Development of a Quality Index to Evaluate the Impact of Abiotic Stress in Saline Soils in the Geothermal Zone of Los Negritos, Michoacán, Mexico
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of Site and Soil Collection
2.2. Soil Quality Indicators
2.3. Development of the Soil Quality Index
2.4. Soil Quality Grades
2.5. Statistical Analysis
3. Results and Discussion
3.1. Soil Quality Indicators
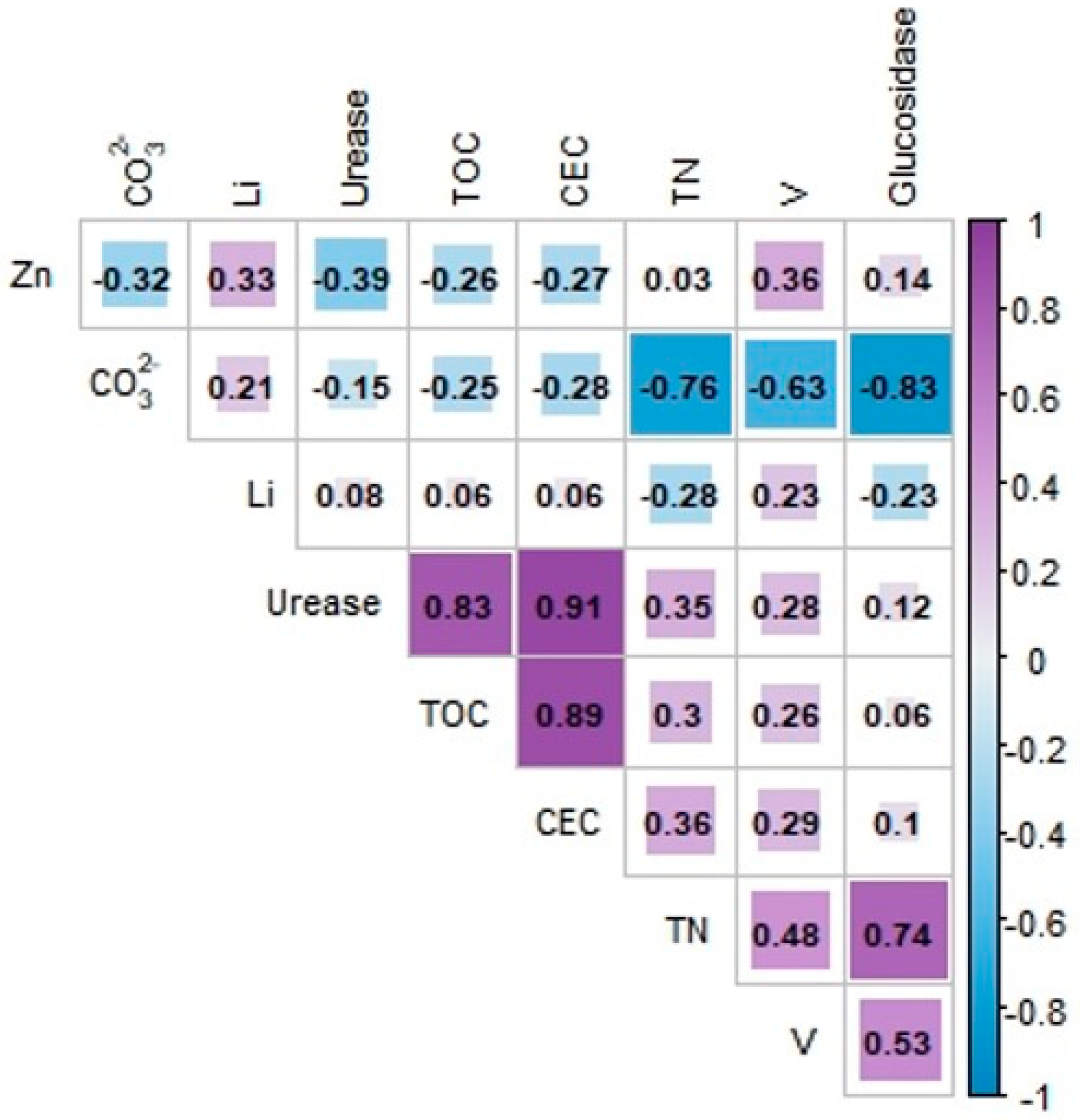
3.2. Development of the Soil Quality Index
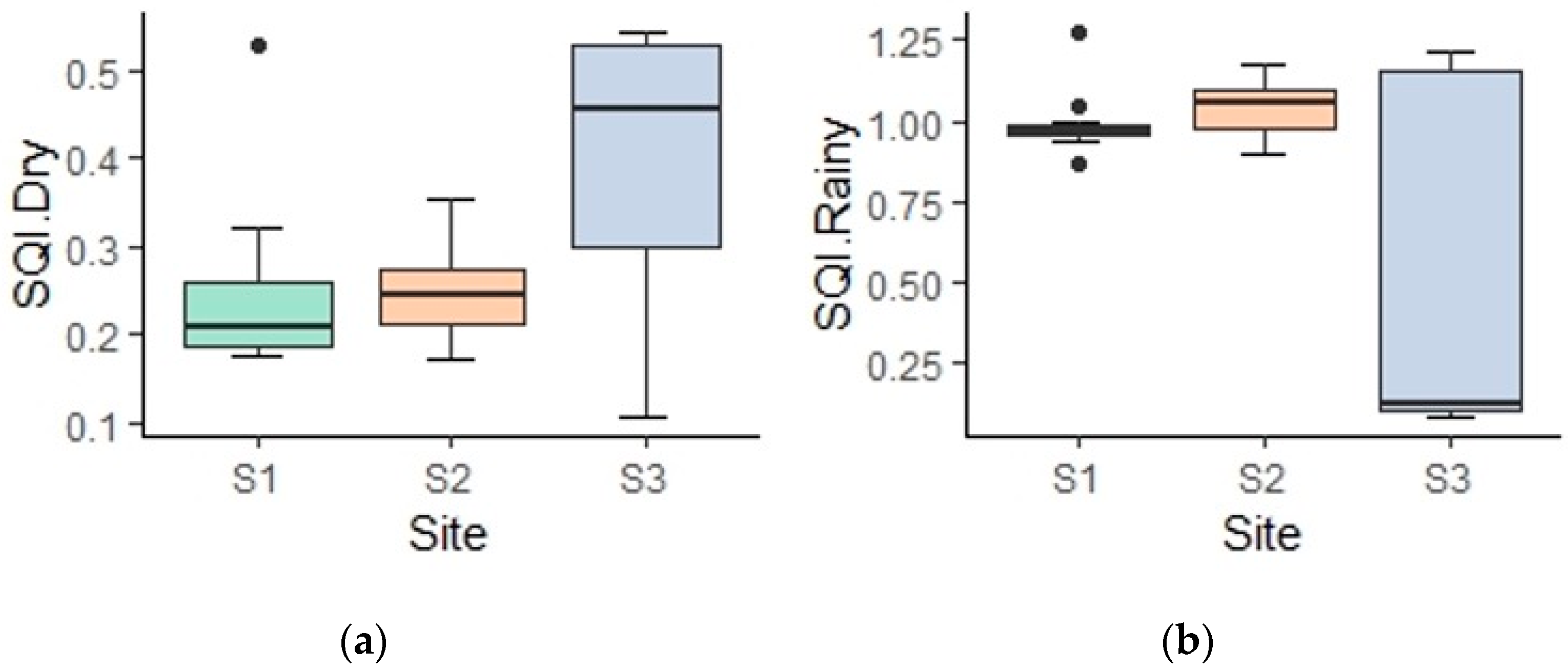
3.3. Soil Quality Grades
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daliakopoulos, I.N.; Tsanis, I.K.; Koutroulis, A.; Kourgialas, N.N.; Varouchakis, A.E.; Karatzas, G.P.; Ritsema, C.J. The threat of soil salinity: A European scale review. Sci. Total Environ. 2016, 573, 727–739. [Google Scholar] [CrossRef]
- Ivushkin, K.; Bartholomeus, H.; Bregt, A.K.; Pulatov, A.; Kempen, B.; De Sousa, L. Global mapping of soil salinity change. Remote Sens. Environ. 2019, 231, 111260. [Google Scholar] [CrossRef]
- Corwin, D.L. Climate change impacts on soil salinity in agricultural areas. Eur. J. Soil Sci. 2020, 72, 842–862. [Google Scholar] [CrossRef]
- Yan, N.; Marschner, P.; Cao, W.; Zuo, C.; Qin, W. Influence of salinity and water content on soil microorganisms. Int. Soil Water Conserv. Res. 2015, 3, 316–323. [Google Scholar] [CrossRef] [Green Version]
- Negacz, K.; Malek, Ž; de Vos, A.; Vellinga, P. Saline soils worldwide: Identifying the most promising areas for saline agriculture. J. Arid Environ. 2022, 203, 104775. [Google Scholar] [CrossRef]
- Organización de las Naciones Unidas para la Alimentación y la Agricultura. Global Map of Salt Affected Soils Version 1.0. Available online: https://www.fao.org/soils-portal/data-hub/soil-maps-and-databases/global-map-of-salt-affected-soils (accessed on 3 December 2021).
