Three Cycles of Continuous Propagation of a Severe PSTVd Strain NicTr-3 in Solanum lycopersicum cv. Rutgers Resulted in Its Attenuation and Very Mild Disease Symptoms in Potato
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Resistance Assessment
2.3. Detection of PSTVd in Potato and Tomato Plants by RT-PCR
2.4. RNA-Seq
2.5. RT-qPCR
2.6. Bioinformatic Analysis
3. Results
3.1. Assessment of Resistance to PSTVd
3.2. Nucleotide Sequence of the PSTVd Strain NicTr-3 Variants Isolated from Tomato Sap
3.3. Transcriptome Analysis
3.4. Gene Ontology Enrichment Analysis of Differentially Expressed Genes
3.5. DEGs Description
4. Conclusions
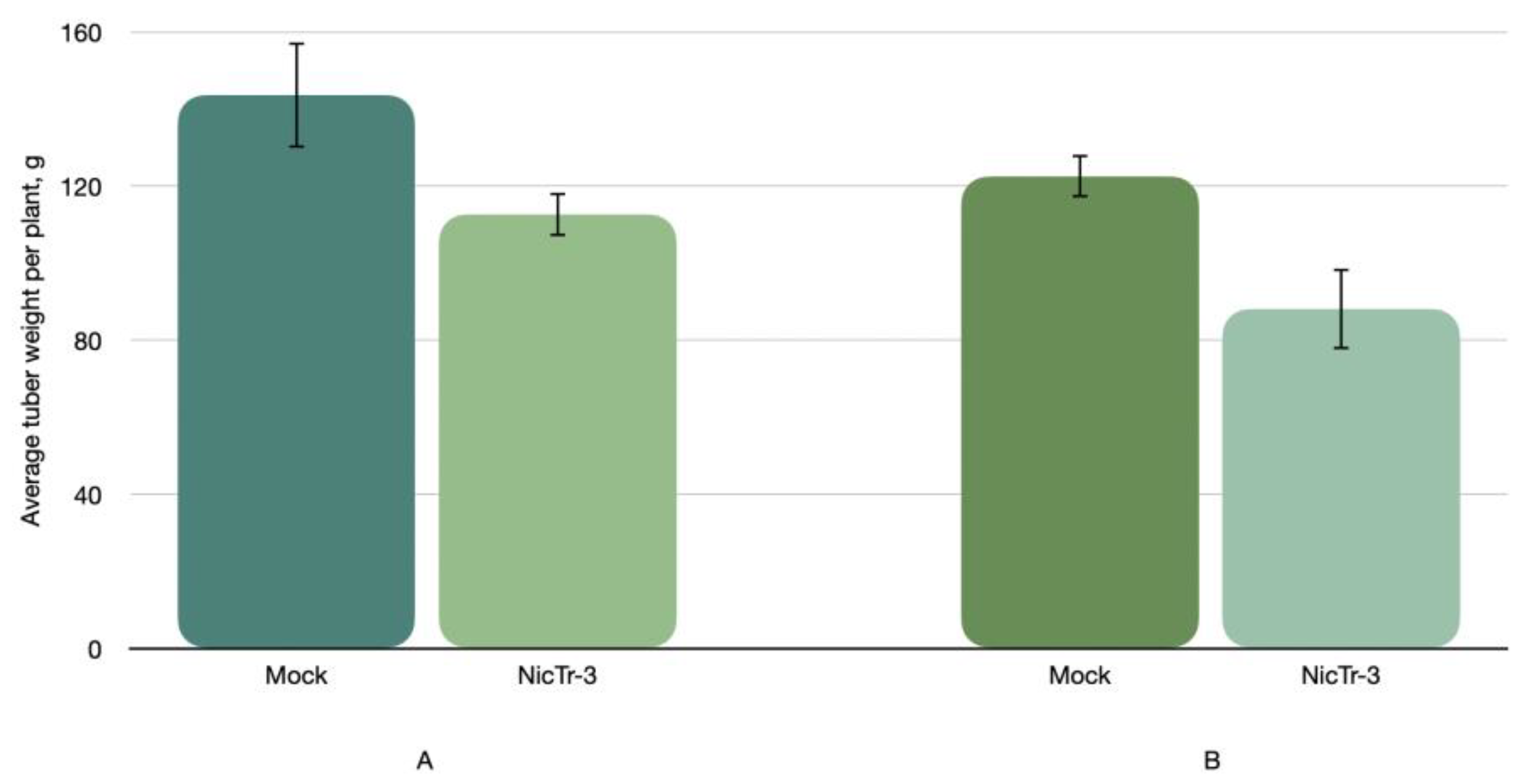
- The PSTVd strain NicTr-3 found in severely affected potato plants of cv. Nikulinskiy was characterized with a high aggressiveness against tomato cv. Rutgers and potato cv. Colomba [5]. However, after three cycles of propagation in tomato cv. Rutgers it became attenuated against potato cv. Colomba and resulted in a statistically significant decrease in tuber weight and a considerable delay of leaf senescence. It was found that the viroid RNAs in tomato sap contained a number of mutated variants that may be connected with the observed attenuation.
