Comprehensive Evaluation of Low Nitrogen Tolerance in Oat (Avena sativa L.) Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Trial Design
2.3. Data Processing and Calculation Methods
3. Results
3.1. CV of Each Trait and Plant N Content of Oat Varieties at the Seedling Stage under Different N Supply Conditions
3.2. Pearson’s Correlation Coefficient and PCA of Traits of Oat Varieties at Different Levels of N Supply
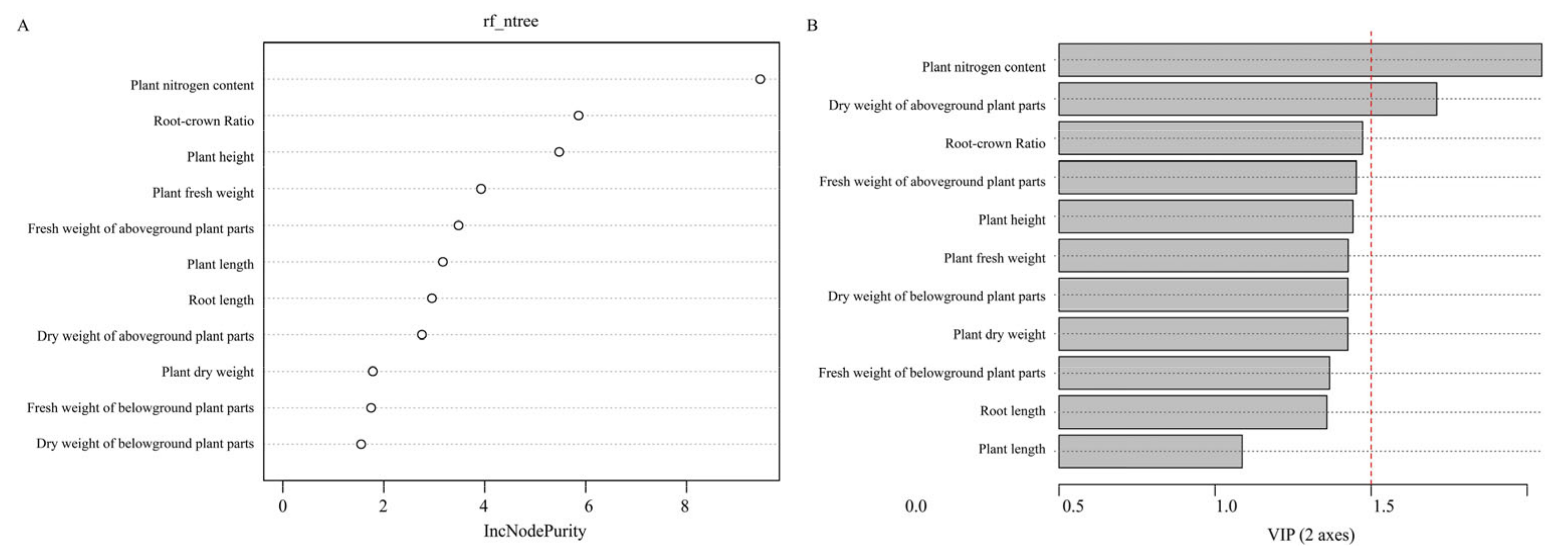
3.3. PLS-DA and Random Forest Analysis of Oat Varieties at Different Levels of N Supply
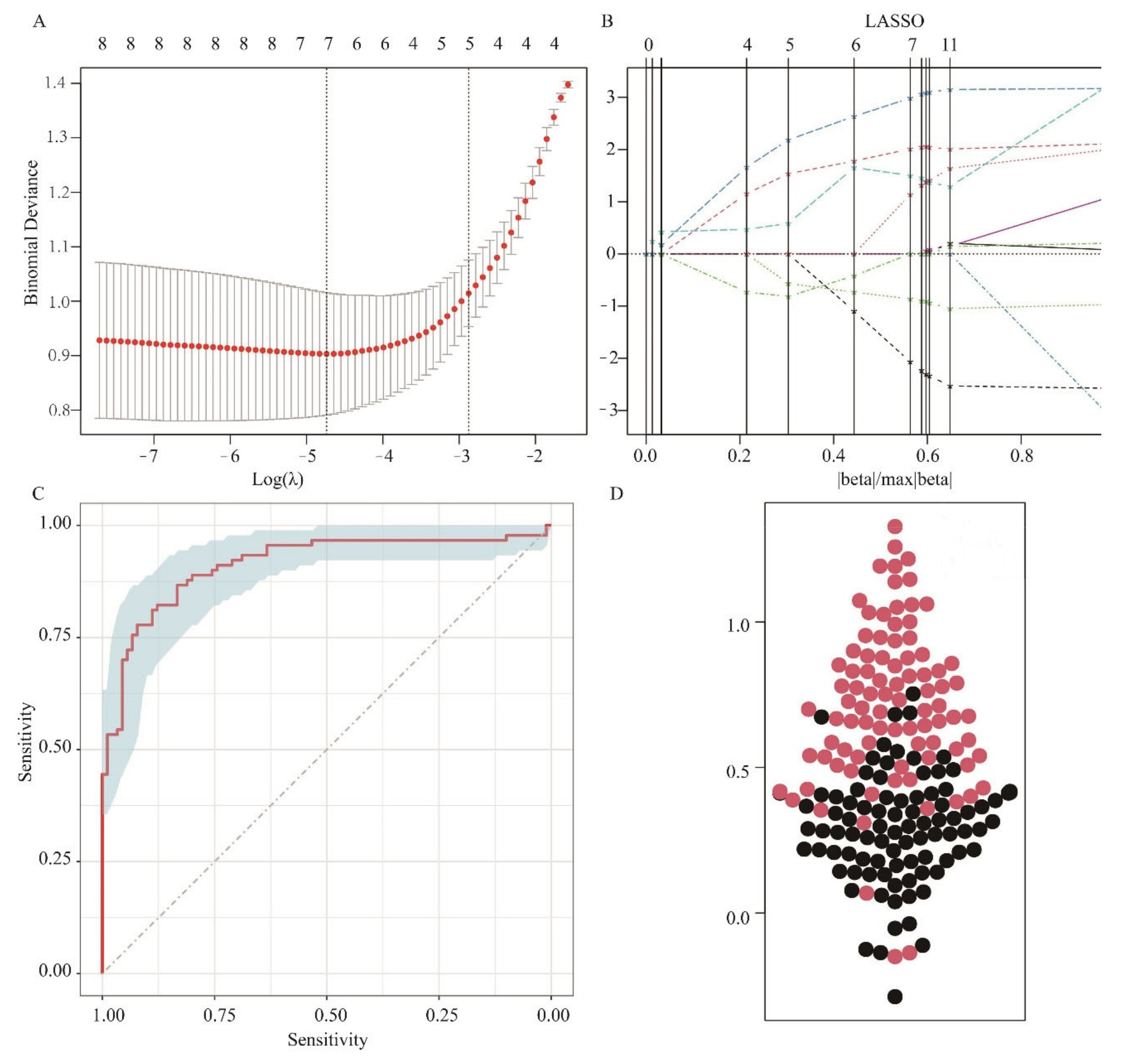
3.4. LASSO Regression Analysis and Model Evaluation of Oat Varieties at Different Levels of N Supply
3.5. Membership Function Analysis to Evaluate the Traits of Oat Seedlings at Different Levels of N Supply
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.X.; Bai, R.; Nan, M.; Ren, W.; Wang, C.M.; Shabala, S.; Zhang, J.L. Evaluation of salt tolerance of oat cultivars and the mechanism of adaptation to salinity. J. Plant. Physiol. 2022, 273, 153708. [Google Scholar] [CrossRef] [PubMed]
- Kamal, N.; Tsardakas Renhuldt, N.; Bentzer, J.; Gundlach, H.; Haberer, G.; Juhasz, A.; Lux, T.; Bose, U.; Tye-Din, J.A.; Lang, D.; et al. The mosaic oat genome gives insights into a uniquely healthy cereal crop. Nature 2022, 606, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Yan, W.; Wang, Y.; Yin, Q.; Liu, J.; Wight, C.; Ma, B. Screening Oat Genotypes for Tolerance to Salinity and Alkalinity. Front. Plant Sci. 2018, 9, 1302. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Tang, X.; Liao, C.; Li, M.; Chen, L.; Lu, G.; Huang, X.; Chen, C.; Gou, W. Effects of Additives on Silage Fermentation Characteristic and In Vitro Digestibility of Perennial Oat at Different Maturity Stages on the Qinghai Tibetan. Microorganisms 2021, 9, 2403. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guan, H.; Jia, Z.; Liu, W.; Ma, X.; Liu, Y.; Wang, H.; Zhou, Q. Freeze-thaw condition limits the fermentation process and accelerates the aerobic deterioration of oat (Avena sativa) silage in the Qinghai-Tibet Plateau. Front. Microbiol. 2022, 13, 944945. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, B.; Pan, L.; Chen, L.; Fu, X.; Li, K. Overexpression of Arabidopsis Dof1, GS1 and GS2 Enhanced Nitrogen Assimilation in Transgenic Tobacco Grown Under Low-Nitrogen Conditions. Plant. Mol. Biol. Rep. 2013, 31, 886–900. [Google Scholar] [CrossRef]
