Allelopathic Effects of the Invasive Prosopis juliflora (Sw.) DC. on Native Plants: Perspectives toward Agrosystems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Seeds Collection
2.3. A Preliminary Field Survey
2.4. Preparation of Aqueous Extract of Leaves of Prosopis juliflora
2.5. Surface Sterilization and Preparation of Seeds Prior to Treatment
2.6. Effect of Aqueous Extracts on Seed Germination and Seedling Growth
2.6.1. Effect on Seed Germination of Native Plants
2.6.2. Effect of Leaf Leachates of P. juliflora on Seedlings Growth of Native Plants
2.6.3. Autotoxicity of the Aqueous Extract
2.7. Data Analysis
3. Results
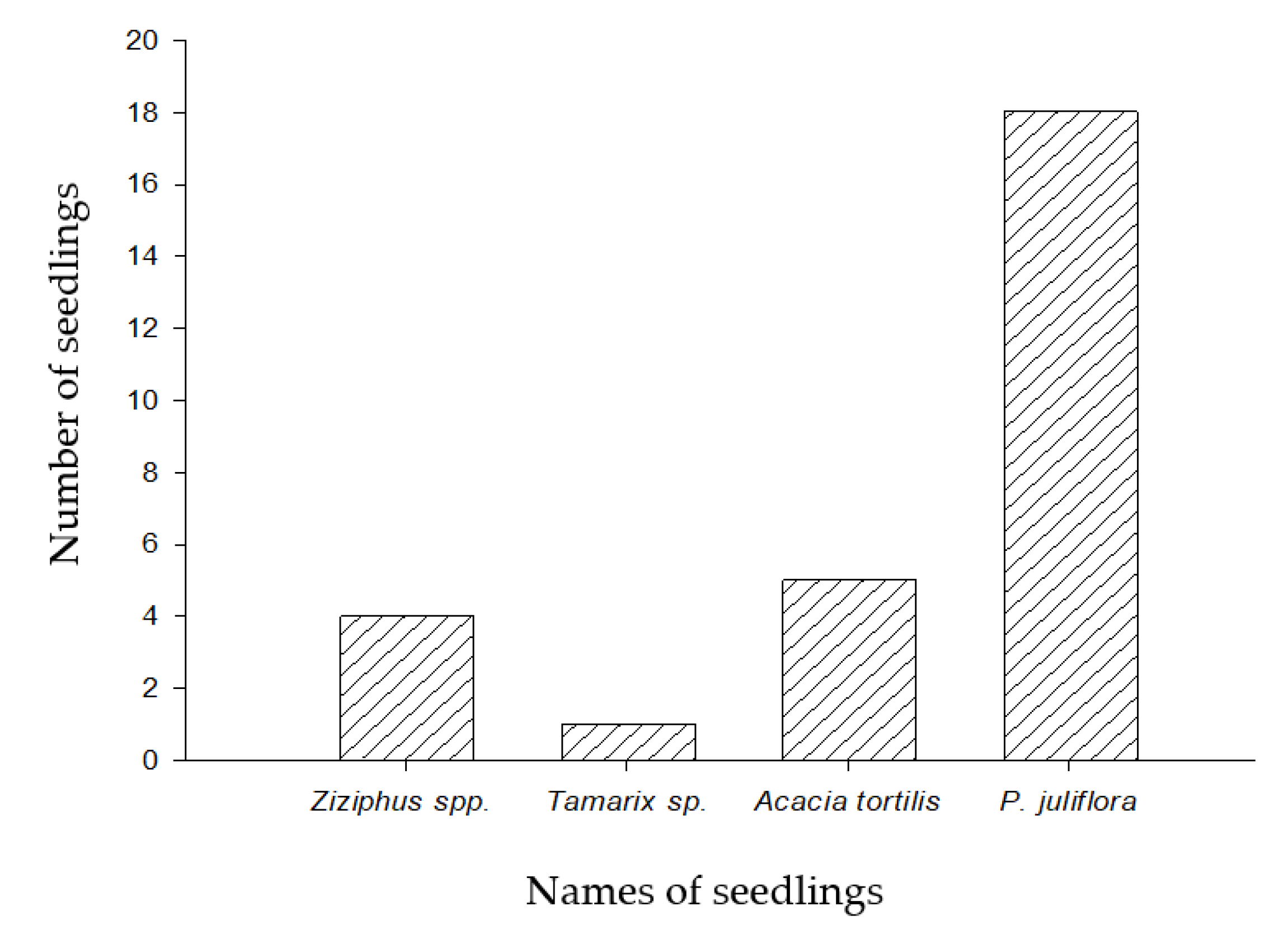
3.1. Field Survey
3.2. Effect of Aqueous Extract of P. juliflora on Seed Germination Potential
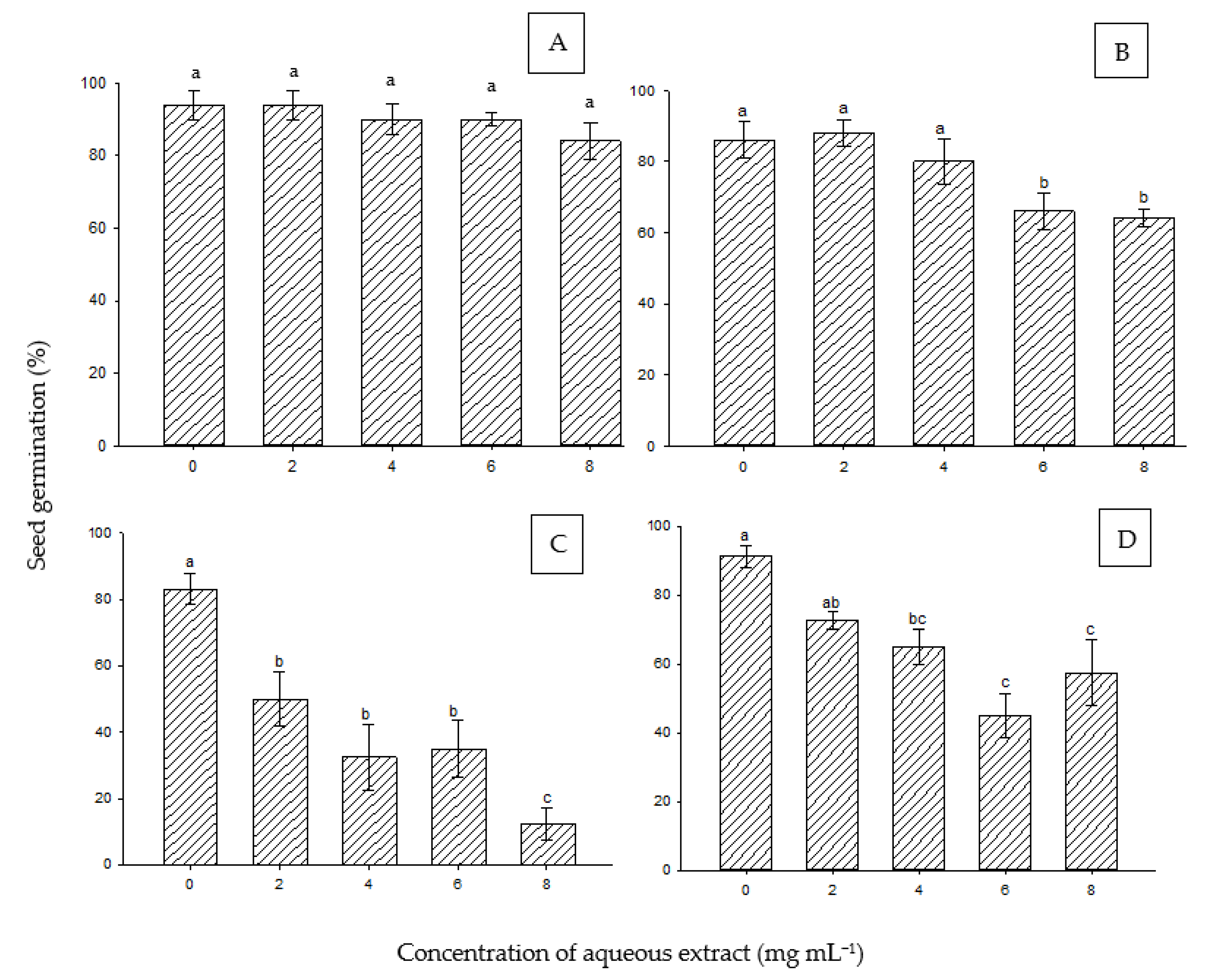
3.2.1. Effect of Aqueous Extract of P. juliflora on Seed Germination of Native Plants
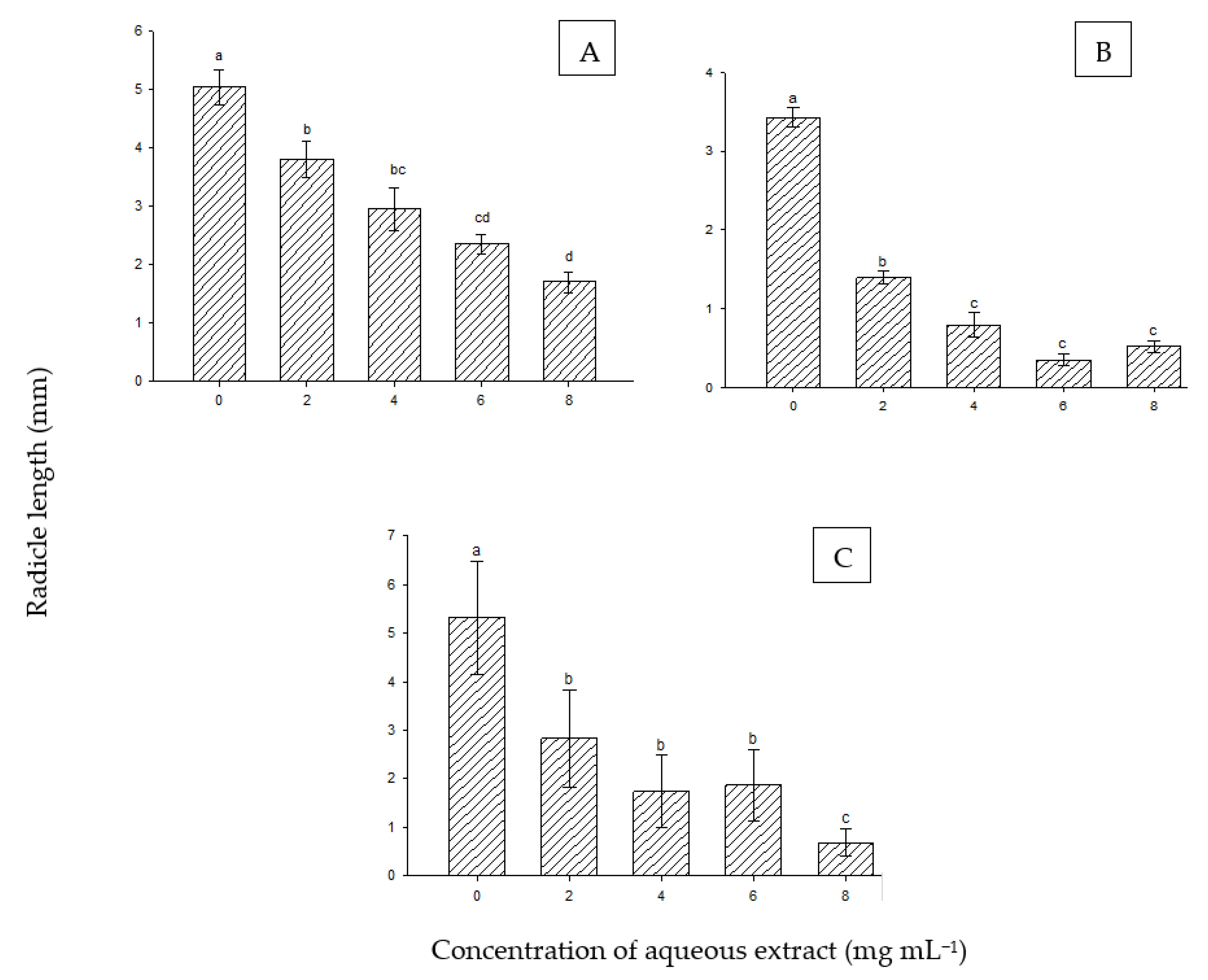
3.2.2. Effect of Aqueous Extract of P. juliflora on Radicle Length of Native Plants
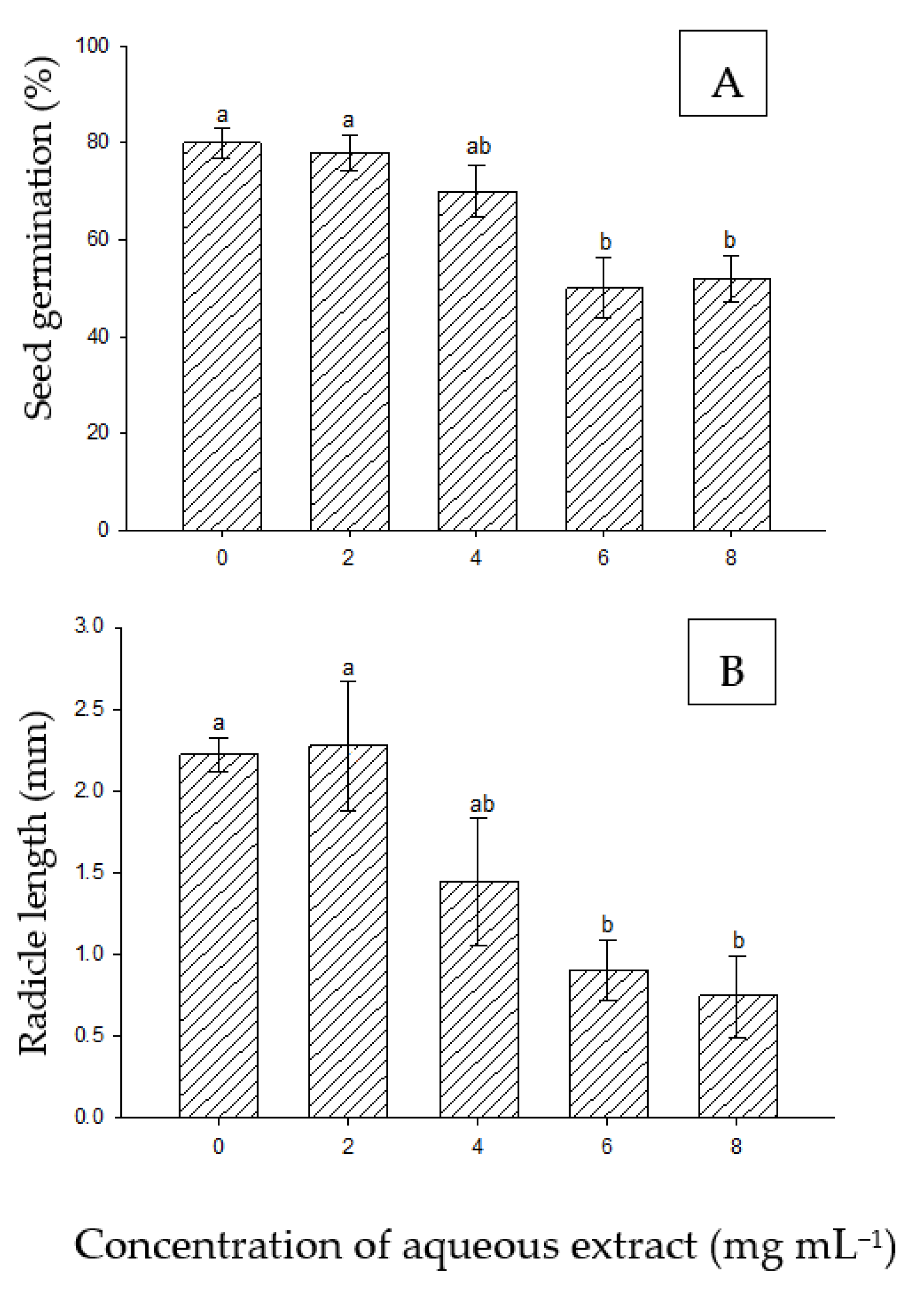
3.2.3. Autotoxicity; Effect of Aqueous Extract of P. juliflora on Germination Potential of P. juliflora
3.3. Effect of Leaf Leachate of P. juliflora on Seedling Growth of Selected Plant Species
