Assembly and Analysis of Plastomes for 15 Potato Cultivars Grown in Russia
Abstract
1. Introduction
2. Materials and Methods
2.1. Nucleotide Sequences
2.2. Plastome Assembly and Annotation
2.3. SNP and InDels Identification
2.4. Cytoplasmic DNA Type Determination
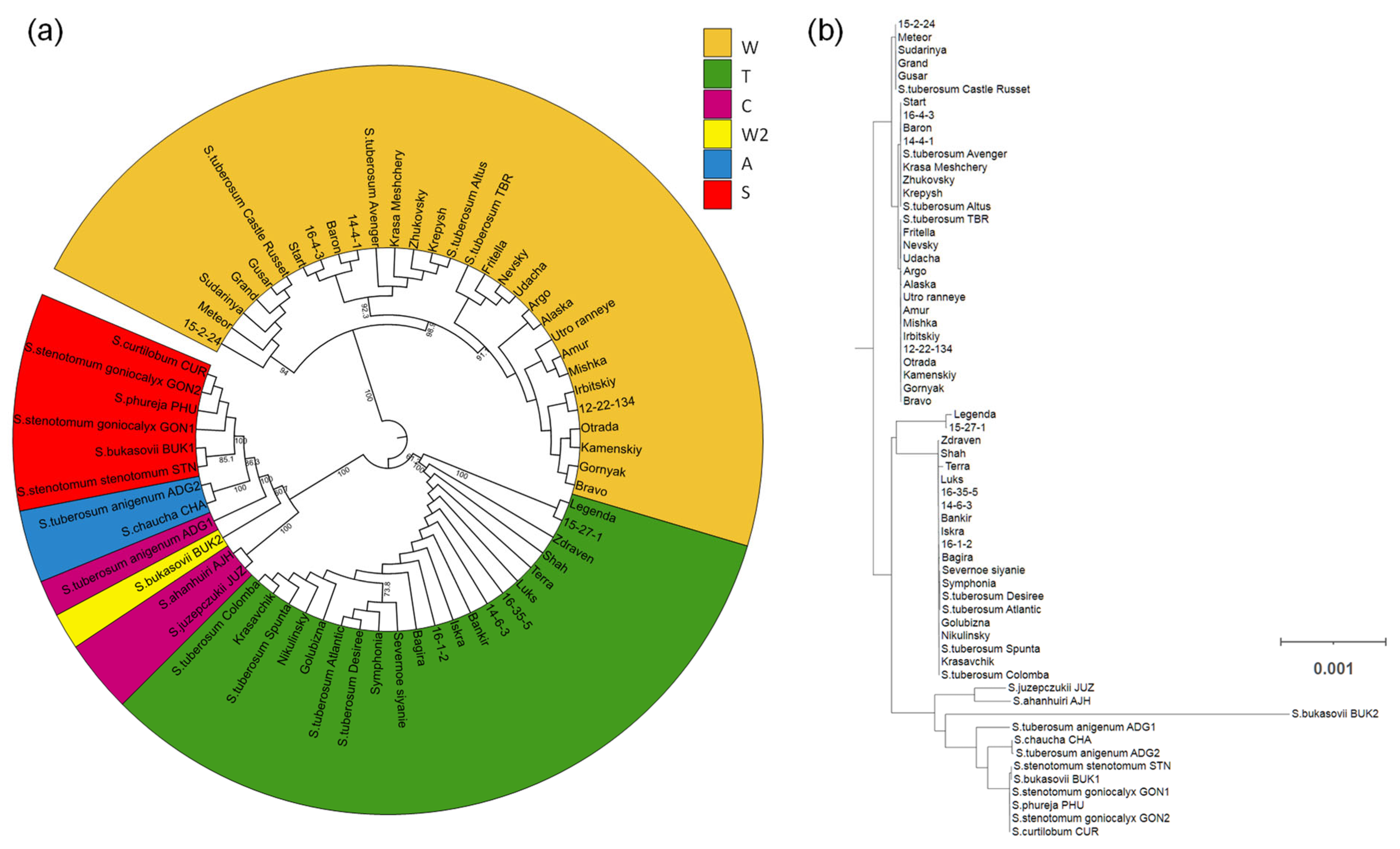
2.5. Phylogenetic Tree Reconstruction
3. Results
3.1. Structural Characteristics of Plastid Genomes and Their Annotations
3.2. Identification of SNPs, Insertions, and Deletions and Assessment of Nucleotide Diversity
3.3. Identification of Chloroplast DNA Types
3.4. Genetic Diversity of Potato Plastomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogalski, M.; do Nascimento Vieira, L.; Fraga, H.P.; Guerra, M.P. Plastid Genomics in Horticultural Species: Importance and Applications for Plant Population Genetics, Evolution, and Biotechnology. Front. Plant Sci. 2015, 6, 586. [Google Scholar] [CrossRef] [PubMed]
- Wicke, S.; Schneeweiss, G.M.; Depamphilis, C.W.; Müller, K.F.; Quandt, D. The evolution of the plastid chromosome in land plants: Gene content, gene order, gene function. Plant Mol. Biol. 2011, 76, 273–297. [Google Scholar] [CrossRef] [PubMed]
- Gitzendanner, M.A.; Soltis, P.S.; Wong, G.K.-S.; Ruhfel, B.R.; Soltis, D.E. Plastid Phylogenomic Analysis of Green Plants: A Billion Years of Evolutionary History. Am. J. Bot. 2018, 105, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Ruess, H.; Liang, Q.; Colleoni, C.; Spooner, D.M. Analyses of 202 Plastid Genomes Elucidate the Phylogeny of Solanum Section Petota. Sci. Rep. 2019, 9, 4454. [Google Scholar] [CrossRef]
- Wheeler, G.L.; Dorman, H.E.; Buchanan, A.; Challagundla, L.; Wallace, L.E. A Review of the Prevalence, Utility, and Caveats of Using Chloroplast Simple Sequence Repeats for Studies of Plant Biology. Appl. Plant Sci. 2014, 2, 1400059. [Google Scholar] [CrossRef]
- Chung, H.-J.; Jung, J.D.; Park, H.-W.; Kim, J.-H.; Cha, H.W.; Min, S.R.; Jeong, W.-J.; Liu, J.R. The Complete Chloroplast Genome Sequences of Solanum Tuberosum and Comparative Analysis with Solanaceae Species Identified the Presence of a 241-Bp Deletion in Cultivated Potato Chloroplast DNA Sequence. Plant Cell Rep. 2006, 25, 1369–1379. [Google Scholar] [CrossRef]