- Analysis of transcriptomes from PSTVd-inoculated potato leaves revealed a significant difference with control cv. Colomba plants. Interestingly, no significant difference was detected between the transcriptomes of the tuber-derived plantlets representing the second vegetative generation. The transcriptome comparison revealed the signs of dysregulation of signal transduction pathways resembling the initiation of dormancy and transient growth delay. It involves the upregulation of 9-cis-epoxycarotenoid dioxygenase gene encoding a rate-limiting enzyme of ABA biosynthesis, induction of AGAMOUS-like MADS-box transcription factor, auxin-repressed/dormancy-associated protein, repression of cytokinin-degrading enzymes, upregulation of tuberization factors CONSTANS, Flowering locus T, Bel5, and so on. The connection between the attenuated viroid strain and the observed changes in potato leaf transcriptomes remains obscure. It is likely that the considerable delay in leaf senescence (Table S2) resulted from this transcriptome dysregulation [48].
- Taking into account the relatively small changes in phenotype and no significant difference being found between the transcriptomes of the second vegetative generation and the non-infected potato plants, the search for potential vaccine-like viroid variants may represent one of possible ways for biocontrol of these harmful pathogenic RNAs. Indeed, there are many threats and obstacles accompanying the development of such technologies, but this way seems to be worth the effort.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Diener, T.O. The viroid: Biological oddity or evolutionary fossil? Adv. Virus Res. 2001, 57, 137–184. [Google Scholar] [CrossRef] [PubMed]
- Mackie, A.E.; Rodoni, B.C.; Barbetti, M.J.; McKirdy, S.J.; Jones, R.A.C. Potato spindle tuber viroid: Alternative host reservoirs and strain found in a remote subtropical irrigation area. Eur. J. Plant Pathol. 2016, 145, 433–446. [Google Scholar] [CrossRef]
- EPPO. National regulatory control systems: Potato spindle tuber viroid on potato. EPPO Bull. 2011, 41, 394–399. [Google Scholar] [CrossRef]
- Mackie, A.E.; Barbetti, M.J.; Rodoni, B.; McKirdy, S.J.; Jones, R.A.C. Effects of a Potato Spindle Tuber Viroid Tomato Strain on the Symptoms, Biomass, and Yields of Classical Indicator and Currently Grown Potato and Tomato Cultivars. Plant Dis. 2019, 103, 3009–3017. [Google Scholar] [CrossRef]
- Afanasenko, O.S.; Lashina, N.M.; Mironenko, N.V.; Kyrova, E.I.; Rogozina, E.V.; Zubko, N.G.; Khiutti, A.V. Evaluation of Responses of Potato Cultivars to Potato Spindle Tuber Viroid and to Mixed Viroid/Viral Infection. Agronomy 2022, 12, 2916. [Google Scholar] [CrossRef]
- Fernow, K.H. Tomato as a test plant for detecting mild strains of potato spindle tuber virus. Phytopathology 1967, 57, 1347–1352. [Google Scholar]
- Matsushita, Y.; Yanagisawa, H.; Khiutti, A.; Mironenko, N.; Ohto, Y.; Afanasenko, O. Genetic diversity and pathogenicity of potato spindle tuber viroid and chrysanthemum stunt viroid isolates in Russia. Eur. J. Plant Pathol. 2021, 161, 529–542. [Google Scholar] [CrossRef]
- Aviña-Padilla, K.; Zambada-Moreno, O.; Herrera-Oropeza, G.E.; Jimenez-Limas, M.A.; Abrahamian, P.; Hammond, R.W.; Hernández-Rosales, M. Insights into the Transcriptional Reprogramming in Tomato Response to PSTVd Variants Using Network Approaches. Int. J. Mol. Sci. 2022, 23, 5983. [Google Scholar] [CrossRef]