- Esvelt Klos, K.; Yimer, B.A.; Howarth, C.J.; McMullen, M.S.; Sorrells, M.E.; Tinker, N.A.; Yan, W.; Beattie, A.D. The Genetic Architecture of Milling Quality in Spring Oat Lines of the Collaborative Oat Research Enterprise. Foods 2021, 10, 2479. [Google Scholar] [CrossRef]
- Kiba, T.; Inaba, J.; Kudo, T.; Ueda, N.; Konishi, M.; Mitsuda, N.; Takiguchi, Y.; Kondou, Y.; Yoshizumi, T.; Ohme-Takagi, M.; et al. Repression of Nitrogen Starvation Responses by Members of the Arabidopsis GARP-Type Transcription Factor NIGT1/HRS1 Subfamily. Plant. Cell 2018, 30, 925–945. [Google Scholar] [CrossRef] [Green Version]
- Martins, M.R.; Jantalia, C.P.; Polidoro, J.C.; Batista, J.N.; Alves, B.J.R.; Boddey, R.M.; Urquiaga, S. Nitrous oxide and ammonia emissions from N fertilization of maize crop under no-till in a Cerrado soil. Soil Tillage Res. 2015, 151, 75–81. [Google Scholar] [CrossRef]
- Van de Wiel, C.C.M.; van der Linden, C.G.; Scholten, O.E. Improving phosphorus use efficiency in agriculture: Opportunities for breeding. Euphytica 2015, 207, 1–22. [Google Scholar] [CrossRef] [Green Version]
- McCabe, C.P.; Burke, J.I. Oat (Avena sativa) yield and grain fill responses to varying agronomic and weather factors. J. Agric. Sci. 2021, 159, 90–105. [Google Scholar] [CrossRef]
- Khaembah, E.N.; Cichota, R.; Vogeler, I. Simulation of management strategies to mitigate nitrogen losses from crop rotations in Southland, New Zealand. J. Sci. Food Agric. 2021, 101, 4241–4249. [Google Scholar] [CrossRef]
- Nazir, M.; Pandey, R.; Siddiqi, T.O.; Ibrahim, M.M.; Qureshi, M.I.; Abraham, G.; Vengavasi, K.; Ahmad, A. Nitrogen-Deficiency Stress Induces Protein Expression Differentially in Low-N Tolerant and Low-N Sensitive Maize Genotypes. Front. Plant Sci. 2016, 7, 298. [Google Scholar] [CrossRef] [Green Version]