- Nguemezi, C.; Tematio, P.; Yemefack, M.; Tsozue, D.; Silatsa, T.B.F. Soil quality and soil fertility status in major soil groups at the Tombel area, South-West Cameroon. Heliyon 2020, 6, e03432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, P.; Wang, J.; Li, C.; Xiao, Q.; Liu, Q.; Sun, Z.; Wang, J.; Cao, C. Soil quality indicators of integrated rice-crayfish farming in the Jianghan Plain, China using a minimum data set. Soil Tillage Res. 2020, 204, 104732. [Google Scholar] [CrossRef]
- Doran, J.W.; Parkin, T.B. Quantitative indicators of soil quality: A minimum data set. In Methods for Assessing Soil Quality; Doran, J.W., Jones, A.J., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 25–37. [Google Scholar] [CrossRef]
- Burns, R.G.; Nannipieri, P.; Benedetti, A.; Hopkins, D.W. Defining soil quality. In Microbiological Methods for Assessing Soil Quality; CABI Publishing: Wallingford, UK, 2006; pp. 15–22. [Google Scholar]
- García, Y.; Ramírez, W.; Sánchez, S. Soil quality indicators: A new way to evaluate this resource. Inf. Express. Pastos Forrajes 2012, 35, 125–138. [Google Scholar]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskensd, L.; Geissend, V.; Kuyperb, T.W.; Mädera, P.; et al. Soil quality–A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Schloter, M.; Nannipieri, P.; Sørensen, S.J.; Van-Elsas, J.D. Microbial indicators for soil quality. Biol. Fertil. Soils 2018, 54, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Mamehpour, N.; Rezapour, S.; Ghaemian, N. Quantitative assessment of soil quality indices for urban croplands in a calcareous semi-arid ecosystem. Geoderma 2021, 382, 114781. [Google Scholar] [CrossRef]
- Tang, D.; Yang, J.; Cheng, P. Comprehensive Evaluation of Soil Substrate Improvement Based on the Minimum Data Set Method. Sustainability 2022, 14, 3939. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, W.; Shen, J.; Li, S.; He, P.; Liang, G. Soil quality assessment of Albic soils with different productivities for eastern China. Soil Tillage Res. 2014, 140, 74–81. [Google Scholar] [CrossRef]
- Guevara-Luna, J.; Hernández-Guzmán, M.; Montoya-Ciriaco, N.; Dendooven, L.; Franco-Hernández, M.O.; Estrada-de los Santos, P.; Vásquez-Murrieta, M.S. The bacterial and archaeal community in saline soils from “Los Negritos” (Mexico) a geothermal area. Pedosphere 2021, 33, 312–320. [Google Scholar] [CrossRef]
- Franco-Hernández, M.O.; Vásquez-Murrieta, M.S.; Patiño-Siciliano, A.; Dendooven, L. Heavy metals concentration in plants growing on mine tailings in Central Mexico. Bioresour. Technol. 2010, 101, 3864–3869. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analysis. Part 2. Microbiological and Biochemical Properties; Weaver, R.W., Angle, J.R., Bottomley, P.S., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 775–833. [Google Scholar]