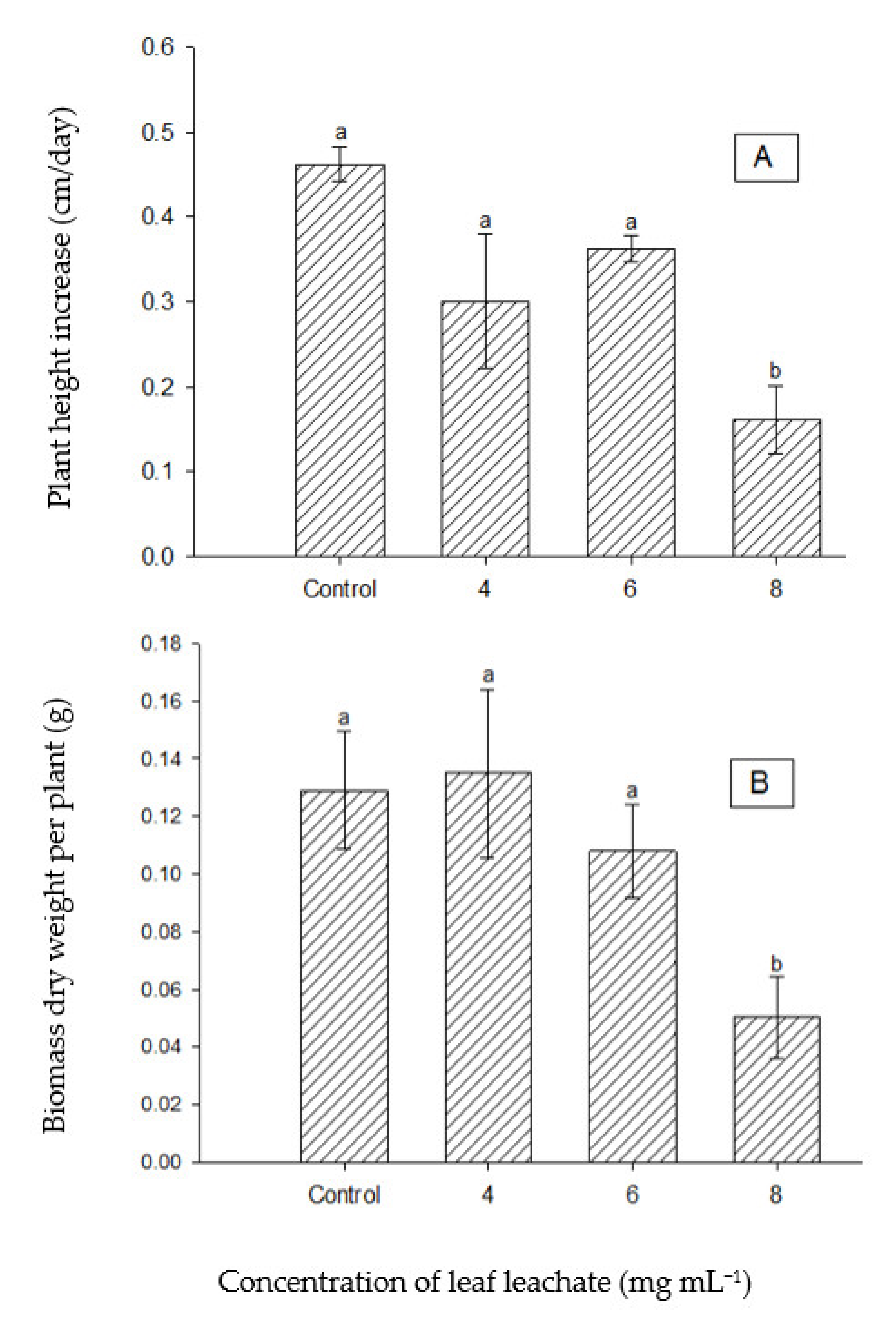
3.3.1. Effect of Leaf Leachate of P. juliflora on Seedling Growth of A. lagopoides
3.3.2. Effect of Leaf Leachate of P. juliflora on Seedling Growth of C. imbricatum
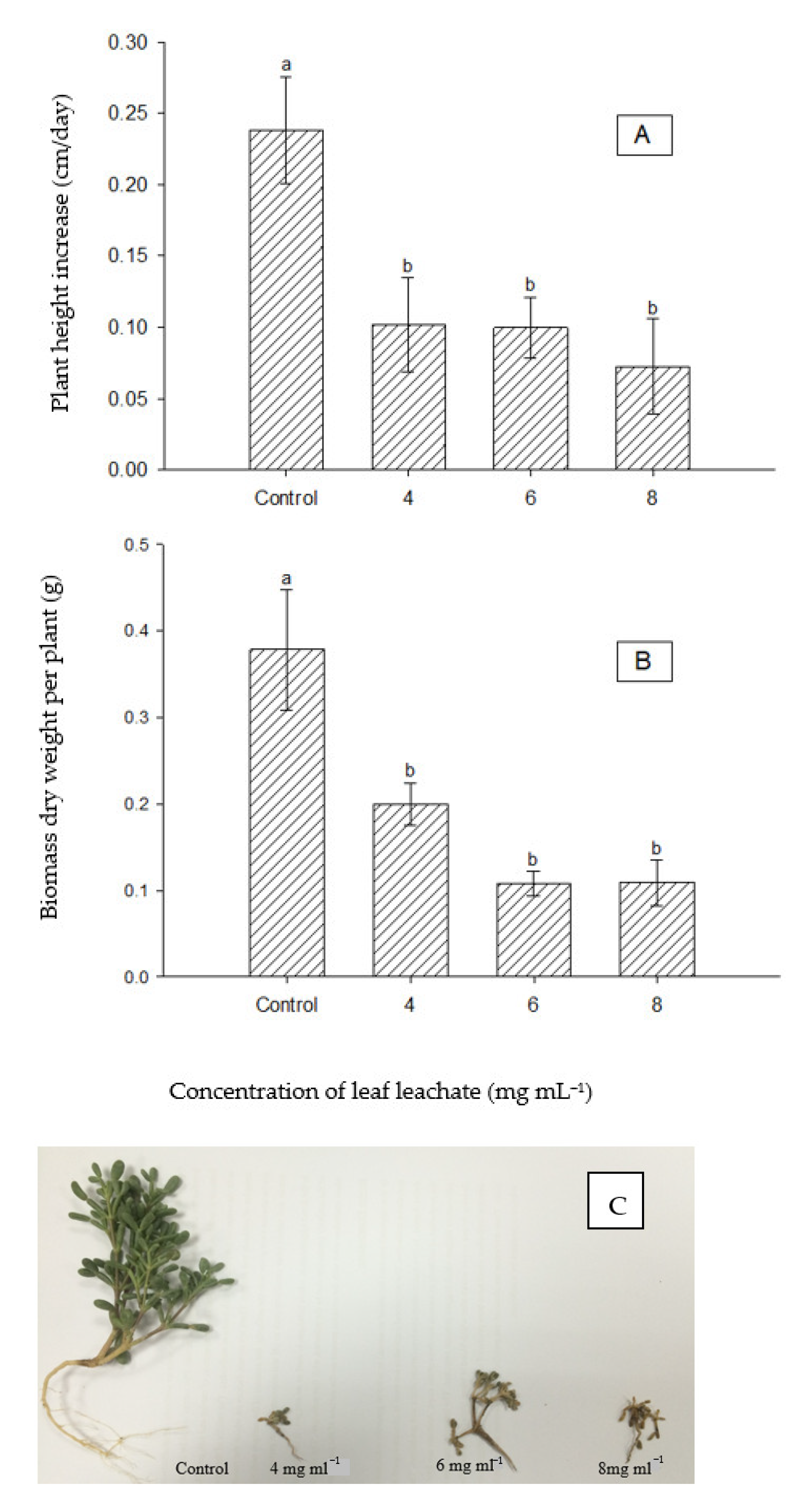
3.3.3. Effect of Leaf Leachate of P. juliflora on Seedling Growth of T. qatarensis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ehlers, B.K.; Charpentier, A.; Grøndahl, E. An allelopathic plant facilitates species richness in the Mediterranean garrigue. J. Ecol. 2014, 102, 176–185. [Google Scholar] [CrossRef]
- Inderjit; Wardle, D.A.; Karban, R.; Callaway, R.M. The ecosystem and evolutionary contexts of allelopathy. Trends Ecol. Evol. 2011, 26, 655–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, J.J.; Rathinasabapathi, B.; Chase, C.A. Allelopathy: How Plants Suppress Other Plants. EDIS 2013, 2013. [Google Scholar] [CrossRef]
- Balcha, L.D. Prosopis juliflora Distribution, Impacts, and Control Methods Available in Ethiopia. Int. J. Nat. Resour. Ecol. Manag. 2022, 7, 132–144. [Google Scholar]