- Achakkagari, S.R.; Kyriakidou, M.; Tai, H.H.; Anglin, N.L.; Ellis, D.; Strömvik, M.V. Complete Plastome Assemblies from a Panel of 13 Diverse Potato Taxa. PLoS ONE 2020, 15, e0240124. [Google Scholar] [CrossRef]
- Yan, L.; Zhu, Z.; Wang, P.; Fu, C.; Guan, X.; Kear, P.; Zhang, C.; Zhu, G. Comparative Analysis of 343 Plastid Genomes of Solanum Section Petota: Insights into Potato Diversity, Phylogeny, and Species Discrimination. J Syst. Evol. 2022. [Google Scholar] [CrossRef]
- Gavrilenko, T.; Antonova, O.; Shuvalova, A.; Krylova, E.; Alpatyeva, N.; Spooner, D.M.; Novikova, L. Genetic diversity and origin of cultivated potatoes based on plastid microsatellite polymorphism. Genet. Resour. Crop. Evol. 2013, 60, 1997–2015. [Google Scholar] [CrossRef]
- Anisimova, I.N.; Gavrilenko, T.A. Cytoplasmic Male Sterility and Prospects for Its Utilization in Breeding, Genetic Studies and Seed Production of Potato. Vavilov. J. Genet. Breed 2017, 21, 83–95. [Google Scholar] [CrossRef]
- Lössl, A.; Adler, N.; Horn, R.; Frei, U.; Wenzel, G. Chondriome-Type Characterization of Potato: Mt α, β, γ, δ, ε and Novel Plastid-Mitochondrial Configurations in Somatic Hybrids. Theor. Appl. Genet. 1999, 98, 1–10. [Google Scholar] [CrossRef]
- Hosaka, K. Who Is the Mother of the Potato? Restriction Endonuclease Analysis of Chloroplast DNA of Cultivated Potatoes. Theor. Appl. Genet. 1986, 72, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, K. Successive Domestication and Evolution of the Andean Potatoes as Revealed by Chloroplast DNA Restriction Endonuclease Analysis. Theor. Appl. Genet. 1995, 90, 356–363. [Google Scholar] [CrossRef]
- Gavrilenko, T.A.; Klimenko, N.S.; Alpatieva, N.V.; Kostina, L.I.; Lebedeva, V.A.; Evdokimova, Z.Z.; Apalikova, O.V.; Novikova, L.Y.; Antonova, O.Y. Cytoplasmic Genetic Diversity of Potato Varieties Bred in Russia and FSU Countries. Vestn. VOGiS 2019, 23, 753–764. [Google Scholar] [CrossRef]
- Hosaka, K.; Sanetomo, R. Development of a Rapid Identification Method for Potato Cytoplasm and Its Use for Evaluating Japanese Collections. Theor. Appl. Genet. 2012, 125, 1237–1251. [Google Scholar] [CrossRef]
