchois, L.; Garcia Cidad, V.; Hermy, M.; Mathijs, E.; Muys, B.; Reijnders, J.; et al. SAFE—A Hierarchical Framework for Assessing the Sustainabilityof Agricultural Systems. Agric. Ecosyst. Environ. 2007, 120, 229–242. [Google Scholar] [CrossRef]
- Dytham, C.; Travis, J.M.J.; Mustin, K.; Benton, T.G. Changes in Species’ Distributions during and after Environmental Change: Which Eco-Evolutionary Processes Matter More? Ecography 2014, 37, 1210–1217. [Google Scholar] [CrossRef] [Green Version]
- Benton, T.G.; Vickery, J.A.; Wilson, J.D. Farmland Biodiversity: Is Habitat Heterogeneity the Key? Trends Ecol. Evol. 2003, 18, 182–188. [Google Scholar] [CrossRef]
- Mantyka-pringle, C.S.; Martin, T.G.; Rhodes, J.R. Interactions between Climate and Habitat Loss Effects on Biodiversity: A Systematic Review and Meta-Analysis. Glob. Chang. Biol. 2012, 18, 1239–1252. [Google Scholar] [CrossRef] [Green Version]
- Graf, R.; Müller, M.; Korner, P.; Jenny, M.; Jenni, L. 20% Loss of Unimproved Farmland in 22 Years in the Engadin, Swiss Alps. Agric. Ecosyst. Environ. 2014, 185, 48–58. [Google Scholar] [CrossRef]
- Zimmermann, B.; Claß-Mahler, I.; Von Cossel, M.; Lewandowski, I.; Weik, J.; Spiller, A.; Nitzko, S.; Lippert, C.; Krimly, T.; Pergner, I.; et al. Mineral-Ecological Cropping Systems—A New Approach to Improve Ecosystem Services by Farming without Chemical Synthetic Plant Protection. Agronomy 2021, 11, 1710. [Google Scholar] [CrossRef]
- Busari, M.A.; Kukal, S.S.; Kaur, A.; Bhatt, R.; Dulazi, A.A. Conservation Tillage Impacts on Soil, Crop and the Environment. Int. Soil Water Conserv. Res. 2015, 3, 119–129. [Google Scholar] [CrossRef] [Green Version]
- Bennett, J.M.; Roberton, S.D.; Jensen, T.A.; Antille, D.L.; Hall, J. A Comparative Study of Conventional and Controlled Traffic in Irrigated Cotton: I. Heavy Machinery Impact on the Soil Resource. Soil Tillage Res. 2017, 168, 143–154. [Google Scholar] [CrossRef]
- Pfiffner, L.; Ostermaier, M.; Stoeckli, S.; Müller, A. Wild Bees Respond Complementarily to ‘High-Quality’Perennial and Annual Habitats of Organic Farms in a Complex Landscape. J. Insect Conserv. 2018, 22, 551–562. [Google Scholar] [CrossRef]
- Grass, I.; Loos, J.; Baensch, S.; Batáry, P.; Librán-Embid, F.; Ficiciyan, A.; Klaus, F.; Riechers, M.; Rosa, J.; Tiede, J.; et al. Land-Sharing/-Sparing Connectivity Landscapes for Ecosystem Services and Biodiversity Conservation. People Nat. 2019, 1, 262–272. [Google Scholar] [CrossRef] [Green Version]
- Fischer, J.; Abson, D.J.; Butsic, V.; Chappell, M.J.; Ekroos, J.; Hanspach, J.; Kuemmerle, T.; Smith, H.G.; von Wehrden, H. Land Sparing versus Land Sharing: Moving Forward. Conserv. Lett. 2014, 7, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Tscharntke, T.; Grass, I.; Wanger, T.C.; Westphal, C.; Batáry, P. Beyond Organic Farming—Harnessing Biodiversity-Friendly Landscapes. Trends Ecol. Evol. 2021, 36, 919–930. [Google Scholar] [CrossRef]
- Librán-Embid, F.; Klaus, F.; Tscharntke, T.; Grass, I. Unmanned Aerial Vehicles for Biodiversity-Friendly Agricultural Landscapes—A Systematic Review. Sci. Total Environ. 2020, 732, 139204. [Google Scholar] [CrossRef] [PubMed]
- Altieri, M.A.; Nicholls, C.I.; Henao, A.; Lana, M.A. Agroecology and the Design of Climate Change-Resilient Farming Systems. Agron. Sustain. Dev. 2015, 35, 869–890. [Google Scholar] [CrossRef] [Green Version]
- Mockshell, J.; Kamanda, J. Beyond the Agroecological and Sustainable Agricultural Intensification Debate: Is Blended Sustainability the Way Forward? Int. J. Agric. Sustain. 2017, 16, 127–149. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I.; Montalba, R. Technological Approaches to Sustainable Agriculture at a Crossroads: An Agroecological Perspective. Sustainability 2017, 9, 349. [Google Scholar] [CrossRef] [Green Version]
- Baldock, D.; Beaufoy, G.; Clark, J. The Nature of Farming: Low Intensity Farming Systems in Nine European Countries; Institute for European Environmental Policy: London, UK, 1994. [Google Scholar]
- Lewandowski, I.; Lippe, M.; Montoya, J.C.; Dickhöfer, U.; Langenberger, G.; Pucher, J.; Schließmann, U.; Derwenskus, F.; Schmid-Staiger, U.; Lippert, C. Primary Production. In Bioeconomy: Shaping the Transition to a Sustainable, Biobased Economy; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-68151-1. [Google Scholar]