- Howari, F.M.; Sharma, M.; Nazzal, Y.; El-Keblawy, A.; Mir, S.; Xavier, C.M.; Salem, I.B.; Al-Taani, A.A.; Alaydaroos, F. Changes in the Invasion Rate of Prosopis juliflora and Its Impact on Depletion of Groundwater in the Northern Part of the United Arab Emirates. Plants 2022, 11, 682. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mathur, M. Impact of invasion by Prosopis juliflora on plant communities in arid grazing lands Network Project on Guggul View project Assessing adaptability and utilization potential of Opuntia ficus-indica in arid and semi-arid regions of India V. Trop. Ecol. 2014, 55, 33–46. [Google Scholar]
- Pasiecznik, N.M.; Felker, P.; Harris, P.J.C.; Harsh, L.N.; Cruz, G.; Tewari, J.C.; Cadoret, K.; Maldonado, L.J. The Prosopis juliflora-Prosopis pallida Complex: A Monograph; HDRA: Coventry UK, 2002; p. 172. [Google Scholar]
- Shiferaw, W.; Demissew, S.; Bekele, T.; Aynekulu, E.; Pitroff, W. Invasion of Prosopis juliflora and its effects on soil physicochemical properties in Afar region, Northeast Ethiopia. Int. Soil Water Conserv. Res. 2021, 9, 631–638. [Google Scholar] [CrossRef]
- Mehari, Z.H. The invasion of Prosopis juliflora and Afar pastoral livelihoods in the Middle Awash area of Ethiopia. Ecol. Process. 2015, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- De Brito Damasceno, G.A.; Souto, A.L.; da Silva, I.B.; Roque, A.d.A.; Ferrari, M.; Giordani, R.B. Prosopis juliflora: Phytochemical, Toxicological, and Allelochemicals. Ref. Ser. Phytochem. 2020, 521–541. [Google Scholar] [CrossRef]