- Klose, S.; Tabatabai, M.A. Urease activity of microbial biomass in soils. Biol Fertil Soils. 1999, 31, 205–211. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Arylsulfatase activity of soils 1. Soil Sci. Soc. Am. J. 1970, 34, 427–429. [Google Scholar] [CrossRef]
- Andrews, S.S.; Karlen, D.L.; Mitchell, J.P. A comparison of soil quality indexing methods for vegetable production systems in Northern California. Agric. Ecosyst. Environ. 2002, 90, 25–45. [Google Scholar] [CrossRef]
- Li, K.; Wang, C.; Zhang, H.; Zhang, J.; Jiang, R.; Feng, G.; Yu, B. Evaluating the effects of agricultural inputs on the soil quality of smallholdings using improved indices. Catena 2022, 209, 105838. [Google Scholar] [CrossRef]
- Andrews, S.S.; Carroll, C.R. Designing a soil quality assessment tool for sustainable agroecosystem management. Ecol. Appl. 2001, 11, 1573–1585. [Google Scholar] [CrossRef]
- Lima, A.C.R.; Brussaard, L.; Totola, M.R.; Hoogmoed, W.B.; De Goede, R.G.M. A functional evaluation of three indicator sets for assessing soil quality. Appl. Soil Ecol. 2013, 64, 194–200. [Google Scholar] [CrossRef]
- Cherubin, M.R.; Karlen, D.L.; Cerri, C.E.; Franco, A.L.; Tormena, C.A.; Davies, C.A.; Cerri, C.C. Soil quality indexing strategies for evaluating sugarcane expansion in Brazil. PLoS ONE 2016, 11, e0150860. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Liu, S.; Zhang, L.; Li, Q.; Zhou, D. Selecting the minimum data set and quantitative soil quality indexing of alkaline soils under different land uses in northeastern China. Sci. Total Environ. 2018, 616, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Doran, J.W.; Parkin, T.B. Defining and assessing soil quality. In Defining Soil Quality for a Sustainable Environment; Doran, J.W., Coleman, D.C., Bezdicek, D.F., Stewart, B.A., Eds.; SSSA: Madison, WI, USA, 1994; pp. 3–21. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Navarro, A.; Gil-Vázquez, J.M.; Delgado-Iniesta, M.J.; Marín-Sanleandro, P.; Blanco-Bernardeau, A.; Ortiz-Silla, R. Establishing an index and identification of limiting parameters for characterizing soil quality in Mediterranean ecosystems. Catena 2015, 131, 35–45. [Google Scholar] [CrossRef]
- Xian, X.; Pang, M.; Zhang, J.; Zhu, M.; Kong, F.; Xi, M. Assessing the effect of potential water and salt intrusion on coastal wetland soil quality: Simulation study. J. Soils Sediments 2015, 19, 2251–2264. [Google Scholar] [CrossRef]
- Omuto, C.T.; Vargas, R.; Viatkin, K.; Yigini, Y. Mapeo de Suelos Afectados por Salinidad: Lección 4- Modelado Espacial de Suelos Afectados por Salinidad; FAO: Roma, Italy, 2021; pp. 2–15. [Google Scholar]
- Yáñez-Díaz, M.I.; Cantú-Silva, I.; González-Rodríguez, H. Efecto del cambio de uso de suelo en las propiedades químicas de un vertisol. Terra Latinoam. 2018, 36, 369–379. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher-Jenull, J.; Ceccherini, M.T.; Pietramellara, G.; Renella, G.; Schloter, M. Beyond microbial diversity for predicting soil functions: A mini review. Pedosphere 2020, 30, 5–17. [Google Scholar] [CrossRef]