- Kitabayashi, S.; Tsushima, D.; Adkar-Purushothama, C.; Sano, T. Identification and Molecular Mechanisms of Key Nucleotides Causing Attenuation in Pathogenicity of Dahlia Isolate of Potato Spindle Tuber Viroid. Int. J. Mol. Sci. 2020, 21, 7352. [Google Scholar] [CrossRef]
- Adkar-Purushothama, C.R.; Bolduc, F.; Bru, P.; Perreault, J.-P. Insights into Potato Spindle Tuber Viroid Quasi-Species from Infection to Disease. Front. Microbiol. 2020, 11, 1235. [Google Scholar] [CrossRef]
- Fujibayashi, M.; Suzuki, T.; Sano, T. Mechanism underlying potato spindle tuber viroid affecting tomato (Solanum lycopersicum): Loss of control over reactive oxygen species production. J. Gen. Plant Pathol. 2021, 87, 226–235. [Google Scholar] [CrossRef]
- Naoi, T.; Hataya, T. Tolerance Even to Lethal Strain of Potato Spindle Tuber Viroid Found in Wild Tomato Species Can Be Introduced by Crossing. Plants 2021, 10, 575. [Google Scholar] [CrossRef]
- Adkar-Purushothama, C.R.; Perreault, J.-P. Alterations of the viroid regions that interact with the host defense genes attenuate viroid infection in host plant. RNA Biol. 2018, 15, 955–966. [Google Scholar] [CrossRef]
- Bao, S.; Owens, R.A.; Sun, Q.; Song, H.; Liu, Y.; Eamens, A.L.; Feng, H.; Tian, H.; Wang, M.-B.; Zhang, R. Silencing of transcription factor encoding gene StTCP23 by small RNAs derived from the virulence modulating region of potato spindle tuber viroid is associated with symptom development in potato. PLOS Pathog. 2019, 15, e1008110. [Google Scholar] [CrossRef] [Green Version]
- Cisneros, A.E.; Lisón, P.; Campos, L.; López-Tubau, J.M.; Altabella, T.; Ferrer, A.; Daròs, J.-A.; Carbonell, A. Down-regulation of tomato STEROL GLYCOSYLTRANSFERASE 1 perturbs plant development and facilitates viroid infection. J. Exp. Bot. 2022, erac361. [Google Scholar] [CrossRef]
- Prol, F.V.; López-Gresa, M.P.; Rodrigo, I.; Bellés, J.M.; Lisón, P. Ethylene is Involved in Symptom Development and Ribosomal Stress of Tomato Plants upon Citrus Exocortis Viroid Infection. Plants 2020, 9, 582. [Google Scholar] [CrossRef]
- Navarro, B.; Gisel, A.; Serra, P.; Chiumenti, M.; Di Serio, F.; Flores, R. Degradome Analysis of Tomato and Nicotiana benthamiana Plants Infected with Potato Spindle Tuber Viroid. Int. J. Mol. Sci. 2021, 22, 3725. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- The Potato Genome Sequencing Consortium. Genome sequence and analysis of the tuber crop potato. Nature 2011, 475, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Yates, A.D.; Allen, J.; Amode, R.M.; Azov, A.G.; Barba, M.; Becerra, A.; Bhai, J.; Campbell, L.I.; Martinez, M.C.; Chakiachvili, M.; et al. Ensembl Genomes 2022: An expanding genome resource for non-vertebrates. Nucleic Acids Res. 2022, 50, D996–D1003. [Google Scholar] [CrossRef]
- Lin, H.-N.; Hsu, W.-L. DART: A fast and accurate RNA-seq mapper with a partitioning strategy. Bioinformatics 2018, 34, 190–197. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Smyth, G.K.; Shi, W. The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads. Nucleic Acids Res. 2019, 47, e47. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Tian, T.; Liu, Y.; Yan, H.; You, Q.; Yi, X.; Du, Z.; Xu, W.; Su, Z. agriGO v2.0: A GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res. 2017, 45, W122–W129. [Google Scholar] [CrossRef] [Green Version]
- Mironenko, N.V.; Orina, A.S.; Lashina, N.M.; Afanasenko, O.S. Expression of transcription factor encoding StTCP23 gene in potato plants infected with tuber spindle viroid. Proc. Appl. Bot. Genet. Breed, 2023; 184, in press. [Google Scholar]