- Saengwilai, P.; Tian, X.; Lynch, J.P. Low crown root number enhances nitrogen acquisition from low-nitrogen soils in maize. Plant. Physiol. 2014, 166, 581–589. [Google Scholar] [CrossRef] [Green Version]
- Mosier, A.R.; Syers, J.K.; Freney, J.R. Global assessment of nitrogen fertilizer: The SCOPE/IGBP nitrogen fertilizer rapid assessment project. Sci. China C Life Sci. 2005, 48 (Suppl. 2), 759–766. [Google Scholar] [CrossRef]
- Wu, M.; Li, G.; Li, W.; Liu, J.; Liu, M.; Jiang, C.; Li, Z. Nitrogen Fertilizer Deep Placement for Increased Grain Yield and Nitrogen Recovery Efficiency in Rice Grown in Subtropical China. Front. Plant Sci. 2017, 8, 1227. [Google Scholar] [CrossRef]
- Hu, B.L.; Li, X.; Wan, Y.; Qiu, Z.H.; Nie, Y.Y.; Xie, J.K. Index screening and comprehensive evaluation of phenotypic traits of low nitrogen tolerance using BILs population derived from Dongxiang wild rice (Oryza rufipogon Griff). Ying Yong Sheng Tai Xue Bao 2015, 26, 2346–2352. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Deng, X.; Zhang, Z.; Yin, L. Comprehensive evaluation of physiological traits under nitrogen stress and participation of linolenic acid in nitrogen-deficiency response in wheat seedlings. BMC Plant Biol. 2020, 20, 501. [Google Scholar] [CrossRef]
- Liu, C.; Gong, X.; Wang, H.; Dang, K.; Deng, X.; Feng, B. Low-nitrogen tolerance comprehensive evaluation and physiological response to nitrogen stress in broomcorn millet (Panicum miliaceum L.) seedling. Plant. Physiol. Biochem. 2020, 151, 233–242. [Google Scholar] [CrossRef]
- Miao, J.; Shi, F.; Li, W.; Zhong, M.; Li, C.; Chen, S. Comprehensive screening of low nitrogen tolerant maize based on multiple traits at the seedling stage. PeerJ 2022, 10, e14218. [Google Scholar] [CrossRef]
- Li, D.; Liu, J.; Guo, H.; Zong, J.; Li, J.; Wang, J.; Li, L.; Chen, J. Effects of low nitrogen supply on nitrogen uptake, assimilation and remobilization in wild bermudagrass. Plant. Physiol. Biochem. 2022, 191, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, A.; Xie, K.; Yang, X.; Luo, Z.; Chen, J.; Zeng, D.; Ren, Y.; Yang, C.; Wang, L.; et al. Functional analysis of the OsNPF4.5 nitrate transporter reveals a conserved mycorrhizal pathway of nitrogen acquisition in plants. Proc. Natl. Acad. Sci. USA 2020, 117, 16649–16659. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.L.; Wan, H.P.; Wu, X.M.; Dai, X.G.; Chen, J.D.; Ji, Q.Q.; Qian, F. Genome-wide association study of low nitrogen tolerance traits at the seedling stage of rapeseed. Biol. Plant. 2021, 65, 10–18. [Google Scholar] [CrossRef]
- Raza, S.; Miao, N.; Wang, P.; Ju, X.; Chen, Z.; Zhou, J.; Kuzyakov, Y. Dramatic loss of inorganic carbon by nitrogen-induced soil acidification in Chinese croplands. Glob. Change Biol. 2020, 26, 3738–3751. [Google Scholar] [CrossRef]
- Bibi, H.; Hameed, S.; Iqbal, M.; Al-Barty, A.; Darwish, H.; Khan, A.; Anwar, S.; Mian, I.A.; Ali, M.; Zia, A.; et al. Evaluation of exotic oat (Avena sativa L.) varieties for forage and grain yield in response to different levels of nitrogen and phosphorous. PeerJ 2021, 9, e12112. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Liu, L.; Qian, K.; Chen, J.; Zhang, Y.; Xie, P.; Xu, M.; Hu, Z.; Yan, W.; Wu, Y.; et al. Plant DNA methylation is sensitive to parent seed N content and influences the growth of rice. BMC Plant Biol. 2021, 21, 211. [Google Scholar] [CrossRef]
- Sun, X.; Chen, F.; Yuan, L.; Mi, G. The physiological mechanism underlying root elongation in response to nitrogen deficiency in crop plants. Planta 2020, 251, 84. [Google Scholar] [CrossRef]
- Ohkubo, Y.; Kuwata, K.; Matsubayashi, Y. A type 2C protein phosphatase activates high-affinity nitrate uptake by dephosphorylating NRT2.1. Nat. Plants 2021, 7, 310–316. [Google Scholar] [CrossRef]
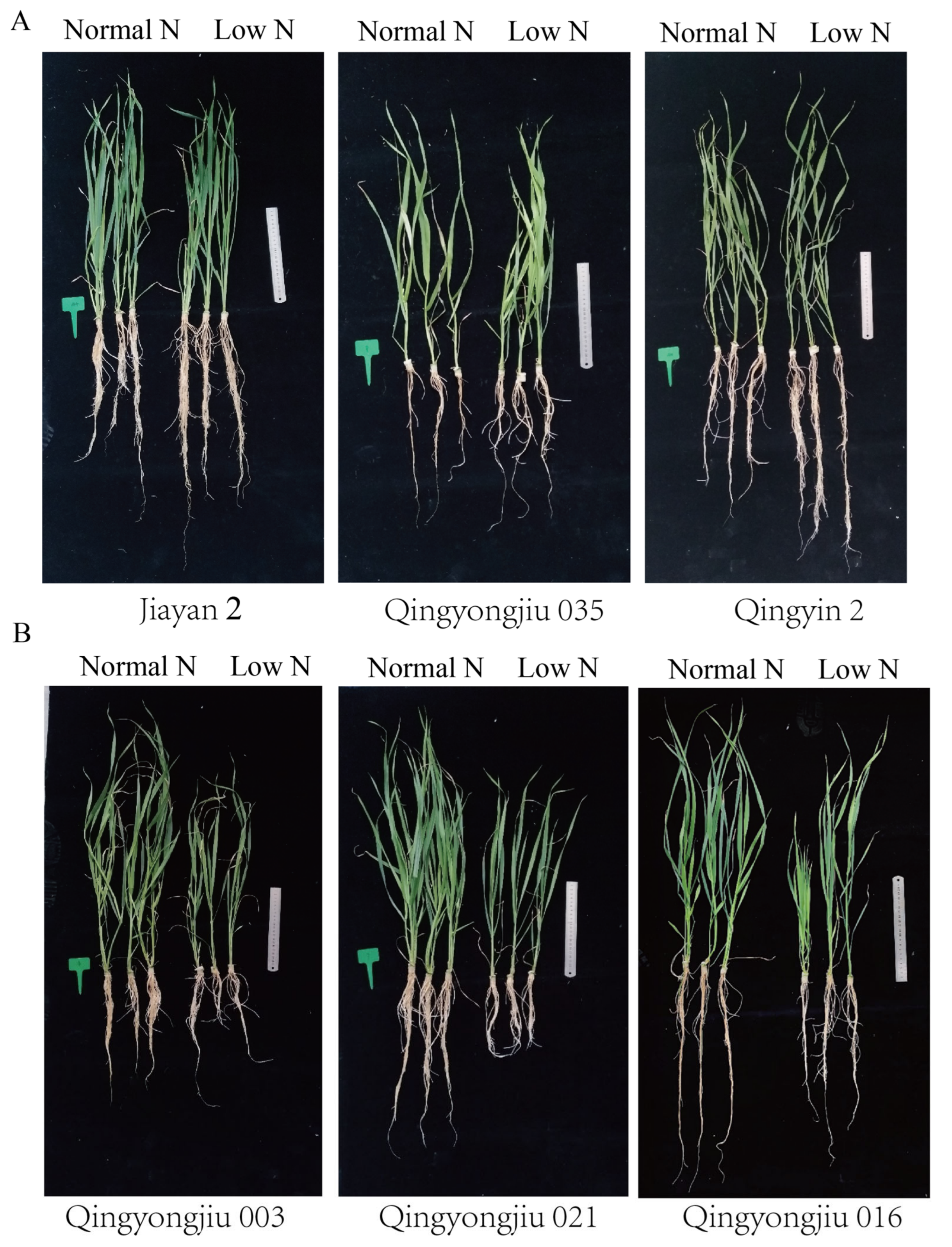
| Number | Name | Source | Number | Name | Source |
|---|---|---|---|---|---|
| 1 | Qingyongjiu 086 | Switzerland | 16 | Qingyongjiu 044 | Canada |
| 2 | Qingyongjiu 068 | Hungary | 17 | Qinghai 444 | China |
| 3 | Qingyongjiu 028 | Soviet Union | 18 | Qingyin 2 | China |
| 4 | Qingyongjiu 021 | China | 19 | Qingyan 1 | China |
| 5 | Qingyongjiu 067 | China | 20 | Qingyongjiu 872 | China |
| 6 | Qingyongjiu 016 | China | 21 | Qingyongjiu 112 | China |