- Dauber, J.; Miyake, S. To Integrate or to Segregate Food Crop and Energy Crop Cultivation at the Landscape Scale? Perspectives on Biodiversity Conservation in Agriculture in Europe. Energy Sustain. Soc. 2016, 6, 25. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Almeyda, J.; Elbersen, B.; Monti, A.; Staritsky, I.; Panoutsou, C.; Alexopoulou, E.; Schrijver, R.; Elbersen, W. Assessing the Potentials for Nonfood Crops. In Modeling and Optimization of Biomass Supply Chains; Elsevier: Amsterdam, The Netherlands, 2017; pp. 219–251. [Google Scholar]
- Laiolo, P.; Tella, J.L. Fate of Unproductive and Unattractive Habitats: Recent Changes in Iberian Steppes and Their Effects on Endangered Avifauna. Environ. Conserv. 2006, 33, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Sklenicka, P. Classification of Farmland Ownership Fragmentation as a Cause of Land Degradation: A Review on Typology, Consequences, and Remedies. Land Use Policy 2016, 57, 694–701. [Google Scholar] [CrossRef] [Green Version]
- Tryjanowski, P.; Hartel, T.; Báldi, A.; Szymański, P.; Tobółka, M.; Herzon, I.; Goławski, A.; Konvicka, M.; Hromada, M.; Jerzak, L.; et al. Conservation of Farmland Birds Faces Different Challenges in Western and Central-Eastern Europe. Acta Ornithol. 2011, 46, 1–12. [Google Scholar] [CrossRef] [Green Version]
- European Environment Agency High Nature Value (HNV) Farmland. Available online: https://www.eea.europa.eu/data-and-maps/data/high-nature-value-farmland (accessed on 20 May 2021).
- Arunrat, N.; Sereenonchai, S. Assessing Ecosystem Services of Rice–Fish Co-Culture and Rice Monoculture in Thailand. Agronomy 2022, 12, 1241. [Google Scholar] [CrossRef]
- Assembly, I.G. Definition of Organic Agriculture; IFOAM-Organics International: Bonn, Germany, 2008. [Google Scholar]
- Youngberg, G.; DeMuth, S.P. Organic Agriculture in the United States: A 30-Year Retrospective. Renew. Agric. Food Syst. 2013, 28, 294–328. [Google Scholar] [CrossRef] [Green Version]
- Zikeli, S.; Gruber, S. Reduced Tillage and No-Till in Organic Farming Systems, Germany—Status Quo, Potentials and Challenges. Agriculture 2017, 7, 35. [Google Scholar] [CrossRef] [Green Version]
- Batáry, P.; Matthiesen, T.; Tscharntke, T. Landscape-Moderated Importance of Hedges in Conserving Farmland Bird Diversity of Organic vs. Conventional Croplands and Grasslands. Biol. Conserv. 2010, 143, 2020–2027. [Google Scholar] [CrossRef]
- Meyer, R. Diversity of European Farming Systems and Pathways to Sustainable Intensification. SCHWERPUNKT Technikfolgenabschätzung Theor. Prax. 2014, 23, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Elbersen, B.; Eupen, V.E.; Mantel, S.; Verzandvoort, S.; Boogaard, H.; Mucher, S.; Cicarreli, T.; Elbersen, W.; Bai, Z.; Iqbal, Y.; et al. Deliverable 2.1 Definition and Classification of Marginal Lands Suitable for Industrial Crops in Europe; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- Mishra, S.K.; Negri, M.C.; Kozak, J.; Cacho, J.F.; Quinn, J.; Secchi, S.; Ssegane, H. Valuation of Ecosystem Services in Alternative Bioenergy Landscape Scenarios. GCB Bioenergy 2019, 11, 748–762. [Google Scholar] [CrossRef] [Green Version]
- Nölting, B.; Mann, C. Governance Strategy for Sustainable Land Management and Water Reuse: Challenges for Transdisciplinary Research. Sustain. Dev. 2018, 26, 691–700. [Google Scholar] [CrossRef]
- Fargione, J.; Hill, J.; Tilman, D.; Polasky, S.; Hawthorne, P. Land Clearing and the Biofuel Carbon Debt. Science 2008, 319, 1235–1238. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.J.; Kling, C.L.; Raman, D.R. A Midwest USA Perspective on Von Cossel et al.’s Prospects of Bioenergy Cropping Systems for a MoreSocial-Ecologically Sound Bioeconomy. Agronomy 2020, 10, 1658. [Google Scholar] [CrossRef]
- Van Wesemael, B.; Rambaud, X.; Poesen, J.; Muligan, M.; Cammeraat, E.; Stevens, A. Spatial Patterns of Land Degradation and Their Impacts on the Water Balance of Rainfed Treecrops: A Case Study in South East Spain. Geoderma 2006, 133, 43–56. [Google Scholar] [CrossRef] [Green Version]