- Kumar Mehar, S. Assessment of effect of Prosopis juliflora litter extract on seed germination and growth of rice. Food Sci. Qual Manag. 2011, 2. [Google Scholar]
- Almaraz-Abarca, N.; da Graça Campos, M.; Avila-Reyes, J.A.; Naranjo-Jimenez, N.; Corral, J.H.; Gonzalez-Valdez, L.S. Antioxidant activity of polyphenolic extract of monofloral honeybee-collected pollen from mesquite (Prosopis juliflora, Leguminosae). J. Food Compos. Anal. 2007, 20, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Wassel, G.; Rizk, A.; Abdel-Bary, E. Phytochemical investigation of Prosopis juliflora DCI Flavonoids and free sugars. Qual. Plant. Et Mater. Veg. 1972, 22, 119–121. [Google Scholar] [CrossRef]
- Nee’Shukla, R.V.; Misra, K. Two flavonoid glycosides from the bark of Prosopis juliflora. Phytochemistry 1981, 20, 339–340. [Google Scholar] [CrossRef]
- Ibrahim, M.; Nadir, M.; Ali, A.; Ahmad, V.U.; Rasheed, M. Phytochemical analyses of Prosopis juliflora Swartz DC. Pak. J. Bot 2013, 45, 2101–2104. [Google Scholar]
- Sharmila, S.; Jeyanthi, R.; Saduzzaman, M. Biodegradation of tannery effluent using Prosopis juliflora. Int. J. ChemTech Res. 2013, 5, 2186–2192. [Google Scholar]