- Bi, C.; Chen, Z.; Wang, J.; Zhou, D. Quantitative assessment of soil health under different planting patterns and soil types. Pedosphere 2013, 23, 194–204. [Google Scholar] [CrossRef]
- Marion, L.F.; Schneider, R.; Cherubin, M.R.; Colares, G.S.; Wiesel, P.G.; da Costa, A.B.; Lobo, E.A. Development of a soil quality index to evaluate agricultural cropping systems in southern Brazil. Soil Tillage Res. 2022, 218, 105293. [Google Scholar] [CrossRef]
- Wawire, A.W.; Csorba, Á.; Kovács, E.; Mairura, F.S.; Tóth, J.A.; Michéli, E. Comparing farmers’ soil fertility knowledge systems and scientific assessment in Upper Eastern Kenya. Geoderma 2021, 396, 115090. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Schloter, M.; Zhang, G.; Chen, Q.; Huang, J.; Han, X. Response of the abundance of key soil microbial nitrogen-cycling genes to multi-factorial global changes. PLoS ONE 2013, 8, e76500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopittke, P.M.; Dalal, R.C.; Menzies, N.W. Changes in exchangeable cations and micronutrients in soils and grains of long-term, low input cropping systems of subtropical Australia. Geoderma 2017, 285, 293–300. [Google Scholar] [CrossRef]
- Sanjuan, B.; Gourcerol, B.; Millot, R.; Rettenmaier, D.; Jeandel, E.; Rombaut, A. Lithium-rich geothermal brines in Europe: An up-date about geochemical characteristics and implications for potential Li resources. Geothermics 2022, 101, 102385. [Google Scholar] [CrossRef]
- Armienta, M.A.; Rodríguez, R.; Ceniceros, N.; Cruz, O.; Aguayo, A.; Morales, P.; Cienfuegos, E. Groundwater quality and geothermal energy. The case of Cerro Prieto geothermal field, México. Renew. Energ. 2014, 63, 236–254. [Google Scholar] [CrossRef]
- Tanveer, M.; Hasanuzzaman, M.; Wang, L. Lithium in environment and potential targets to reduce lithium toxicity in plants. J. Plant Growth Regul. 2019, 38, 1574–1586. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Hassan, W.; Shah, A.N.; Anjum, S.A.; Cheema, S.A.; Ali, I. Lithium toxicity in plants: Reasons, mechanisms and remediation possibilities—A review. Plant Physiol. Biochem. 2016, 107, 104–115. [Google Scholar] [CrossRef]
- Nehrani, S.H.; Askari, M.S.; Saadat, S.; Delavar, M.A.; Taheri, M.; Holden, N.M. Quantification of soil quality under semi-arid agriculture in the northwest of Iran. Ecol. Indic. 2020, 108, 105770. [Google Scholar] [CrossRef]
- Qi, Y.; Darilek, J.L.; Huang, B.; Zhao, Y.; Sun, W.; Gu, Z. Evaluating soil quality indices in an agricultural region of Jiangsu Province, China. Geoderma 2009, 149, 325–334. [Google Scholar] [CrossRef]