- Holland, R.A.; Eigenbrod, F.; Muggeridge, A.; Brown, G.; Clarke, D.; Taylor, G. A Synthesis of the Ecosystem Services Impact of Second Generation Bioenergy Crop Production. Renew. Sustain. Energy Rev. 2015, 46, 30–40. [Google Scholar] [CrossRef]
- Puschenreiter, M.; Mench, M.; Bert, V.; Kumpiene, J.; Kidd, P.; Cundy, A.; Vangronsveld, J.; Renella, G.; Friesl-Hanl, W.; Siebielec, G.; et al. The Greenland Project: Gentle Remediation of Trace Element Contaminated Land. In Proceedings of the 4th International Congress EUROSOIL, Soil Science for the Benefit of Mankind and Environment, Bari, Italy, 2–6 July 2012. [Google Scholar]
- Wagner, M.; Mangold, A.; Lask, J.; Petig, E.; Kiesel, A.; Lewandowski, I. Economic and Environmental Performance of Miscanthus Cultivated on Marginal Land for Biogas Production. GCB Bioenergy 2019, 11, 34–49. [Google Scholar] [CrossRef] [Green Version]
- Renwick, A.; Jansson, T.; Verburg, P.H.; Revoredo-Giha, C.; Britz, W.; Gocht, A.; McCracken, D. Policy Reform and Agricultural Land Abandonment in the EU. Land Use Policy 2013, 30, 446–457. [Google Scholar] [CrossRef]
- Schmeller, D.S.; Henry, P.-Y.; Julliard, R.; Gruber, B.; Clobert, J.; Dziock, F.; Lengyel, S.; Nowicki, P.; Déri, E.; Budrys, E.; et al. Advantages of Volunteer-Based Biodiversity Monitoring in Europe. Conserv. Biol. 2009, 23, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Wittmer, H.; van Zyl, H.; Brown, C.; Rode, J.; Ozdemiroglu, E.; Bertrand, N.; ten Brink, P.; Seidl, A.; Kettunen, M.; Mazza, L.; et al. Guidance Manual for TEEB Country Studies (Version 1.0); Institute for European Environmental Policy: Bruxelles, Belgium, 2013. [Google Scholar]
- Skevas, T.; Swinton, S.M.; Hayden, N.J. What Type of Landowner Would Supply Marginal Land for Energy Crops? Biomass Bioenergy 2014, 67, 252–259. [Google Scholar] [CrossRef]
- Panoutsou, C.; Giarola, S.; Ibrahim, D.; Verzandvoort, S.; Elbersen, B.; Sandford, C.; Malins, C.; Politi, M.; Vourliotakis, G.; Zita, V.E.; et al. Opportunities for Low Indirect Land Use Biomass for Biofuels in Europe. Appl. Sci. 2022, 12, 4623. [Google Scholar] [CrossRef]
- Le Provost, G.; Schenk, N.V.; Penone, C.; Thiele, J.; Westphal, C.; Allan, E.; Ayasse, M.; Blüthgen, N.; Boeddinghaus, R.S.; Boesing, A.L.; et al. The Supply of Multiple Ecosystem Services Requires Biodiversity across Spatial Scales. Nat. Ecol. Evol. 2022, 7, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Nahuelhaul, L.; Benra, F.; Laterra, P.; Marin, S.; Arriagada, R.; Jullian, C. Patterns of Ecosystem Services Supply across Farm Properties: Implications for Ecosystem Services-Based Policy Incentives. Sci. Total Environ. 2018, 634, 941–950. [Google Scholar] [CrossRef] [Green Version]
- Sherren, K.; Greenland-Smith, S. Farm Management Fragmentation in Nova Scotia Does Not Affect Farm Habitat Provision. Can. Geogr. 2019, 63, 297–311. [Google Scholar] [CrossRef]
- Cundy, A.B.; Bardos, R.P.; Church, A.; Puschenreiter, M.; Friesl-Hanl, W.; Müller, I.; Neu, S.; Mench, M.; Witters, N.; Vangronsveld, J. Developing Principles of Sustainability and Stakeholder Engagement for “Gentle” Remediation Approaches: The European Context. J. Environ. Manag. 2013, 129, 283–291. [Google Scholar] [CrossRef]
- Van Meerbeek, K.; Appels, L.; Dewil, R.; Calmeyn, A.; Lemmens, P.; Muys, B.; Hermy, M. Biomass of Invasive Plant Species as a Potential Feedstock for Bioenergy Production. Biofuels Bioprod. Biorefin. 2015, 9, 273–282. [Google Scholar] [CrossRef]
- Fernando, A.L.; Boléo, S.; Barbosa, B.; Costa, J.; Duarte, M.P.; Monti, A. Perennial Grass Production Opportunities on Marginal Mediterranean Land. BioEnergy Res. 2015, 8, 1523–1537. [Google Scholar] [CrossRef]
- Scordia, D.; Papazoglou, E.G.; Kotoula, D.; Sanz, M.; Ciria, C.S.; Pérez, J.; Maliarenko, O.; Prysiazhniuk, O.; Von Cossel, M.; Greiner, B.E.; et al. Towards Identifying Industrial Crop Types and Associated Agronomies to Improve Biomass Production from Marginal Lands in Europe. GCB Bioenergy 2022, 14, 710–734. [Google Scholar] [CrossRef]
- Ende, L.M.; Knöllinger, K.; Keil, M.; Fiedler, A.J.; Lauerer, M. Possibly Invasive New Bioenergy Crop Silphium Perfoliatum: Growth and Reproduction Are Promoted in Moist Soil. Agriculture 2021, 11, 24. [Google Scholar] [CrossRef]