| 7 | Qingyongjiu 065 | Soviet Union | 22 | Qingyongjiu 097 | Sweden |
| 8 | Qingyongjiu 008 | China | 23 | Qingyongjiu 087 | Hungary |
| 9 | Qingyongjiu 055 | Romania | 24 | Qingyongjiu 091 | Hungary |
| 10 | Qingyongjiu 003 | China | 25 | Qinghaitianyanmai | China |
| 11 | Qingyongjiu 045 | Canada | 26 | Jiayan 2 | China |
| 12 | Qingyongjiu 035 | Canada | 27 | Qingyongjiu 093 | Hungary |
| 13 | Bayan 3 | China | 28 | Linna | China |
| 14 | Bayan 5 | China | 29 | Qingyongjiu 096 | Sweden |
| 15 | Qingyongjiu 002 | China | 30 | D1 | China |
| Trait | Normal N | Low N | Low N Tolerance Indices | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Range | Mean | CV/% | Range | Mean | CV/% | Range | Mean | CV/% | |
| Plant length (cm) | 84.5~137.3 | 105.55 A | 11.32 | 59.7~139.6 | 99.73 A | 16.93 | 59.7~139.6 | 0.94 | 14.97 |
| Plant height (cm) | 48.1~84.7 | 64.74 A | 11.88 | 35.4~80 | 58.23 B | 15.03 | 35.4~84.7 | 0.90 | 14.40 |
| Root length (cm) | 25.6~70.4 | 40.97 A | 20.84 | 17.6~72.2 | 41.57 A | 30.89 | 17.6~72.2 | 1.01 | 26.53 |
| Plant fresh weight (g) | 3.95~28.17 | 12.47 A | 41.30 | 2.58~17.5 | 9.01 B | 31.63 | 2.58~28.17 | 0.72 | 41.80 |
| Fresh weight of aboveground plant parts (g) | 3.24~18.37 | 8.33 A | 42.86 | 1.02~11.51 | 5.72 B | 34.79 | 1.02~18.37 | 0.69 | 45.16 |
| Fresh weight of belowground plant parts (g) | 0.71~9.8 | 4.14 A | 41.55 | 0.8~5.99 | 3.31 B | 30.82 | 0.71~9.8 | 0.80 | 39.97 |
| Dry weight of belowground plant parts (g) | 0.05~0.56 A | 0.32 A | 31.25 | 0.06~0.55 | 0.30 A | 30.00 | 0.05~0.56 | 0.94 | 29.65 |
| Dry weight of aboveground plant parts (g) | 0.42~2.71 | 1.17 A | 46.15 | 0.12~1.91 | 0.80 B | 38.75 | 0.12~2.71 | 0.68 | 48.31 |
| Plant dry weight (g) | 0.47~3.27 | 1.49 A | 41.61 | 0.3~2.33 | 1.11 B | 34.23 | 0.3~3.27 | 0.74 | 42.09 |
| Root–crown ratio (%) | 0.12~0.59 | 0.30 A | 33.33 | 0.17~1.5 | 0.42 B | 35.71 | 0.12~1.5 | 1.4 | 39.14 |
| Plant N content (g/kg) | 33.74~54.58 | 47.98 A | 7.92 | 37.21~51.58 | 44.99 B | 7.98 | 33.74~54.58 | 0.94 | 8.59 |