- Tesoriere, L.; Butera, D.; Allegra, M.; Fazzari, M.; Livrea, M.A. Distribution of betalain pigments in red blood cells after consumption of cactus pear fruits and increased resistance of the cells to ex vivo induced oxidative hemolysis in humans. J. Agric. Food Chem. 2005, 53, 1266–1270. [Google Scholar] [CrossRef]
- Bhatt, S.; Chovatiya, S.; Shah, A. Evaluation of raw and hydrothermically processed Prosopis juliflora seed meal as supplementary feed for the growth of Labeo rohita fingerlings. Aquac. Nutr. 2011, 17, e164–e173. [Google Scholar] [CrossRef]
- Wink, M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 2003, 64, 3–19. [Google Scholar] [CrossRef]
- Nakano, H.; Fujii, Y.; Suzuki, T.; Yamada, K.; Kosemura, S.; Yamamura, S.; Suzuki, T.; Hasegawa, K. A growth-inhibitory substance exuded from freeze-dried mesquite (Prosopis juliflora (Sw.) DC.) leaves. Plant Growth Regul. 2001, 33, 165–168. [Google Scholar] [CrossRef]
- Nakano, H.; Fujii, Y.; Yamada, K.; Kosemura, S.; Yamamura, S.; Hasegawa, K.; Suzuki, T. Isolation and identification of plant growth inhibitors as candidate (s) for allelopathic substance (s), from aqueous leachate from mesquite (Prosopis juliflora (Sw.) DC.) leaves. Plant Growth Regul. 2002, 37, 113–117. [Google Scholar] [CrossRef]