- Campbell, M.; Suttle, J.; Douches, D.S.; Buell, C.R. Treatment of potato tubers with the synthetic cytokinin 1-(α-ethylbenzyl)-3-nitroguanidine results in rapid termination of endodormancy and induction of transcripts associated with cell proliferation and growth. Funct. Integr. Genom. 2014, 14, 789–799. [Google Scholar] [CrossRef]
- Senning, M.; Sonnewald, U.; Sonnewald, S. Deoxyuridine triphosphatase expression defines the transition from dormant to sprouting potato tuber buds. Mol. Breed. 2010, 26, 525–531. [Google Scholar] [CrossRef]
- Van Sandt, V.S.T.; Suslov, D.; Verbelen, J.-P.; Vissenberg, K. Xyloglucan Endotransglucosylase Activity Loosens a Plant Cell Wall. Ann. Bot. 2007, 100, 1467–1473. [Google Scholar] [CrossRef] [Green Version]
- Gimeno-Gilles, C.; Lelièvre, E.; Viau, L.; Malik-Ghulam, M.; Ricoult, C.; Niebel, A.; Leduc, N.; Limami, A.M. ABA-Mediated Inhibition of Germination Is Related to the Inhibition of Genes Encoding Cell-Wall Biosynthetic and Architecture: Modifying Enzymes and Structural Proteins in Medicago truncatula Embryo Axis. Mol. Plant 2009, 2, 108–119. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Li, C.; Ge, J.; Xu, M.; Zhu, Q.; Wu, T.; Guo, A.; Xie, J.; Dong, H. Recessive Mutation Identifies Auxin-Repressed Protein ARP1, Which Regulates Growth and Disease Resistance in Tobacco. Mol. Plant Microbe Interact. 2014, 27, 638–654. [Google Scholar] [CrossRef] [Green Version]
- De Souza, G.B.; Mendes, T.A.D.O.; Fontes, P.P.; Barros, V.D.A.; Gonçalves, A.B.; Ferreira, T.D.F.; Costa, M.D.-B.L.; Alves, M.S.; Fietto, L.G. Genome-wide identification and expression analysis of dormancy-associated gene 1/auxin repressed protein (DRM1/ARP) gene family in Glycine max. Prog. Biophys. Mol. Biol. 2019, 146, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Aleith, F.; Richter, G. Gene expression during induction of somatic embryogenesis in carrot cell suspensions. Planta 1991, 183, 17–24. [Google Scholar] [CrossRef]
- Bacete, L.; Schulz, J.; Engelsdorf, T.; Bartosova, Z.; Vaahtera, L.; Yan, G.; Gerhold, J.M.; Tichá, T.; Øvstebø, C.; Gigli-Bisceglia, N.; et al. THESEUS1 modulates cell wall stiffness and abscisic acid production in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2022, 119, e2119258119. [Google Scholar] [CrossRef]
- Gorgues, L.; Li, X.; Maurel, C.; Martinière, A.; Nacry, P. Root osmotic sensing from local perception to systemic responses. Stress Biol. 2022, 2, 36. [Google Scholar] [CrossRef]
- Hussain, Q.; Asim, M.; Zhang, R.; Khan, R.; Farooq, S.; Wu, J. Transcription Factors Interact with ABA through Gene Expression and Signaling Pathways to Mitigate Drought and Salinity Stress. Biomolecules 2021, 11, 1159. [Google Scholar] [CrossRef]
- Blume, R.; Yemets, A.; Korkhovyi, V.; Radchuk, V.; Rakhmetov, D.; Blume, Y. Genome-wide identification and analysis of the cytokinin oxidase/dehydrogenase (ckx) gene family in finger millet (Eleusine coracana). Front. Genet. 2022, 13, 963789. [Google Scholar] [CrossRef]
- Shi, Z.; Halaly-Basha, T.; Zheng, C.; Weissberg, M.; Ophir, R.; Galbraith, D.W.; Pang, X.; Or, E. Transient induction of a subset of ethylene biosynthesis genes is potentially involved in regulation of grapevine bud dormancy release. Plant Mol. Biol. 2018, 98, 507–523. [Google Scholar] [CrossRef] [Green Version]
- Mou, W.; Li, D.; Luo, Z.; Li, L.; Mao, L.; Ying, T. SlAREB1 transcriptional activation of NOR is involved in abscisic acid-modulated ethylene biosynthesis during tomato fruit ripening. Plant Sci. 2018, 276, 239–249. [Google Scholar] [CrossRef]