| Trait | Plant Length | Plant Height | Root Length | Plant Fresh Weight | Fresh Weight of Aboveground Plant Parts | Fresh Weight of Belowground Plant Parts | Dry weight of Belowground Plant Parts | Dry Weight of Aboveground Plant Parts | Root-Crown Ratio | Plant N Content | Plant Dry Weight |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant length | 1 | ||||||||||
| Plant height | 0.631 ** | 1 | |||||||||
| Root length | 0.907 ** | 0.434 * | 1 | ||||||||
| Plant fresh weight | 0.491 ** | 0.629 ** | 0.390 * | 1 | |||||||
| Fresh weight of aboveground plant parts | 0.557 ** | 0.629 ** | 0.461 * | 0.975 ** | 1 | ||||||
| Fresh weight of belowground plant parts | 0.259 | 0.518 ** | 0.169 | 0.881 ** | 0.757 ** | 1 | |||||
| Dry weight of belowground plant parts | 0.398 * | 0.505 ** | 0.339 | 0.843 ** | 0.775 ** | 0.849 ** | 1 | ||||
| Dry weight of aboveground plant parts | 0.559 ** | 0.614 ** | 0.461 * | 0.958 ** | 0.970 ** | 0.771 ** | 0.759 ** | 1 | |||
| Root-crown ratio | −0.327 | −0.412 * | −0.397 * | −0.501 ** | −0.575 ** | −0.262 | −0.084 | −0.579 ** | 1 | ||
| Plant N content | 0.109 | 0.077 | 0.195 | 0.008 | 0.117 | −0.217 | −0.224 | 0.021 | −0.484 ** | 1 | |
| Plant dry weight | 0.551 ** | 0.614 ** | 0.456 * | 0.973 ** | 0.970 ** | 0.815 ** | 0.843 ** | 0.989 ** | −0.498 ** | −0.030 | 1 |
| Factor | Eigenvalues | % of Variance (Rotated) | ||||
|---|---|---|---|---|---|---|
| Eigenvalue (Unrotated) | % of Variance | Cumulative % of Variance | Eigenvalue (Unrotated) | % of Variance | Cumulative % of Variance | |
| 1 | 6.685 | 60.771 | 60.771 | 6.685 | 60.771 | 60.771 |
| 2 | 1.842 | 16.748 | 77.518 | 1.842 | 16.748 | 77.518 |
| 3 | 1.169 | 10.63 | 88.148 | 1.169 | 10.63 | 88.148 |
| 4 | 0.512 | 4.655 | 92.803 | |||
| 5 | 0.443 | 4.029 | 96.832 | |||
| 6 | 0.2 | 1.815 | 98.647 | |||
| 7 | 0.098 | 0.887 | 99.534 | |||
| 8 | 0.032 | 0.294 | 99.828 | |||
| 9 | 0.017 | 0.158 | 99.986 | |||
| 10 | 0.001 | 0.011 | 99.997 | |||
| 11 | 0 | 0.003 | 100 | |||
| Varieties | Plant Length | Plant Height | Root Length | Plant Fresh Weight | Fresh Weight of Aboveground Plant Parts | Fresh Weight of Belowground Plant Parts | Dry Weight of Belowground Plant Parts | Dry Weight of Aboveground Plant Parts | Root-Crown Ratio | Plant N Content | Plant Dry Weight | Mean | Low N Tolerance Ranking |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D1 | 0.339 | 0.174 | 0.395 | 0.189 | 0.258 | 0.123 | 0.225 | 0.293 | 0.737 | 0.515 | 0.252 | 0.318 | 24 |