- Nakano, H.; Nakajima, E.; Fujii, Y.; Yamada, K.; Shigemori, H.; Hasegawa, K. Leaching of the allelopathic substance,-tryptophan from the foliage of mesquite (Prosopis juliflora (Sw.) DC.) plants by water spraying. Plant Growth Regul. 2003, 40, 49–52. [Google Scholar] [CrossRef]
- Inderjit; Seastedt, T.R.; Callaway, R.M.; Pollock, J.L.; Kaur, J. Allelopathy and plant invasions: Traditional, congeneric, and bio-geographical approaches. Biol. Invasions 2008, 10, 875–890. [Google Scholar] [CrossRef]
- Pardo-Muras, M.; Puig, C.G.; Pedrol, N. Complex Synergistic Interactions among Volatile and Phenolic Compounds Underlie the Effectiveness of Allelopathic Residues Added to the Soil for Weed Control. Plants 2022, 11, 1114. [Google Scholar] [CrossRef] [PubMed]
- Ghazanfar, S.A. Invasive Prosopis in sultanate of Oman. Aliens 1996, 3, 10. [Google Scholar]
- Western, A.R. The Flora of the United Arab Emirates: An Introduction; United Arab Emirates University: Al-Ain, United Arab Emirates, 1989. [Google Scholar]
- Sergeev, A.V. Prosopis juliflora—Flora of Qatar. 2008. Available online: https://www.floraofqatar.com/prosopis_juliflora.htm (accessed on 5 October 2022).