- Spencer, J.L.; Raghu, S. Refuge or Reservoir? The Potential Impacts of the Biofuel Crop Miscanthus x Giganteus on a Major Pest of Maize. PLoS ONE 2009, 4, e8336. [Google Scholar] [CrossRef] [PubMed]
- Koellner, T.; Scholz, R.W. Assessment of Land Use Impacts on the Natural Environment—Part 2: Generic Characterization Factors for Local Species Diversity in Central Europe. Int. J. Life Cycle Assess. 2006, 20, 1–20. [Google Scholar]
- Raghu, S.; Anderson, A.S.; Daehler, C.; Davis, A.S.; Wiedenmann, R.N.; Simberloff, D.; Mack, R. ECOLOGY: Adding Biofuels to the Invasive Species Fire? Science 2006, 313, 1742. [Google Scholar] [CrossRef]
- Ceauşu, S.; Carver, S.; Verburg, P.H.; Kuechly, H.U.; Hölker, F.; Brotons, L.; Pereira, H.M. European Wilderness in a Time of Farmland Abandonment. In Rewilding European Landscapes; Springer: Cham, Switzerland, 2015; pp. 22–46. [Google Scholar]
- Battaglia, M.; Thomason, W.; Fike, J.H.; Evanylo, G.K.; Von Cossel, M.; Babur, E.; Iqbal, Y.; Diatta, A.A. The Broad Impacts of Corn Stover and Wheat Straw Removal for Biofuel Production on Crop Productivity, Soil Health and Greenhouse Gas Emissions: A Review. GCB Bioenergy 2021, 13, 45–57. [Google Scholar] [CrossRef]
- Celma, S.; Sanz, M.; Ciria, P.; Maliarenko, O.; Prysiazhniuk, O.; Daugaviete, M.; Lazdina, D.; Von Cossel, M. Yield Performance of Woody Crops on Marginal Agricultural Land in Latvia, Spain and Ukraine. Agronomy 2022, 12, 908. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Krzyżaniak, M.; Szczukowski, S.; Tworkowski, J.; Załuski, D.; Bieniek, A.; Gołaszewski, J. Effect of Increased Soil Fertility on the Yield and Energy Value of Short-Rotation Woody Crops. BioEnergy Res. 2015, 8, 1136–1147. [Google Scholar] [CrossRef] [Green Version]
- Stolarski, M.J.; Stachowicz, P. Black Locust, Poplar or Willow? Yield and Energy Value in Three Consecutive Four-Year Harvest Rotations. Ind. Crops Prod. 2023, 193, 116197. [Google Scholar] [CrossRef]
- Zalesny Jr, R.S.; Berndes, G.; Dimitriou, I.; Fritsche, U.; Miller, C.; Eisenbies, M.; Ghezehei, S.; Hazel, D.; Headlee, W.L.; Mola-Yudego, B.; et al. Positive Water Linkages of Producing Short Rotation Poplars and Willows for Bioenergy and Phytotechnologies. WIREs Energy Environ. 2019, 8, e345. [Google Scholar] [CrossRef]
- Vanbeveren, S.P.P.; Ceulemans, R. Biodiversity in Short-Rotation Coppice. Renew. Sustain. Energy Rev. 2019, 111, 34–43. [Google Scholar] [CrossRef]
- Baum, S.; Bolte, A.; Weih, M. High Value of Short Rotation Coppice Plantations for Phytodiversity in Rural Landscapes. GCB Bioenergy 2012, 4, 728–738. [Google Scholar] [CrossRef]
- Bressler, A.; Vidon, P.; Hirsch, P.; Volk, T. Valuation of Ecosystem Services of Commercial Shrub Willow (Salix Spp.) Woody Biomass Crops. Environ. Monit. Assess. 2017, 189, 137. [Google Scholar] [CrossRef] [PubMed]
- Ostaff, D.P.; Mosseler, A.; Johns, R.C.; Javorek, S.; Klymko, J.; Ascher, J.S. Willows (Salix Spp.) as Pollen and Nectar Sources for Sustaining Fruit and Berry Pollinating Insects. Can. J. Plant Sci. 2015, 95, 505–516. [Google Scholar] [CrossRef]
- Barbosa, B.; Boléo, S.; Sidella, S.; Costa, J.; Duarte, M.P.; Mendes, B.; Cosentino, S.L.; Fernando, A.L. Phytoremediation of Heavy Metal-Contaminated Soils Using the Perennial Energy Crops Miscanthus Spp. and Arundo donax L. BioEnergy Res. 2015, 8, 1500–1511. [Google Scholar] [CrossRef]
- Nsanganwimana, F.; Pourrut, B.; Mench, M.; Douay, F. Suitability of Miscanthus Species for Managing Inorganic and Organic Contaminated Land and Restoring Ecosystem Services. A Review. J. Environ. Manag. 2014, 143, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Laval-Gilly, P.; Henry, S.; Mazziotti, M.; Bonnefoy, A.; Comel, A.; Falla, J. Miscanthus x Giganteus Composition in Metals and Potassium After Culture on Polluted Soil and Its Use as Biofuel. BioEnergy Res. 2017, 10, 846–852. [Google Scholar] [CrossRef]
- Pulighe, G.; Bonati, G.; Fabiani, S.; Barsali, T.; Lupia, F.; Vanino, S.; Nino, P.; Arca, P.; Roggero, P.P. Assessment of the Agronomic Feasibility of Bioenergy Crop Cultivation on Marginal and Polluted Land: A GIS-Based Suitability Study from the Sulcis Area, Italy. Energies 2016, 9, 895. [Google Scholar] [CrossRef] [Green Version]