- Dubois, M.; Van den Broeck, L.; Inzé, D. The Pivotal Role of Ethylene in Plant Growth. Trends Plant Sci. 2018, 23, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Yang, R.; Bartels, D.; Dong, T.; Duan, H. Roles of Abscisic Acid and Gibberellins in Stem/Root Tuber Development. Int. J. Mol. Sci. 2022, 23, 4955. [Google Scholar] [CrossRef]
- Cao, D.; Lin, Z.; Huang, L.; Damaris, R.N.; Li, M.; Yang, P. A CONSTANS-LIKE gene of Nelumbo nucifera could promote potato tuberization. Planta 2021, 253, 65. [Google Scholar] [CrossRef]
- Navarro, C.; Cruz-Oró, E.; Prat, S. Conserved function of FLOWERING LOCUS T (FT) homologues as signals for storage organ differentiation. Curr. Opin. Plant Biol. 2015, 23, 45–53. [Google Scholar] [CrossRef]
- Więsyk, A.; Lirski, M.; Fogtman, A.; Zagórski-Ostoja, W.; Góra-Sochacka, A. Differences in gene expression profiles at the early stage of Solanum lycopersicum infection with mild and severe variants of potato spindle tuber viroid. Virus Res. 2020, 286, 198090. [Google Scholar] [CrossRef]
- Joubert, M.; Berg, N.V.D.; Theron, J.; Swart, V. Transcriptomics Advancement in the Complex Response of Plants to Viroid Infection. Int. J. Mol. Sci. 2022, 23, 7677. [Google Scholar] [CrossRef]
- Chi, S.; Zhang, J.; Li, H.; Wang, P.; Feng, L.; Ren, Y. RNA-seq Profiling Reveals the Transcriptional Response Against Potato Spindle Tuber Viroid in Different Potato Cultivars and Developmental Stages. Potato Res. 2022, 65, 979–990. [Google Scholar] [CrossRef]
- Prol, F.V.; Márquez-Molins, J.; Rodrigo, I.; López-Gresa, M.; Bellés, J.; Gómez, G.; Pallás, V.; Lisón, P. Symptom Severity, Infection Progression and Plant Responses in Solanum Plants Caused by Three Pospiviroids Vary with the Inoculation Procedure. Int. J. Mol. Sci. 2021, 22, 6189. [Google Scholar] [CrossRef]
- Góra-Sochacka, A.; Więsyk, A.; Fogtman, A.; Lirski, M.; Zagórski-Ostoja, W. Root Transcriptomic Analysis Reveals Global Changes Induced by Systemic Infection of Solanum lycopersicum with Mild and Severe Variants of Potato Spindle Tuber Viroid. Viruses 2019, 11, 992. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Gao, J.; Kim, J.I.; Chen, K.; Bressan, R.A.; Zhu, J.-K. Control of Plant Water Use by ABA Induction of Senescence and Dormancy: An Overlooked Lesson from Evolution. Plant Cell Physiol. 2017, 58, 1319–1327. [Google Scholar] [CrossRef] [Green Version]

| Comparison | Number of DEGs | |
|---|---|---|
| Upregulated | Downregulated | |
| 14 dpi versus 0 dpi | 101 | 8 |
| 30 dpi versus 14 dpi | 107 | 506 |
| 30 dpi versus 0 dpi | 1909 | 2660 |
| Comparison | Upregulated | Downregulated |
|---|---|---|
| 14 dpi vs. 0 dpi | 1 | 0 |
| 30 dpi vs. 14 dpi | 0 | 30 |
| 30 dpi vs. 0 dpi | 40 | 76 |
| Comparison | Upregulated GO Terms and Associated Processes | Downregulated GO Terms and Associated Processes |
|---|---|---|
| 30 dpi vs. 0 dpi | Biological Process: 21 significant terms, including: Response to various stimuli and oxidation stress: oxidation reduction, transcription, multicellular organismal process, response to external and endogenous stimulus, response to organic substances, macromolecule modification. Metabolic processes: phosphate metabolic process, photosynthesis | Biological Process: 32 significant terms, including: Cell division and cycle-related terms: cell cycle process Metabolic processes: phosphate, steroid, lipid, carbohydrate, glucan, polysaccharide, response to organic substances Regulation and response to stimuli: Response to endogenous stimulus and hormones, development, multicellular organismal process, macromolecule modification |