- Lössl, A.; Götz, M.; Braun, A.; Wenzel, G. Molecular Markers for Cytoplasm in Potato: Male Sterility and Contribution of Different Plastid-Mitochondrial Configurations to Starch Production. Euphytica 2000, 116, 221–230. [Google Scholar] [CrossRef]
- Sanetomo, R.; Gebhardt, C. Cytoplasmic Genome Types of European Potatoes and Their Effects on Complex Agronomic Traits. BMC Plant Biol. 2015, 15, 162. [Google Scholar] [CrossRef]
- Vanishree, G.; Patil, V.U.; Kaur, R.P.; Bhardwaj, V.; Chakrabarti, S.K.; Kumar, M. Cytoplasmic Types of Indian Potato Cultivars and Their Effect on Important Agronomic Traits. Agric. Res. 2022, 11, 390–397. [Google Scholar] [CrossRef]
- Tiwari, J.K.; Devi, S.; Chandel, P.; Ali, N.; Bhardwaj, V.; Singh, B.P. Organelle Genome Analysis in Somatic Hybrids Between Solanum Tuberosum and S. Pinnatisectum Revealed Diverse Cytoplasm Type in Potato. Agric. Res. 2016, 5, 22–28. [Google Scholar] [CrossRef]
- Martyrosyan, E.V.; Ryzhova, N.N.; Kochieva, E.Z. Polymorphism of Chloroplast Microsatellite DNA Loci in Russian Potato Cultivars. Russ. J. Genet. 2007, 43, 1325–1327. [Google Scholar] [CrossRef]
- Smyda-Dajmund, P.; Śliwka, J.; Janiszewska, M.; Zimnoch-Guzowska, E. Cytoplasmic Diversity of Potato Relatives Preserved at Plant Breeding and Acclimatization Institute in Poland. Mol. Biol. Rep. 2020, 47, 3929–3935. [Google Scholar] [CrossRef] [PubMed]
- Karetnikov, D.I.; Vasiliev, G.V.; Toshchakov, S.V.; Shmakov, N.A.; Genaev, M.A.; Nesterov, M.A.; Ibragimova, S.M.; Rybakov, D.A.; Gavrilenko, T.A.; Salina, E.A.; et al. Analysis of Genome Structure and Its Variations in Potato Cultivars Grown in Russia. Int. J. Mol. Sci. 2023, 24, 5713. [Google Scholar] [CrossRef] [PubMed]
- Lihodeevskiy, G.A.; Shanina, E.P. Structural Variations in the Genome of Potato Varieties of the Ural Selection. Agronomy 2021, 11, 1703. [Google Scholar] [CrossRef]
- Hoopes, G.; Meng, X.; Hamilton, J.P.; Achakkagari, S.R.; de Alves Freitas Guesdes, F.; Bolger, M.E.; Coombs, J.J.; Esselink, D.; Kaiser, N.R.; Kodde, L.; et al. Phased, Chromosome-Scale Genome Assemblies of Tetraploid Potato Reveal a Complex Genome, Transcriptome, and Predicted Proteome Landscape Underpinning Genetic Diversity. Mol. Plant 2022, 15, 520–536. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 1 February 2023).