- Lask, J.; Magenau, E.; Ferrarini, A.; Kiesel, A.; Wagner, M.; Lewandowski, I. Perennial Rhizomatous Grasses: Can They Really Increase Species Richness and Abundance in Arable Land?—A Meta-Analysis. GCB Bioenergy 2020, 12, 968–978. [Google Scholar] [CrossRef]
- Pidlisnyuk, V.; Stefanovska, T.; Lewis, E.E.; Erickson, L.E.; Davis, L.C. Miscanthus as a Productive Biofuel Crop for Phytoremediation. Crit. Rev. Plant Sci. 2014, 33, 1–19. [Google Scholar] [CrossRef]
- Shang, J.; Zhu, Q.; Zhang, W. Advancing Soil Physics for Securing Food, Water, Soil and Ecosystem Services. Vadose Zone J. 2018, 17, 180207. [Google Scholar] [CrossRef] [Green Version]
- Lask, J.; Martínez Guajardo, A.; Weik, J.; Von Cossel, M.; Lewandowski, I.; Wagner, M. Comparative Environmental and Economic Life Cycle Assessment of Biogas Production from Perennial Wild Plant Mixtures and Maize (Zea mays L.) in Southwest Germany. GCB Bioenergy 2020, 18, 571–585. [Google Scholar] [CrossRef]
- Christen, B.; Dalgaard, T. Buffers for Biomass Production in Temperate European Agriculture: A Review and Synthesis on Function, Ecosystem Services and Implementation. Biomass Bioenergy 2013, 55, 53–67. [Google Scholar] [CrossRef]
- Ferrarini, A.; Serra, P.; Almagro, M.; Trevisan, M.; Amaducci, S. Multiple Ecosystem Services Provision and Biomass Logistics Management in Bioenergy Buffers: A State-of-the-Art Review. Renew. Sustain. Energy Rev. 2017, 73, 277–290. [Google Scholar] [CrossRef]
- Agostini, A.; Serra, P.; Giuntoli, J.; Martani, E.; Ferrarini, A.; Amaducci, S. Biofuels from Perennial Energy Crops on Buffer Strips: A Win-Win Strategy. J. Clean. Prod. 2021, 297, 126703. [Google Scholar] [CrossRef]
- Stutter, M.I.; Richards, S. Relationships between Soil Physicochemical, Microbiological Properties, and Nutrient Release in Buffer Soils Compared to Field Soils. J. Environ. Qual. 2012, 41, 400–409. [Google Scholar] [CrossRef] [Green Version]
- Sieber, S.; Pannell, D.; Müller, K.; Holm-Müller, K.; Kreins, P.; Gutsche, V. Modelling Pesticide Risk: A Marginal Cost-Benefit Analysis of an Environmental Buffer-Zone Programme. Land Use Policy 2010, 27, 653–661. [Google Scholar] [CrossRef]
- Roux, D.J.; Nel, J.L.; Fisher, R.-M.; Barendse, J. Top-down Conservation Targets and Bottom-up Management Action: Creating Complementary Feedbacks for Freshwater Conservation. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 364–380. [Google Scholar] [CrossRef]
- Laurent, M.; Dickie, M.; Becker, M.; Serrouya, R.; Boutin, S. Evaluating the Mechanisms of Landscape Change on White-Tailed Deer Populations. J. Wildl. Manag. 2021, 85, 340–353. [Google Scholar] [CrossRef]
- Santangeli, A.; Chen, Y.; Boorman, M.; Sales Ligero, S.; Albert García, G. Semi-Automated Detection of Tagged Animals from Camera Trap Images Using Artificial Intelligence. Ibis 2022, 164, 1123–1131. [Google Scholar] [CrossRef]
- Vollrath, B.; Werner, A.; Degenbeck, M.; Marzini, K. Energetische Verwertung von kräuterreichen Ansaaten in der Agrarlandschaft—Eine ökologische und wirtschaftliche Alternative bei der Biogasproduktion (Phase II). In Energie aus Wildpflanzen; Bayerische Landesanstalt für Weinbau und Gartenbau: Veitshöchheim, Germany, 2016; p. 241. [Google Scholar]
- Haan, N.L.; Landis, D.A. Pest Suppression Potential Varies across 10 Bioenergy Cropping Systems. GCB Bioenergy 2023, 15, 765–775. [Google Scholar] [CrossRef]
- Varela Pérez, P.; Greiner, B.E.; Von Cossel, M. Socio-Economic and Environmental Implications of Bioenergy Crop Cultivation on Marginal African Drylands and Key Principles for a Sustainable Development. Earth 2022, 3, 652–682. [Google Scholar] [CrossRef]
- Winkler, B.; Lemke, S.; Ritter, J.; Lewandowski, I. Integrated Assessment of Renewable Energy Potential: Approach and Application in Rural South Africa. Environ. Innov. Soc. Transit. 2017, 24, 17–31. [Google Scholar] [CrossRef]
- Ferrarini, A.; Fornasier, F.; Serra, P.; Ferrari, F.; Trevisan, M.; Amaducci, S. Impacts of Willow and Miscanthus Bioenergy Buffers on Biogeochemical N Removal Processes along the Soil–Groundwater Continuum. GCB Bioenergy 2017, 9, 246–261. [Google Scholar] [CrossRef] [Green Version]