- Siddiqui, S.; Bhardwar, S.; Khan, S.K.; Meghvanshi, M.K. Allelopathic effect of different conentration of water extract of Prosopis Juliflora leaf on seed germination and radicle length of wheat. J. Sci. Res. 2009, 4, 81–84. [Google Scholar]
- Noor, M.; Salam, U.; Khan; Ajmal, M. Allelopathic effects of Prosopis juliflora Swartz. J. Arid. Environ. 1995, 31, 83–90. [Google Scholar] [CrossRef]
- Jayasinghe, A.G.C.S.; Perera, G.A.D. Allelopathic Effect of Prosopis juliflora (Mesquite) on Seed Germination of Native Coastal Dry Forest Species. In Proceedings of the Peradeniya University Research Sessions, Peradeniya, Sri Lanka, 24 November 2011; Volume 16, p. 180. [Google Scholar]
- Saadaoui, E.; Martin, J.J.; Ghazel, N.; Ben Romdhane, C.; Massoudi, N.; Cervantes, E. Allelopathic Effects of Aqueous Extracts of Ricinus communis L. on the Germination of Six Cultivated Species. Int. J. Plant Soil Sci. 2015, 7, 220–227. [Google Scholar] [CrossRef]
- Khan, D.; Shahid Shaukat, S. Phytotoxic effects of P. juliflora Swartz. DC. against some of its field associates and the cultivated species. Int. J. Biol Biotech 2006, 3, 353–366. [Google Scholar]
- El-Keblawy, A.; Al-Rawai, A. Impacts of the invasive exotic Prosopis juliflora (Sw.) D.C. on the native flora and soils of the UAE. Plant Ecol. 2007, 190, 23–35. [Google Scholar] [CrossRef]
- Iffat Alam, S.; Hammoda, H.; Khan, F.; Al Enazi, R.; Goktepe, I. Electrical Conductivity, pH, Organic Matter and Texture of Selected Soils Around the Qatar University Campus. Res. Agric. Livest. Fish. 2020, 7, 403–409. [Google Scholar] [CrossRef]
- Amin, S.; Mansur, I.; Pamoengkas, P.; Yusmur, A. Germination Test of Two Different Types of Seed to Accelerate Reclamation and Rehabilitation of Ex-limestone Mining Land. Proc. IOP Conf. Ser. Earth Environ. Sci. 2021, 959, 012050. [Google Scholar] [CrossRef]
- Omer, H.H.M.; Mohammed, I.S. Allelopathic effects of mesquite (Prosopis juliflora) aqueous extracts on seeds germination and seedlings growth of alfalfa, sesame and sorghum. Cell Biol. Development. 2017, 1, 51–54. [Google Scholar] [CrossRef]
- Ramandi, A.; Javan, I.Y.; Tazehabadi, F.M.; Asl, G.I.; Khosravanian, R.; Ebrahimzadeh, M.H. Improvement in seed surface sterilization and in vitro seed germination of ornamental and medicinal plant-Catharanthus roseus (L.). Chiang Mai J. Sci. 2019, 46, 1107–1112. [Google Scholar]
- Alhaddad, F.A.; Abu-Dieyeh, M.H.; ElAzazi, E.S.M.; Ahmed, T.A. Salt tolerance of selected halophytes at the two initial growth stages for future management options. Sci. Rep. 2021, 11, 10194. [Google Scholar] [CrossRef]
- Statwick, J.M. Germination pretreatments to break hard-seed dormancy in Astragalus cicer L. (Fabaceae). PeerJ 2016, 4, e2621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najib, T.; Heydari, M.M.; Meda, V. Combination of germination and innovative microwave-assisted infrared drying of lentils: Effect of physicochemical properties of different varieties on water uptake, germination, and drying kinetics. Appl. Food Res. 2022, 2, 100040. [Google Scholar] [CrossRef]
- Setia, R.; Dai, Z.; Nickerson, M.T.; Sopiwnyk, E.; Malcolmson, L.; Ai, Y. Impacts of short-term germination on the chemical compositions, technological characteristics and nutritional quality of yellow pea and faba bean flours. Food Res. Int. 2019, 122, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Wymore, A.S.; Compson, Z.G.; McDowell, W.H.; Potter, J.D.; Hungate, B.A.; Whitham, T.G.; Marks, J.C. Leaf-litter leachate is distinct in optical properties and bioavailability to stream heterotrophs. Freshw. Sci. 2015, 34, 857–866. [Google Scholar] [CrossRef] [Green Version]
- Strauss, E.A.; Lamberti, G.A. Effect of dissolved organic carbon quality on microbial decomposition and nitrification rates in stream sediments. Freshw. Biol. 2002, 47, 65–74. [Google Scholar] [CrossRef]
- Gianinetti, A. Basic features of the analysis of germination data with generalized linear mixed models. Data 2020, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Bertsouklis, K.; Vlachou, G.; Trigka, M.; Papafotiou, M. In Vitro Studies on Seed Germination of the Mediterranean Species Anthyllis barba-jovis to Facilitate Its Introduction into the Floriculture Industry. Horticulturae 2022, 8, 889. [Google Scholar] [CrossRef]