- Jin, J.-J.; Yu, W.-B.; Yang, J.-B.; Song, Y.; de Pamphilis, C.W.; Yi, T.-S.; Li, D.-Z. GetOrganelle: A Fast and Versatile Toolkit for Accurate de Novo Assembly of Organelle Genomes. Genom. Biol. 2020, 21, 241. [Google Scholar] [CrossRef]
- Tillich, M.; Lehwark, P.; Pellizzer, T.; Ulbricht-Jones, E.S.; Fischer, A.; Bock, R.; Greiner, S. GeSeq—Versatile and Accurate Annotation of Organelle Genomes. Nucleic Acids Res. 2017, 45, W6–W11. [Google Scholar] [CrossRef]
- Amiryousefi, A.; Hyvönen, J.; Poczai, P. IRscope: An Online Program to Visualize the Junction Sites of Chloroplast Genomes. Bioinformatics 2018, 34, 3030–3031. [Google Scholar] [CrossRef]
- Beier, S.; Thiel, T.; Münch, T.; Scholz, U.; Mascher, M. MISA-Web: A Web Server for Microsatellite Prediction. Bioinformatics 2017, 33, 2583–2585. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Page, A.J.; Taylor, B.; Delaney, A.J.; Soares, J.; Seemann, T.; Keane, J.A.; Harris, S.R. SNP-Sites: Rapid Efficient Extraction of SNPs from Multi-FASTA Alignments. Microb. Genom. 2016, 2, e000056. [Google Scholar] [CrossRef] [PubMed]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Krzywinski, M.; Schein, J.; Birol, İ.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An Information Aesthetic for Comparative Genomics. Genom. Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- San Millán, R.M.; Martínez-Ballesteros, I.; Rementeria, A.; Garaizar, J.; Bikandi, J. Online Exercise for the Design and Simulation of PCR and PCR-RFLP Experiments. BMC Res. Notes 2013, 6, 513. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (ITOL) v5: An Online Tool for Phylogenetic Tree Display and Annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Rybakov, D.A.; Antonova, O.Y.; Chukhina, I.G.; Fomina, N.A.; Klimenko, N.S.; Zheltova, V.V.; Meleshin, A.A.; Kochieva, E.Z.; Oves, E.V.; Apshev, K.K.; et al. Nomenclatural Standards and Genetic Passports of Potato Cultivars Bred in the A.G. Lorkh All-Russian Research Institute of Potato Farming. Biotehnol. I Sel. Rastenij 2021, 3, 5–52. [Google Scholar] [CrossRef]