- Krzyżaniak, M.; Stolarski, M.J.; Warmiński, K. Life Cycle Assessment of Giant Miscanthus: Production on Marginal Soil with Various Fertilisation Treatments. Energies 2020, 13, 1931. [Google Scholar] [CrossRef] [Green Version]
- Kalt, G.; Mayer, A.; Theurl, M.C.; Lauk, C.; Erb, K.-H.; Haberl, H. Natural Climate Solutions versus Bioenergy: Can Carbon Benefits of Natural Succession Compete with Bioenergy from Short Rotation Coppice? GCB Bioenergy 2019, 11, 1283–1297. [Google Scholar] [CrossRef] [Green Version]
- Naeem, S.; Duffy, J.E.; Zavaleta, E.S. The Functions of Biological Diversity in an Age of Extinction. Science 2012, 336, 1401–1406. [Google Scholar] [CrossRef] [Green Version]
- Batáry, P.; Dicks, L.V.; Kleijn, D.; Sutherland, W.J. The role of agri-environment schemes in conservation and environmental management. Conserv. Biol. 2015, 29, 1006–1016. [Google Scholar] [CrossRef] [Green Version]
- FNR. Anbau Nachwachsender Rohstoffe 2021 Konstant (Translation: Cultivation of Renewable Raw Materials Constant in 2021); Fachagentur Nachwachsende Rohstoffe: Gülzow-Prüzen, Germany, 2022. [Google Scholar]
- Herrmann, C.; Idler, C.; Heiermann, M. Biogas Crops Grown in Energy Crop Rotations: Linking Chemical Composition and Methane Production Characteristics. Bioresour. Technol. 2016, 206, 23–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Term | Definition | Reference |
|---|---|---|
| Unproductive land | Land that is unproductive in terms of agricultural production. | [18] |
| Marginal land | “Lands having limitations which in aggregate are severe for sustained application of a given use and/or are sensitive to land degradation, as a result of inappropriate human intervention, and/or have lost already part or all of their productive capacity as a result of inappropriate human intervention and also include contaminated and potentially contaminated sites that form a potential risk to humans, water, ecosystems, or other receptors”. | [35] |
| Less favored areas | Classified as areas where conditions are present that make farming more difficult due to natural constraints, which increase the costs of production or reduce opportunities for agricultural ventures. | [26] |
| Fallow land | Arable land that is used in a crop rotation system or is otherwise well maintained and is in good agricultural and environmental condition. Fallow land must be left to recover from the processes of agricultural production, usually for at least one entire crop year. This definition includes bare land that maintains no crops at all, land with natural growth that can be used as feed or ploughed, and green fallow areas intended for the production of green manure. | [36] |
| Mountainous areas | Classified as areas in Nordic areas that experience temperatures similar to or lower than the highest peaks of the Alps, non-mountainous areas that form part of mountain ranges, municipalities that are 50% mountainous, but excluding isolated mountain areas that are comprised of less than 5 km2. | [37] |
| Abandoned land | Abandoned land was once used for a particular purpose, such as industrial, silvicultural, or agricultural activities but has since been abandoned. Abandoned land is not the same as fallow land, since there is no intention to eventually resume activities. This land type is difficult to map. | [38] |
| Wasteland | Previous management, natural processes, or other events have rendered this land type unused, unstable, and without agricultural potential. This type includes active dunes, salt flats, rock outcrops, deserts, ice caps, and arid mountain areas. | [39] |
| Brownfields | Land that may be affected by contamination or pollution issues; may be seen as a subset of degraded land. | [23] |
| Degraded land | Land where productive use has been limited by anthropogenic activities. Generally, at least some degraded lands are seen as marginal. | [23] |
| Buffer strips | Small marginal areas near rivers, roadways, or other urban places. | [40] |
| Term | Definition | References |
|---|---|---|