- Santos-Francés, F.; Martínez-Graña, A.; Ávila-Zarza, C.; Criado, M.; Sánchez, Y. Comparison of methods for evaluating soil quality of semiarid ecosystem and evaluation of the effects of physico-chemical properties and factor soil erodibility (Northern Plateau, Spain). Geoderma 2019, 354, 113872. [Google Scholar] [CrossRef]
- Goswami, M.; Suresh, D.E.K.A. Plant growth-promoting rhizobacteria—Alleviators of abiotic stresses in soil: A review. Pedosphere 2020, 30, 40–61. [Google Scholar] [CrossRef]
- Jia, Z.; Myrold, D.D.; Conrad, R. Soil biodiversity in a rapidly changing world. Pedosphere 2020, 30, 1–4. [Google Scholar] [CrossRef]
- Parihar, C.M.; Singh, A.K.; Jat, S.L.; Dey, A.; Nayak, H.S.; Mandal, B.N.; Yadav, O.P. Soil quality and carbon sequestration under conservation agriculture with balanced nutrition in intensive cereal-based system. Soil Tillage Res. 2020, 202, 104653. [Google Scholar] [CrossRef]
- He, B.; Cai, Y.; Ran, W.; Jiang, H. Spatial and seasonal variations of soil salinity following vegetation restoration in coastal saline land in eastern China. Catena 2014, 118, 147–153. [Google Scholar] [CrossRef]
- Cantú, M.P.; Becker, A.R.; Bedano, J.C.; Schiviano, H.F.; Parra, B.J. Evaluation of the impact of land use and management change by means of soil quality indicators, Cordoba, Argentina. Cadernos Lab. Xeoloxico Laxe Coruna 2009, 34, 203–214. [Google Scholar]
- Karaca, S.; Dengiz, O.; Turan, İ.D.; Özkan, B.; Dedeoğlu, M.; Gülser, F.; Ay, A. An assessment of pasture soils quality based on multi-indicator weighting approaches in semi-arid ecosystem. Ecol. Indic. 2021, 121, 107001. [Google Scholar] [CrossRef]



| Site | S1 | S2 | S3 | S1 | S2 | S3 | |
|---|---|---|---|---|---|---|---|
| Indicators | Unit | Dry Season | Rainy Season | ||||
| Moisture content | % | 7.30 ± 1.11 Bb | 11.21 ± 1.80 aB | 10.11 ± 2.45 aB | 22.72 ± 1.29 bA | 24.65 ± 1.43 aA | 16.05 ± 0.97 cA |
| WHC | mg kg−1 | 925.7 ± 116.2 bA | 1204.9 ± 63.7 aA | 991.1 ± 84.4 bA | 919.17 ± 15.48 bA | 1010.1 ± 56.8 aB | 864.50 ± 21.07 cB |
| pH | 6.63 ± 0.29 cB | 6.96 ± 0.12 bB | 9.12 ± 0.14 aB | 7.74 ± 0.09 bB | 7.61 ± 0.13 cA | 9.31 ± 0.02 aA | |
| EC | dS m−1 at 25 °C | 2.23 ± 0.41 cA | 12.41 ± 1.86 bA | 34.38 ± 2.77 aA | 1.18 ± 0.53 cB | 10.2 ± 2.26 bB | 26.97 ± 4.32 aB |
| CEC | cmolc kg−1 | 7.01 ± 4.30 aB | 5.06 ± 2.34 aB | 1.31 ± 0.65 bB | 43.33 ± 2.72 bA | 54.58 ± 6.09 aA | 31.81 ± 3.25 cA |
| TOC | mg kg−1 | 149.33 ± 4.45 aB | 126.93 ± 4.56 bB | 28.80 ± 5.37 cB | 527.84 ± 15.29 aA | 586.4 ± 184.9 aA | 496.48 ± 17.48 aA |
| TN | 1.63 ± 0.17 bA | 2.07 ± 0.29 aA | 0.049 ± 0.38 cB | 1.74 ± 0.18 aA | 1.76 ± 0.12 aB | 0.213 ± 0.06 bA | |
| NH4+ | 32.21 ± 13.90 bA | 85.01 ± 38.7 aA | 3.33 ± 1.04 cB | 15.99 ± 0.76 bB | 18.97 ± 1.98 aB | 5.72 ± 1.15 cA | |
| NO2− | 95.98 ± 6.91 aA | 91.31 ± 1.98 aA | 92.59 ± 1.16aA | 66.91 ± 5.11 bB | 78.46 ± 2.44 aB | 68.02 ± 3.93 bB | |
| NO3− | 1908 ± 947 aA | 55.23 ± 7.94 bB | 98.42 ± 25.62bB | 94.8 ± 33.0 bB | 287.6 ± 46.6 aA | 349.5 ± 132.3 aA | |