| Molecular Function: 19 significant terms, including: Binding of: protein, polysaccharide, pattern, heme, tetrapyrrole Activity of: oxidoreductases, (protein)kinases and transferases Electron transporter | Molecular Function: 26 significant terms, including: Binding of: protein, NAD or NADH, carbohydrate, coenzyme, chaperone Activity of: motor, oxidoreductase, hydrolase, kinase and transferase | |
| Cellular Component: no significant terms | Cellular Component: 18 significant terms, including: organelle, macromolecular complex, microtubule, nucleosome, chromatin, cell wall | |
| 30 dpi vs. 14 dpi | Biological Process: no significant terms | Biological Process: 4 significant terms, including: DNA replication, lipid biosynthesis process, oxidation reduction, microtubule-based process |
| Molecular Function: no significant terms | Molecular Function: 14 significant terms, including: Binding of: protein, NAD or NADH, copper ion Activity of: oxidoreductase, catalytic, hydrolase, transferase, protein kinase | |
| Cellular Component: no significant terms | Cellular Component: 12 significant terms, including: Chromosome, cytoskeleton, organelle |
| Gene | Description | logFC | FDR | |
|---|---|---|---|---|
| Upregulated | ||||
| 1 | PGSC0003DMG400004312 | 9-cis-epoxycarotenoid dioxygenase | 4.82 | 0.000611605 |
| 2 | PGSC0003DMG400004081 | Agamous-like MADS-box protein AGL8 homolog | 10.30 | 0.000611605 |
| 3 | PGSC0003DMG400013680 | Pectinesterase | 4.51 | 0.001384518 |
| 4 | PGSC0003DMG400015755 | DnaJ isoform | 3.97 | 0.001717415 |
| 5 | PGSC0003DMG400001969 | Carotenoid cleavage dioxygenase 4 | 4.69 | 0.001914105 |
| Downregulated | ||||
| 1 | PGSC0003DMG400037894 | Aspartic proteinase nepenthesin-1 | −4.72 | 0.001717415 |
| 2 | PGSC0003DMG400020481 | 14 kDa proline-rich protein DC2,15 | −12.28 | 0.001914105 |
| 3 | PGSC0003DMG400025030 | Receptor kinase THESEUS 1 | −3.52 | 0.001914105 |
| 4 | PGSC0003DMG400023361 | Methylenetetrahydrofolate reductase | −4.65 | 0.001914105 |
| 5 | PGSC0003DMG401014997 | Zinc finger protein | −4.90 | 0.001914105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kochetov, A.V.; Shmakov, N.; Afonnikov, D.A.; Vasiliev, G.V.; Shatskaya, N.V.; Egorova, A.A.; Mironenko, N.V.; Lashina, N.M.; Khiutti, A.V.; Afanasenko, O.S. Three Cycles of Continuous Propagation of a Severe PSTVd Strain NicTr-3 in Solanum lycopersicum cv. Rutgers Resulted in Its Attenuation and Very Mild Disease Symptoms in Potato. Agronomy 2023, 13, 684. https://doi.org/10.3390/agronomy13030684
Kochetov AV, Shmakov N, Afonnikov DA, Vasiliev GV, Shatskaya NV, Egorova AA, Mironenko NV, Lashina NM, Khiutti AV, Afanasenko OS. Three Cycles of Continuous Propagation of a Severe PSTVd Strain NicTr-3 in Solanum lycopersicum cv. Rutgers Resulted in Its Attenuation and Very Mild Disease Symptoms in Potato. Agronomy. 2023; 13(3):684. https://doi.org/10.3390/agronomy13030684
Chicago/Turabian StyleKochetov, Alex V., Nikolay Shmakov, Dmitry A. Afonnikov, Gennady V. Vasiliev, Natalja V. Shatskaya, Anastasiya A. Egorova, Nina V. Mironenko, Nina M. Lashina, Alexander V. Khiutti, and Olga S. Afanasenko. 2023. "Three Cycles of Continuous Propagation of a Severe PSTVd Strain NicTr-3 in Solanum lycopersicum cv. Rutgers Resulted in Its Attenuation and Very Mild Disease Symptoms in Potato" Agronomy 13, no. 3: 684. https://doi.org/10.3390/agronomy13030684