- Klimenko, N.S.; Gavrilenko, T.A.; Chukhina, I.G.; Gadzhiev, N.M.; Evdokimova, Z.Z.; Lebedeva, V.A. Nomenclatural Standards and Genetic Passports of Potato Cultivars Bred at the Leningrad Research Institute for Agriculture “Belogorka”. Biotehnol. I Sel. Rastenij 2021, 3, 18–54. [Google Scholar] [CrossRef]
- Antonova, O.Y.; Shvachko, N.A.; Novikova, L.Y.; Shuvalov, O.Y.; Kostina, L.I.; Klimenko, N.S.; Shuvalova, A.R.; Gavrilenko, T.A. Genetic Diversity of Potato Varieties Bred in Russia and Its Neighboring Countries Based on the Polymorphism of SSR-Loci and Markers Associated with Resistance R-genes. Russ. J. Genet. Appl. Res. 2017, 7, 489–500. [Google Scholar] [CrossRef]
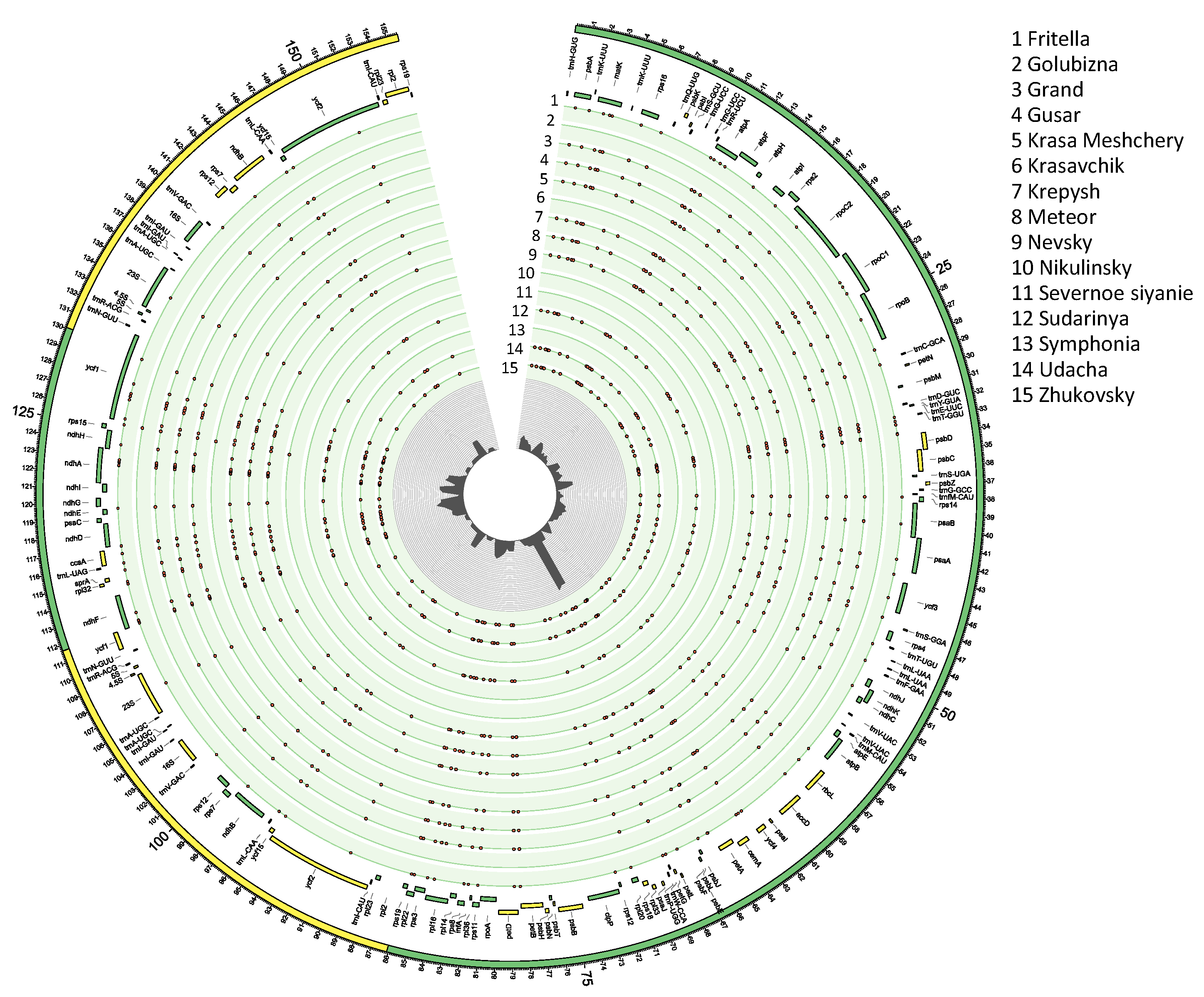
| Cultivar | cpDNA | LSC | IRb | SSC | IRa | ||||
|---|---|---|---|---|---|---|---|---|---|
| size, bp | location | size, bp | location | size, bp | location | size, bp | location | size, bp | |
| Fritella | 155,565 | 1–86,006 | 86,006 | 86,007–111,599 | 25,593 | 111,598–129,972 | 18,375 | 129,973–155,565 | 25,593 |
| Golubizna | 155,296 | 1–85,737 | 85,737 | 85,738–111,330 | 25,593 | 111,329–129,703 | 18,375 | 129,704–155,296 | 25,593 |
| Grand | 155,549 | 1–85,991 | 85,991 | 85,992–111,583 | 25,592 | 111,582–129,957 | 18,376 | 129,958–155,549 | 25,592 |
| Gusar | 155,549 | 1–85,991 | 85,991 | 85,992–111,583 | 25,592 | 111,582–129,957 | 18,376 | 129,958–155,549 | 25592 |
| Krasa Meshchery | 155,562 | 1–86,003 | 85,991 | 86,004–111,596 | 25,593 | 111,595–129,969 | 18,375 | 129,970–155,562 | 25,593 |
| Krasavchik | 155,296 | 1–85,737 | 85,737 | 85,738–111,330 | 25593 | 111329–129703 | 18,375 | 129,704–155,296 | 25,593 |
| Krepysh | 155,562 | 1–86,003 | 86,003 | 86,004–111,596 | 25,593 | 111,595–129,969 | 18,375 | 129,970–155,562 | 25,593 |
| Meteor | 155,549 | 1–85,991 | 85991 | 85,992–111,583 | 25,592 | 111,582–129,957 | 18,376 | 129,958–155,549 | 25,592 |
| Nevsky | 155,565 | 1–86,006 | 86,006 | 86,007–111,599 | 25,593 | 111,598–129,972 | 18,375 | 129,973–155,565 | 25,593 |