| Biodiversity | “Biodiversity is a state of attribute of a site or area and specifically refers to the variety within and among living organisms, assemblages of living organisms, biotic communities, and biotic processes, whether naturally occurring or modified by humans. Biodiversity can be measured in terms of genetic diversity and the identity and number of different types of species, assemblages of species, biotic communities, and biotic processes, and the amount (e.g., abundance, biomass, cover, rate) and structure of each. It can be observed and measured at any spatial scale ranging from microsites and habitat patches to the entire biosphere”. | [7] |
| Biodiversity parameters | Features of biodiversity that will be used within the characterization matrix produced in this study. Similar to “indicators” used within biodiversity monitoring schemes. | |
| Ecosystem processes | Intrinsic ecosystem characteristics that maintain the ecosystem’s integrity. These processes include, for example, decomposition, production, nutrient and energy cycles or fluxes. | [8] |
| Ecosystem services | Benefits that humans obtain from ecosystems. Includes provisioning services (such as food or water), regulating services (such as flood or disease control), cultural services (such as spiritual, cultural, or recreational services), and supporting services (such as nutrient cycling). | [8] |
| Ecological resilience | The measure of the ability of an ecosystem to absorb changes and continue to persist [49]. In addition, the level of disturbance an ecosystem can experience, without shifting to a different structure with different outputs, depends on ecological dynamics and the organizational/institutional capacity to understand, manage, and respond to those dynamics. | [8] |
| Response diversity | The variety of species that can provision different ecosystem functions, that also have different capacities to respond to disturbance which makes the entire ecosystem more resilient. | [50] |
| Ecological stability | The ability of an ecosystem to quickly return to a state of equilibrium after temporary disturbances. | [49] |
| Biodiversity surrogates | Can be used to indirectly capture biodiversity changes. However, changes must be inferred instead of directly observed. | [51] |
| Species richness | The number of species in an area (in total or at a specific organizational level) but does not reflect abundance or distribution. | [9] |
| Alpha/beta/gamma diversity | Alpha diversity is the diversity in an ecosystem expressed in species richness, beta diversity compares communities using habitat gradients, and gamma diversity is the sum of the alpha values of all the communities in a landscape as well as the differentiation of their beta values. | [52] |
| Functional diversity | Functional diversity is the variety of physiological, morphological, and ecological species traits of an area. In addition, the range, value, and relative abundance of traits present in organisms within a community. | [8,53] |
| Transition zones/overlap zones | Areas of transitions between two biomes or other abiotic differences (elevation, for example). | |
| Ecotones | Areas of transitions between ecological communities. | [54] |
| Temporal turnover | The rate of temporal changes in ecological communities, which occurs as a consequence of shifting community composition and changes in species abundance. | [55] |
| CBD Focal Area | Headline Indicator | SEBI 2010 Specific Indicator |
|---|---|---|
| Status and trends of the components of biological diversity | Trends in the abundance and distribution of selected species | Abundance and distribution of selected species Birds Butterflies |
| Change in status of threatened and/or protected species | Red List index for European species | |
| Species of European interest | ||
| Trends in extent of selected biomes, ecosystems, and habitats | Ecosystem coverage | |
| Habitats of European interest | ||
| Trends in genetic diversity of domesticated animals, cultivated plants, and fish species of major socioeconomic importance | Livestock genetic diversity | |
| Coverage of protected areas | Nationally designated protected areas | |
| Sites designated under the EU Habitats and Birds Directives | ||
| Threats to biodiversity | Nitrogen deposition | Critical load exceedance for nitrogen |
| Trends in invasive alien species (numbers and costs of invasive alien species) | Invasive alien species in Europe | |