| Bayan 3 | 0.394 | 0.453 | 0.312 | 0.35 | 0.395 | 0.316 | 0 | 0.446 | 1 | 0 | 0.302 | 0.361 | 21 |
| Bayan 5 | 0.229 | 0.442 | 0.115 | 0.261 | 0.23 | 0.486 | 0.409 | 0.241 | 0.347 | 0.7 | 0.23 | 0.335 | 22 |
| Qingyin 2 | 0.556 | 0.665 | 0.388 | 0.852 | 0.995 | 0.611 | 0.596 | 0.987 | 0.853 | 0.478 | 0.876 | 0.714 | 3 |
| Linna | 0.713 | 0.527 | 0.617 | 0.611 | 0.652 | 0.556 | 0.663 | 0.677 | 0.648 | 0.419 | 0.657 | 0.613 | 8 |
| Qinghai 444 | 0.901 | 0.625 | 0.849 | 0.305 | 0.29 | 0.387 | 0.494 | 0.327 | 0.364 | 0.616 | 0.333 | 0.499 | 14 |
| Qinghaitianyanmai | 0.721 | 0.454 | 0.649 | 0.525 | 0.581 | 0.449 | 0.322 | 0.636 | 0.922 | 0.689 | 0.537 | 0.589 | 10 |
| Qingyan 1 | 0.696 | 0.552 | 0.59 | 0.662 | 0.691 | 0.617 | 0.43 | 0.85 | 0.856 | 0.568 | 0.723 | 0.658 | 5 |
| Jiayan 2 | 0.956 | 1 | 0.662 | 0.824 | 0.832 | 0.788 | 0.48 | 0.992 | 0.961 | 0.749 | 0.837 | 0.825 | 1 |
| Qingyongjiu 002 | 0.558 | 0.472 | 0.53 | 0.536 | 0.419 | 0.847 | 0.641 | 0.571 | 0.46 | 1.000 | 0.567 | 0.6 | 9 |
| Qingyongjiu 003 | 0.213 | 0.066 | 0.349 | 0.069 | 0.078 | 0.141 | 0.001 | 0.095 | 0.754 | 0.541 | 0.04 | 0.213 | 28 |
| Qingyongjiu 008 | 0.26 | 0.209 | 0.395 | 0.012 | 0.054 | 0.015 | 0.059 | 0.091 | 0.528 | 0.85 | 0.049 | 0.229 | 27 |
| Qingyongjiu 021 | 0.542 | 0 | 0.204 | 0.014 | 0.054 | 0 | 0.044 | 0.128 | 0 | 0.951 | 0.083 | 0.184 | 29 |
| Qingyongjiu 016 | 0 | 0.24 | 0 | 0 | 0 | 0.069 | 0.139 | 0 | 0.145 | 0.598 | 0 | 0.108 | 30 |
| Qingyongjiu 035 | 0.605 | 0.587 | 0.488 | 1 | 1 | 0.97 | 1 | 1 | 0.596 | 0.789 | 1 | 0.821 | 2 |
| Qingyongjiu 044 | 0.154 | 0.377 | 0.096 | 0.188 | 0.164 | 0.357 | 0.113 | 0.214 | 0.652 | 0.603 | 0.164 | 0.28 | 26 |
| Qingyongjiu 045 | 0.466 | 0.551 | 0.339 | 0.697 | 0.591 | 1 | 0.623 | 0.671 | 0.652 | 0.746 | 0.629 | 0.633 | 7 |
| Qingyongjiu 055 | 0.531 | 0.592 | 0.395 | 0.652 | 0.671 | 0.626 | 0.503 | 0.898 | 0.872 | 0.865 | 0.779 | 0.671 | 4 |