| Nikulinsky | 155,296 | 1–85,737 | 85,737 | 85,738–111,330 | 25,593 | 111,329–129,703 | 18,375 | 129,704–155,296 | 25,593 |
| Severnoe siyanie | 155,296 | 1–85,737 | 85,737 | 85,738–111,330 | 25,593 | 111,329–129,703 | 18,375 | 129,704–155,296 | 25,593 |
| Sudarinya | 155,549 | 1–85,991 | 85,991 | 85,992–111,583 | 25,592 | 111582–129957 | 18,376 | 129,958–155,549 | 25,592 |
| Symphonia | 155,296 | 1–85,737 | 85,737 | 85,738–111,330 | 25,593 | 111,329–129,703 | 18,375 | 129,704–155,296 | 25,593 |
| Udacha | 155,565 | 1–86,006 | 86,006 | 86,007–111,599 | 25,593 | 111,598–129,972 | 18,375 | 129,973–155,565 | 25,593 |
| Zhukovsky | 155,562 | 1–86,003 | 86,003 | 86,004–111,596 | 25,593 | 111595–129969 | 18,375 | 129,970–155,562 | 25,593 |
| Cultivar | Number of SNP | Number of SNP in Genes | Genes with SNPs |
|---|---|---|---|
| Fritella | 121 | 55 | 23S, atpA, ccsA, infA, matK, ndhA, ndhB, ndhF, ndhH, ndhK, petD, psaA, psaB, psbA, rbcL, rpl16, rpl20, rpl22, rpoB, rpoC1, rpoC2, rps11, rps14, rps16, rps3, rps8, ycf1, ycf2, ycf3 |
| Golubizna | 1 | 1 | - |
| Grand | 114 | 52 | 23S, atpA, ccsA, infA, matK, ndhA, ndhB, ndhF, ndhH, ndhK, petD, psaA, psaB, psbA, rbcL, rpl14, rpl16, rpl20, rpl22, rpoB, rpoC1, rpoC2, rps11, rps14, rps16, rps3, ycf1, ycf2, ycf3 |
| Gusar | 114 | 52 | 23S, atpA, ccsA, infA, matK, ndhA, ndhB, ndhF, ndhH, ndhK, petD, psaA, psaB, psbA, rbcL, rpl14, rpl16, rpl20, rpl22, rpoB, rpoC1, rpoC2, rps11, rps14, rps16, rps3, ycf1, ycf2, ycf3 |
| Krasa Meshchery | 121 | 54 | 23S, atpA, ccsA, infA, matK, ndhA, ndhB, ndhD, ndhF, ndhH, ndhK, petD, psaA, psaB, psbA, rbcL, rpl16, rpl20, rpl22, rpoB, rpoC1, rpoC2, rps11, rps14, rps16, rps3, ycf1, ycf2, ycf3 |
| Krasavchik | 1 | 1 | - |
| Krepysh | 121 | 54 | 23S, atpA, ccsA, infA, matK, ndhA, ndhB, ndhD, ndhF, ndhH, ndhK, petD, psaA, psaB, psbA, rbcL, rpl16, rpl20, rpl22, rpoB, rpoC1, rpoC2, rps11, rps14, rps16, rps3, ycf1, ycf2, ycf3 |