| PO43− | 103.8 ± 42.5 bA | 218.2 ± 40.3 aA | 76.28 ± 14.56bA | 34.40 ± 5.58 bB | 33.18 ± 2.27 bB | 61.95 ± 6.32 aB | |
| CO32− | ND | ND | 186.70 ± 17.71B | ND | ND | 319.4 ± 11.7 A | |
| HCO3− | 111.85 ± 13.21 bB | 130.49 ± 8.04 aB | 32.20 ± 17.79 cB | 230.5 ± 40.7 bA | 325.4 ± 30.5 aA | 122.0 ± 68.2 cA | |
| SO42− | 723.9 ± 61.0 cA | 1372.7 ± 236.7 bA | 1917.4 ± 116.4 aA | 303.3 ± 142.4 cB | 429.81 ± 15.61 bB | 1832 ± 74.6 aA | |
| Cl− | 110.93 ± 22.90 cA | 205.95 ± 8.02 bA | 450.19 ± 3.65 aA | 20.25 ± 0.0 cB | 225.02 ± 26.2 bA | 475.9 ± 88.9 aA | |
| Sand | 516.31 ± 25.0 bB | 536.31 ± 10.0 aB | 542.97 ± 10.0 aB | 593.48 ± 10.0 aA | 596.8 ± 596.8 aA | 583.48 ± 25.0 aA | |
| Clay | 326.95 ± 5.0 aA | 43.62 ± 5.00 cB | 260.3 ± 30.0 bA | 106.59 ± 10.0 bB | 63.19 ± 10.0 cA | 243.19 ± 10.0 aA | |
| Silt | 156.74 ± 27.84 cB | 420.07 ± 8.66 aA | 196.74 ± 20.0 bA | 299.93 ± 17.32 bA | 340.0 ± 39.7 aB | 173.33 ± 21.79 cA | |
| As | mg kg−1 | 118.39 ± 67.0 aA | 61.9 ± 44.1 abA | 20.7 ± 32.4 bA | 157.1 ± 45.3 aA | 19.2 ± 57.5 bA | 17.7 ± 35.5 bA |
| Ca | 10198 ± 6438 bA | 14381 ± 3989 bA | 47741 ± 24224 aA | 18638 ± 15578 bA | 11605 ± 1461 bA | 49495 ± 17203 aA | |
| Cd | 7.96 ± 2.70 abA | 7.52 ± 1.65 bA | 13.79 ± 8.36 aA | 10.12 ± 7.29 aA | 6.21 ± 0.62 abB | 2.84 ± 3.37 bB | |
| Co | 8.64 ± 4.35 abA | 11.47 ± 1.02 aA | 7.49 ± 2.46 bA | 10.79 ± 1.30 aA | 11.60 ± 1.31 aA | 3.97 ± 4.93 bA | |
| Cr | 51.32 ± 28.41 aA | 70.52 ± 10.40 aA | 55.03 ± 5.76 aA | 70.82 ± 6.81 aA | 77.93 ± 11.67 aA | 27.6 ± 33.3 bB | |
| Cu | 31.94 ± 17.34 bA | 45.26 ± 3.50 aA | 37.45 ± 8.06 abA | 42.91 ± 1.48 aA | 43.71 ± 9.32 aA | 17.27 ± 20.70 bB | |
| Fe | 14213 ± 8124 bA | 21353 ± 1951 aA | 9431 ± 4811 bA | 18173 ± 3697 aA | 22844 ± 3210 aA | 11027 ± 5014 bA | |
| Li | 45.21 ± 15.85 bB | 57.11 ± 3.85 abA | 73.09 ± 26.18 aA | 61.50 ± 7.57 aA | 60.53 ± 6.18 aA | 70.70 ± 40.1 aA | |
| Mg | 6879 ± 3910 bA | 10290 ± 980 bA | 19113 ± 8582 aA | 11497 ± 5583 bA | 11316 ± 1607 bA | 23635 ± 5384 aA | |
| Mn | 353.0 ± 200.9 aA | 388.5 ± 91.8 aA | 309.7 ± 117.2 aA | 457.9 ± 44.5 aA | 333.3 ± 72.5 bA | 267.6 ± 51.8 bA | |
| Mo | 15.34 ± 6.82 aB | 12.5 ± 5.32 aA | 4.84 ± 5.88 bA | 44.02 ± 36.6 aA | 38.0 ± 48.1 abA | 1.59 ± 4.77 bA | |
| Ni | 36.6 ± 33.8 aA | 44.86 ± 22.77 aA | 22.18 ± 5.27 aA | 28.99 ± 3.61 aA | 30.96 ± 7.05 aA | 12.32 ± 14.88 bA | |
| Sr | 84.2 ± 48.8 bA | 147.99 ± 24.62 bA | 521 ± 271 aA | 206.7 ± 177.8 bA | 159.83 ± 25.16 bA | 549.7 ± 171.9 aA | |
| Ti | 465.0 ± 264.8 bA | 974.6 ± 191.9 aA | 360.2 ± 211.3 bA | 548.5 ± 98.2 bA | 1050.0 ± 230.8 aA | 420.4 ± 290.3 bA | |
| V | 46.96 ± 22.66 aA | 59.58 ± 3.42 aA | 26.88 ± 12.18 bA | 54.51 ± 12.20 aA | 58.90 ± 6.42 aA | 14.82 ± 21.20 bA | |
| Zn | 73.7 ± 39.9 aA | 88.53 ± 12.05 aA | 100.4 ± 32.7 aA | 96.45 ± 10.74 aA | 75.47 ± 10.68 aB | 29.7 ± 35.4 bB | |