| Qingyongjiu 065 | 0.266 | 0.173 | 0.323 | 0.575 | 0.585 | 0.585 | 0.476 | 0.665 | 0.832 | 0.384 | 0.589 | 0.496 | 15 |
| Qingyongjiu 067 | 0.192 | 0.324 | 0.155 | 0.218 | 0.189 | 0.354 | 0.327 | 0.286 | 0.471 | 0.809 | 0.265 | 0.326 | 23 |
| Qingyongjiu 068 | 0.371 | 0.34 | 0.342 | 0.55 | 0.533 | 0.597 | 0.466 | 0.607 | 0.717 | 0.714 | 0.567 | 0.528 | 12 |
| Qingyongjiu 086 | 0.163 | 0.183 | 0.204 | 0.247 | 0.25 | 0.288 | 0.178 | 0.286 | 0.712 | 0.652 | 0.246 | 0.31 | 25 |
| Qingyongjiu 087 | 0.594 | 0.69 | 0.413 | 0.409 | 0.52 | 0.253 | 0.229 | 0.5 | 0.891 | 0.466 | 0.412 | 0.489 | 16 |
| Qingyongjiu 091 | 0.681 | 0.551 | 0.552 | 0.249 | 0.266 | 0.26 | 0.288 | 0.3 | 0.617 | 0.375 | 0.297 | 0.403 | 20 |
| Qingyongjiu 093 | 0.758 | 0.738 | 0.561 | 0.352 | 0.441 | 0.229 | 0.383 | 0.468 | 0.69 | 0.529 | 0.433 | 0.507 | 13 |
| Qingyongjiu 096 | 0.725 | 0.43 | 0.721 | 0.313 | 0.429 | 0.139 | 0.267 | 0.452 | 0.772 | 0.354 | 0.384 | 0.453 | 18 |
| Qingyongjiu 097 | 0.673 | 0.454 | 0.602 | 0.214 | 0.257 | 0.194 | 0.187 | 0.281 | 0.704 | 0.683 | 0.234 | 0.408 | 19 |
| Qingyongjiu 112 | 1 | 0.376 | 1 | 0.529 | 0.66 | 0.361 | 0.403 | 0.807 | 0.92 | 0.425 | 0.684 | 0.651 | 6 |
| Qingyongjiu 872 | 0.674 | 0.488 | 0.597 | 0.597 | 0.605 | 0.586 | 0.404 | 0.45 | 0.7 | 0.435 | 0.415 | 0.541 | 11 |
| Qingyongjiu 028 | 0.52 | 0.647 | 0.357 | 0.353 | 0.351 | 0.381 | 0.236 | 0.401 | 0.826 | 0.611 | 0.344 | 0.457 | 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, K.; Liang, G.; Jia, Z.; Ju, Z.; Ma, X.; Zhou, Q. Comprehensive Evaluation of Low Nitrogen Tolerance in Oat (Avena sativa L.) Seedlings. Agronomy 2023, 13, 604. https://doi.org/10.3390/agronomy13020604
Wang Y, Liu K, Liang G, Jia Z, Ju Z, Ma X, Zhou Q. Comprehensive Evaluation of Low Nitrogen Tolerance in Oat (Avena sativa L.) Seedlings. Agronomy. 2023; 13(2):604. https://doi.org/10.3390/agronomy13020604
Chicago/Turabian StyleWang, Yue, Kaiqiang Liu, Guoling Liang, Zhifeng Jia, Zeliang Ju, Xiang Ma, and Qingping Zhou. 2023. "Comprehensive Evaluation of Low Nitrogen Tolerance in Oat (Avena sativa L.) Seedlings" Agronomy 13, no. 2: 604. https://doi.org/10.3390/agronomy13020604