| Meteor | 114 | 52 | 23S, atpA, ccsA, infA, matK, ndhA, ndhB, ndhF, ndhH, ndhK, petD, psaA, psaB, psbA, rbcL, rpl14, rpl16, rpl20, rpl22, rpoB, rpoC1, rpoC2, rps11, rps14, rps16, rps3, ycf1, ycf2, ycf3 |
| Nevsky | 121 | 55 | 23S, atpA, ccsA, infA, matK, ndhA, ndhB, ndhF, ndhH, ndhK, petD, psaA, psaB, psbA, rbcL, rpl16, rpl20, rpl22, rpoB, rpoC1, rpoC2, rps11, rps14, rps16, rps3, rps8, ycf1, ycf2, ycf3 |
| Nikulinsky | 1 | 1 | - |
| Severnoe siyanie | 0 | 0 | - |
| Sudarinya | 114 | 52 | 23S, atpA, ccsA, infA, matK, ndhA, ndhB, ndhF, ndhH, ndhK, petD, psaA, psaB, psbA, rbcL, rpl14, rpl16, rpl20, rpl22, rpoB, rpoC1, rpoC2, rps11, rps14, rps16, rps3, ycf1, ycf2, ycf3 |
| Symphonia | 0 | 0 | - |
| Udacha | 121 | 55 | 23S, atpA, ccsA, infA, matK, ndhA, ndhB, ndhF, ndhH, ndhK, petD, psaA, psaB, psbA, rbcL, rpl16, rpl20, rpl22, rpoB, rpoC1, rpoC2, rps11, rps14, rps16, rps3, rps8, ycf1, ycf2, ycf3 |
| Zhukovsky | 121 | 54 | 23S, atpA, ccsA, infA, matK, ndhA, ndhB, ndhD, ndhF, ndhH, ndhK, petD, psaA, psaB, psbA, rbcL, rpl16, rpl20, rpl22, rpoB, rpoC1, rpoC2, rps11, rps14, rps16, rps3, ycf1, ycf2, ycf3 |
| Cultivar | Insertions | Deletions | Genes with Insertions | Genes with Deletions | ||||
|---|---|---|---|---|---|---|---|---|
| Total Number | >1 bp | Maximal Size | Total Number | >1 bp | Maximal Size | |||
| Fritella | 19 | 12 | 241 | 13 | 9 | 18 | - | petB |
| Golubizna | 0 | 0 | 0 | 0 | 0 | 0 | - | - |
| Grand | 17 | 9 | 241 | 11 | 6 | 18 | - | petB |
| Gusar | 17 | 9 | 241 | 11 | 6 | 18 | - | petB |
| Krasa Meshchery | 20 | 12 | 241 | 13 | 9 | 18 | - | petB |
| Krasavchik | 0 | 0 | 0 | 0 | 0 | 0 | - | - |
| Krepysh | 20 | 12 | 241 | 13 | 9 | 18 | - | petB |
| Meteor | 17 | 9 | 241 | 11 | 6 | 18 | - | petB |
| Nevsky | 19 | 12 | 241 | 13 | 9 | 18 | - | petB |
| Nikulinsky | 0 | 0 | 0 | 0 | 0 | 0 | - | - |
| Severnoe siyanie | 0 | 0 | 0 | 0 | 0 | 0 | - | - |
| Sudarinya | 17 | 9 | 241 | 11 | 6 | 18 | - | petB |
| Symphonia | 0 | 0 | 0 | 0 | 0 | 0 | - | - |
| Udacha | 19 | 12 | 241 | 13 | 9 | 18 | - | petB |