| β-glucosidase | mg p-nitrophenol g−1 h−1 | 64.51 ± 1.43 bA | 68.08 ± 2.03 aA | 55.38 ± 1.31 cA | 61.45 ± 1.10 bB | 66.60 ± 4.12 aA | 52.68 ± 0.93 cB |
| Alkaline phosphatase | 52.92 ± 1.10 bA | 63.04 ± 1.45 aA | 53.52 ± 0.87 bA | 51.67 ± 0.56 cB | 63.00 ± 0.70 aA | 52.76 ± 0.52 aB | |
| Acid phosphatase | 55.35 ± 1.03 bA | 61.48 ± 1.65 aA | 52.60 ± 1.06 cA | 51.65 ± 0.83 bB | 62.19 ± 1.05 aA | 52.20 ± 0.24 aA | |
| Arylsulfatase | 52.49 ± 1.22 bA | 62.65 ± 1.33 aA | 52.87 ± 0.45 bA | 49.70 ± 0.43 cB | 59.36 ± 0.12 aB | 52.11 ± 0.14 aB | |
| Urease | mg NH4+-N kg−1 h−1 | 112.16 ± 4.28 bB | 130.22 ± 3.03 aB | 102.47 ± 1.21 cB | 194.12 ± 0.61 bA | 232.93 ± 0.98 aA | 194.28 ± 0.57 bA |
| Principal Component | PC1 | PC2 | PC3 | PC4 |
| Eigenvalue | 14.41 | 7.44 | 4.96 | 4.52 |
| Variance % | 36.96 | 19.08 | 12.72 | 11.61 |
| Cumulative % | 36.96 | 56.05 | 68.78 | 80.39 |
| CEC | 0.260 | 0.936 | 0.023 | −0.074 |
| TOC | 0.153 | 0.910 | 0.068 | −0.134 |
| NT | 0.897 | −0.033 | 0.264 | −0.151 |
| CO32− | −0.882 | 0.138 | −0.280 | −0.081 |
| Glucosidase | 0.866 | −0.212 | 0.129 | −0.220 |
| Urease | 0.226 | 0.921 | −0.077 | −0.235 |
| Li | −0.170 | 0.165 | −0.641 | 0.515 |
| V | 0.906 | −0.029 | −0.091 | 0.289 |
| Zn | 0.440 | −0.212 | −0.339 | 0.717 |
| Soil Quality | Very Low | Low | Moderate | High | Very High |
|---|---|---|---|---|---|
| Scale | <0.16 | 0.17–0.32 | 0.33–0.48 | 0.49–0.64 | >0.8 |
| Class | I | II | III | IV | V |
| Season Site | Dry | Rainy | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | |
| SQIw | 0.26 bB | 0.24 bB | 0.43 aA | 0.99 aA | 1.04 aA | 0.56 bA |
| Soil Quality Class | Low | Low | Moderate | Very High | Very High | High |
| Site | 1 | 2 | 3 |
|---|---|---|---|
| Global SQIw | 0.62 a | 0.64 a | 0.49 a |
| Soil Quality Class | High | High | High |
| Season | Dry | Rainy |
|---|---|---|
| Global SQIw | 0.31 b | 0.86 a |
| Soil Quality Class | Low | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahena-Osorio, Y.; Franco-Hernández, M.O.; Pueyo, J.J.; Vásquez-Murrieta, M.S. Development of a Quality Index to Evaluate the Impact of Abiotic Stress in Saline Soils in the Geothermal Zone of Los Negritos, Michoacán, Mexico. Agronomy 2023, 13, 1650. https://doi.org/10.3390/agronomy13061650
Bahena-Osorio Y, Franco-Hernández MO, Pueyo JJ, Vásquez-Murrieta MS. Development of a Quality Index to Evaluate the Impact of Abiotic Stress in Saline Soils in the Geothermal Zone of Los Negritos, Michoacán, Mexico. Agronomy. 2023; 13(6):1650. https://doi.org/10.3390/agronomy13061650
Chicago/Turabian StyleBahena-Osorio, Yanely, Marina Olivia Franco-Hernández, José J. Pueyo, and María Soledad Vásquez-Murrieta. 2023. "Development of a Quality Index to Evaluate the Impact of Abiotic Stress in Saline Soils in the Geothermal Zone of Los Negritos, Michoacán, Mexico" Agronomy 13, no. 6: 1650. https://doi.org/10.3390/agronomy13061650