| Impact of climate change on biodiversity | Impact of climatic change on bird populations | |
| Ecosystem integrity and ecosystem goods and services | Marine Trophic Index | Marine Trophic Index of European seas |
| Connectivity/fragmentation of ecosystems | Fragmentation of natural and semi-natural areas | |
| Fragmentation of river systems | ||
| Water quality in aquatic ecosystems | Nutrients in transitional, coastal, and marine waters | |
| Freshwater quality | ||
| Sustainable use | Area of forest, agricultural, fishery, and aquaculture ecosystems under sustainable management | Forest: growing stock, increment, and felling |
| Forest: deadwood | ||
| Agriculture: nitrogen balance | ||
| Agriculture: area under management practices potentially supporting biodiversity | ||
| Fisheries: European commercial fish stocks | ||
| Aquaculture: effluent water quality from finfish farms | ||
| Ecological Footprint of European countries | Ecological footprint of European countries | |
| Status of access and benefits sharing | Percentage of European patent applications for inventions based on genetic resources | Patent applications based on genetic resources |
| Status of resource transfers | Funding to biodiversity | Financing biodiversity management |
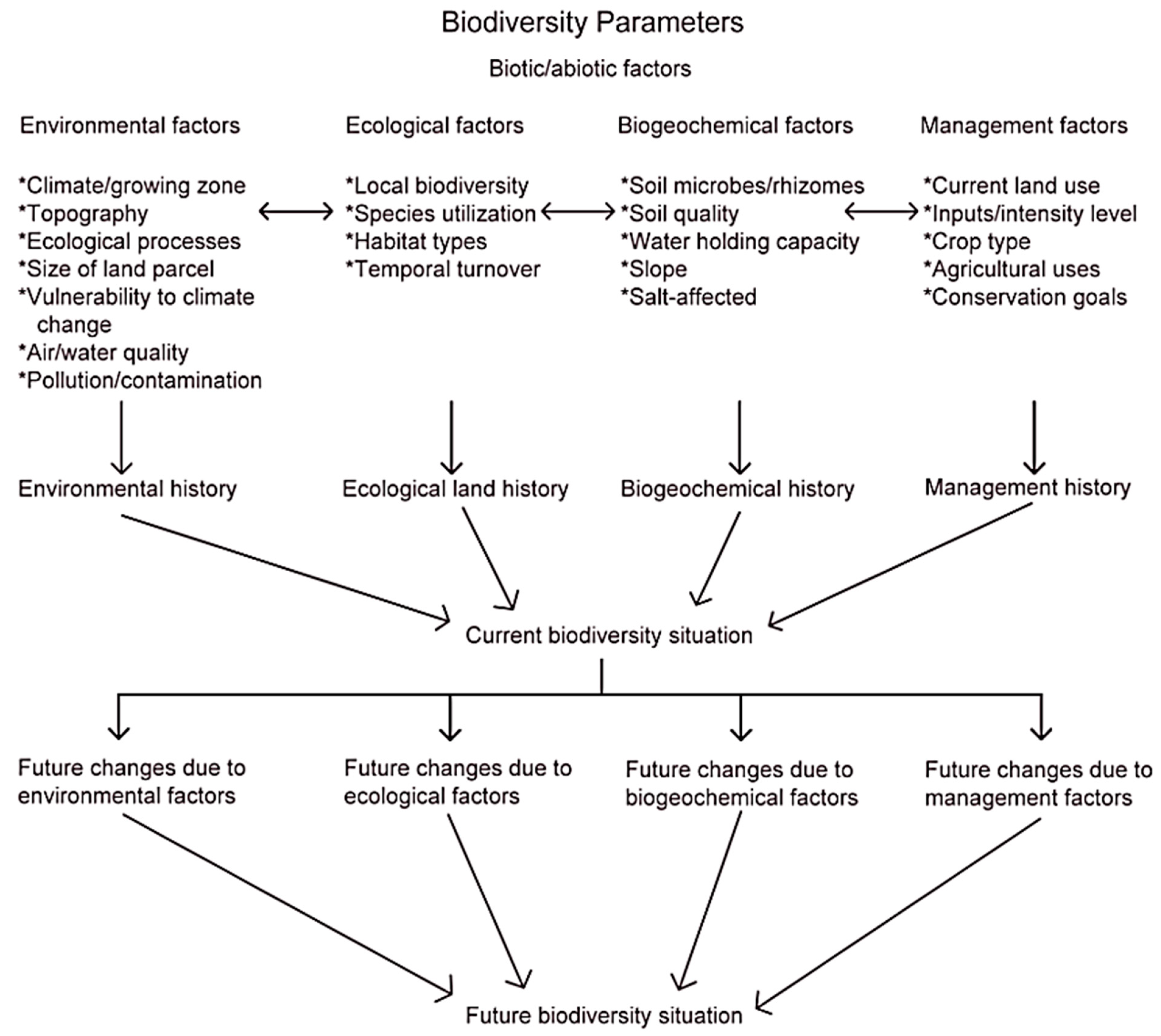
| Public opinion (additional EU focal area) | Public awareness and participation | Public awareness |
| Factor Category | Factor Group within Category | Factors (Where Applicable) | Reference |
|---|---|---|---|
| Ecological factors | Local biodiversity | Abundance/distribution of species | [120] |
| Alpha/beta/gamma diversity | |||
| Functional diversity (at all organizational levels) | [6,121] | ||
| Genetic diversity | [58] | ||
| Species density | |||
| Species utilization | Biotic interactions | [58] | |
| Compatibility with crop | [122] | ||
| Dispersal | |||
| Ecological corridors | [123] | ||
| Food/material availability | |||
| Invasive/non-native species influences | |||
| Migration routes | |||
| Nest sites | |||
| Species dispersal potential/response to climate change | [124] | ||
| Habitat types | Habitat structure/microhabitats | [58,125] | |
| Fragmentation | [78] | ||
| Habitat loss | [126] | ||
| Patch sizes | |||
| Transition zones | [75] | ||
| Temporal turnover | |||
| Biogeochemistry factors | Soil microbes/rhizomes | [107] | |
| Soil quality | Structure/texture | [123] | |
| Nutrient availability | |||
| Depth | |||
| pH | |||
| Productivity | |||
| Level of degradation or compaction | |||
| Vulnerability to erosion | |||
| Water-holding capacity | |||
| Soil type | |||
| Slope | [43] | ||
| Salinity | [43] | ||
| Management factors | Current land use | Pasture | [127] |
| Meadow | |||
| Fallow | |||
| Cropland | |||
| Abandoned | |||
| Inputs (type and level)/intensity level | Fertilizer (mineral/organic) | [128] | |
| Chemical–synthetical plant protection measures | [128] | ||
| Bio-based plant protection measures | [128] | ||
| Biocontrol agents | [128] | ||
| Tillage intensity | [129] | ||
| Number and type of area traffic | [130] | ||
| Crop type | Annual/perennial crop | [131] | |
| Mono-, inter-, mixed-cropping | |||
| Cover cropping | |||
| Types and numbers of flowers | |||
| Conservation management goals | Land sharing | [132,133] | |
| Land sparing | |||
| Habitat networking | [125] | ||
| Agricultural history | Severity of habitat fragmentation (if present) | [134] | |
| Level of degradation/abandonment (if present) | |||
| Farming system | [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burland, A.; von Cossel, M. Towards Managing Biodiversity of European Marginal Agricultural Land for Biodiversity-Friendly Biomass Production. Agronomy 2023, 13, 1651. https://doi.org/10.3390/agronomy13061651
Burland A, von Cossel M. Towards Managing Biodiversity of European Marginal Agricultural Land for Biodiversity-Friendly Biomass Production. Agronomy. 2023; 13(6):1651. https://doi.org/10.3390/agronomy13061651
Chicago/Turabian StyleBurland, Anna, and Moritz von Cossel. 2023. "Towards Managing Biodiversity of European Marginal Agricultural Land for Biodiversity-Friendly Biomass Production" Agronomy 13, no. 6: 1651. https://doi.org/10.3390/agronomy13061651