| Zhukovsky | 20 | 12 | 241 | 13 | 9 | 18 | - | petB |
| Cultivar | NTCP6 | NTCP7 | NTCP8 | NTCP9 | NTCP12 | NTCP14 | NTCP18 | Type |
|---|---|---|---|---|---|---|---|---|
| Fritella | 174 | 174 | 255 | 310 | 125 | 150 | 188 | C/W |
| Golubizna | 173 | 173 | 252 | 279 | 125 | 149 | 188 | W/T |
| Grand | 175 | 174 | 254 | 280 | 125 | 150 | 188 | W |
| Gusar | 175 | 174 | 254 | 280 | 125 | 150 | 188 | W |
| Krasa Meshchery | 174 | 174 | 254 | 310 | 125 | 151 | 188 | A/W |
| Krasavchik | 173 | 173 | 252 | 279 | 125 | 149 | 188 | W/T |
| Krepysh | 174 | 174 | 254 | 310 | 125 | 151 | 188 | A/W |
| Meteor | 175 | 174 | 254 | 280 | 125 | 150 | 188 | W |
| Nevsky | 174 | 174 | 255 | 310 | 125 | 150 | 188 | W |
| Nikulinsky | 173 | 173 | 252 | 279 | 125 | 149 | 188 | W/T |
| Severnoe siyanie | 173 | 173 | 252 | 279 | 125 | 149 | 188 | W/T |
| Sudarinya | 175 | 174 | 254 | 280 | 125 | 150 | 188 | W |
| Symphonia | 173 | 173 | 252 | 279 | 125 | 149 | 188 | W/T |
| Udacha | 174 | 174 | 255 | 310 | 125 | 150 | 188 | C/W |
| Zhukovsky | 174 | 174 | 254 | 310 | 125 | 151 | 188 | A/W |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karetnikov, D.I.; Salina, E.A.; Kochetov, A.V.; Afonnikov, D.A. Assembly and Analysis of Plastomes for 15 Potato Cultivars Grown in Russia. Agronomy 2023, 13, 1454. https://doi.org/10.3390/agronomy13061454
Karetnikov DI, Salina EA, Kochetov AV, Afonnikov DA. Assembly and Analysis of Plastomes for 15 Potato Cultivars Grown in Russia. Agronomy. 2023; 13(6):1454. https://doi.org/10.3390/agronomy13061454
Chicago/Turabian StyleKaretnikov, Dmitry I., Elena A. Salina, Alex V. Kochetov, and Dmitry A. Afonnikov. 2023. "Assembly and Analysis of Plastomes for 15 Potato Cultivars Grown in Russia" Agronomy 13, no. 6: 1454. https://doi.org/10.3390/agronomy13061454
APA StyleKaretnikov, D. I., Salina, E. A., Kochetov, A. V., & Afonnikov, D. A. (2023). Assembly and Analysis of Plastomes for 15 Potato Cultivars Grown in Russia. Agronomy, 13(6), 1454. https://doi.org/10.3390/agronomy13061454