- Haregeweyn, N.; Tsunekawa, A.; Tsubo, M.; Meshesha, D.; Melkie, A. Analysis of the invasion rate, impacts and control measures of Prosopis juliflora: A case study of Amibara District, Eastern Ethiopia. Environ. Monit. Assess. 2013, 185, 7527–7542. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.M.; Soliman, W.S.; Mahmoud, M.; Youssif, M.F.; Mansour, A.; Taher, E.; Hassan, I.N.; Mansour, H.; Abdelkareem, M. Predicting the Spatial Spread of Invasive Prosopis juliflora (SW.) D.C along Environmental Gradients in Gabel Elba National Park, Egypt. Int. J. Sci. Eng. Res. 2016, 7, 596–599. [Google Scholar] [CrossRef]
- Al-Humaid, A.I.; Warrag, M.O.A. Allelopathic effects of mesquite (Prosopis juliflora) foliage on seed germination and seedling growth of bermudagrass (Cynodon dactylon). J. Arid Environ. 1998, 38, 237–243. [Google Scholar] [CrossRef]
- Getachew, S.; Demissew, S.; Woldemariam, T.; Dana, E. Allelopathic effects of the invasive Prosopis juliflora (Sw.) DC. on selected native plant species in Middle Awash, Southern Afar Rift of Ethiopia. Manag. Biol. Invasions 2012, 3, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Mei, L.; Tang, J. Allelopathic effects of invasive Solidago canadensis on germination and root growth of native Chinese plants. In Proceedings of the 4th World Congress on Allelopathy, Wagga Wagga, NSW, Australia, 21–26 August 2005; Charles Sturt University: Wagga Wagga, NSW, Australia, 2005; pp. 43–49. [Google Scholar]
- Saxena, S.K. Banni grassland and halophytes. In Halophytes as a Resource for Livestock and for Rehabilitation of Degraded Lands; Springer: Dordrecht, The Netherlands, 1994; Volume 32, pp. 217–222. [Google Scholar] [CrossRef]
- Ungar, I.A. Are Biotic Factors Significant in Influencing the Distribution of Halophytes in Saline Habitats? Bot. Review. 1998, 64, 176–199. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kohli, R.K. Autotoxicity: Concept, Organisms, and Ecological Significance. Crit. Rev. Plant Sci. 2010, 18, 757–772. [Google Scholar] [CrossRef]
- Bokrezion, H. The Ecological and Socio Economic Role of Prosopis juliflora in Eritrea an Analytical Assessment within the Context of Rural Development in the Horn of Africa; Johannes Gutenberg-Universität Mainz: Mainz, Germany, 2008. [Google Scholar]
- Mohsenzadeh, S.; Malboobi, M.A.; Razavi, K.; Farrahi-Aschtiani, S. Physiological and molecular responses of Aeluropus lagopoides (Poaceae) to water deficit. Environ. Exp. Bot. 2006, 56, 314–322. [Google Scholar] [CrossRef]
- Goel, U.; Saxena, D.B.; Kumar, B. Comparative study of allelopathy as exhibited byProsopis Juliflora swartz and Prosopis cineraria (L) druce. J. Chem. Ecol. 1989, 15, 591–600. [Google Scholar] [CrossRef]
- Kaur, R.; Gonzáles, W.L.; Llambi, L.D.; Soriano, P.J.; Callaway, R.M.; Rout, M.E.; Gallaher, T.J.; Inderjit. Community Impacts of Prosopis juliflora Invasion: Biogeographic and Congeneric Comparisons. PLoS ONE 2012, 7, e44966. [Google Scholar] [CrossRef]
- Kaur, R.; Callaway, R.M.; Inderjit. Soils and the conditional allelopathic effects of a tropical invader. Soil Biol. Biochem. 2014, 78, 316–325. [Google Scholar] [CrossRef]
- Mustafa, H.F.; Bushra, R.A. Effect of Leaf Aqueous Extract of Prosopis juliflora (Swartz) DC and Acacia raddiana (Brenan) on Early Growth of Sorghum sudanese (Piper Stapf). IOSR J. Agric. Vet. Sci. 2018, 11, 26–31. [Google Scholar]
- Hall, M.; Llewellyn, O.A.; Miller, A.G.; Al-Abbasi, T.M.; Al-Wetaid, A.H.; Al-Harbi, R.J.; Al-Shammari, K.F. Important plant areas in the arabian peninsula: 2. farasan archipelago. Edinb. J. Bot. 2010, 67, 189–208. [Google Scholar] [CrossRef]
- Badri, A.M.; Garbi, M.I.; Gmaraldeen, S.M.; Magzoub, A.A.; Ibrahim, I.T.; Saleh, M.S.; Kabbashi, A.S.; Mohamed, S.G. Antioxidant activity and phytochemical screening of Prosopis juliflora leaves extract. Adv. Med. Plant Res 2017, 5, 37–40. [Google Scholar] [CrossRef] [Green Version]
- Sathiya, M.; Muthuchelian, K. Investigation of Phytochemical Profile and Antibacterial Potential of Ethanolic Leaf Extract of Prosopis juliflora DC. Ethnobot. Leafl. 2008, 2008, 167. [Google Scholar]
- Saleh, I.; Ahmed, T.; Halboosi, R.; Abu-Dieyeh, M. Genetic diversity of Prosopis juliflora in the state of Qatar and its valuable use against postharvest pathogen of mango fruits. Sci. Rep. 2022, 12, 1–13. [Google Scholar] [CrossRef]
- Saleh, I.; Abu-Dieyeh, M.H. Novel Prosopis juliflora leaf ethanolic extract as natural antimicrobial agent against food spoiling microorganisms. Sci. Rep. 2021, 11, 1–17. [Google Scholar] [CrossRef]
- Saleh, I.; Abu-Dieyeh, M. Novel Prosopis juliflora leaf ethanolic extract coating for extending postharvest shelf-life of strawberries. Food Control. 2022, 133, 108641. [Google Scholar] [CrossRef]
- Saleh; Abu-Dieyeh. Evaluation of novel Prosopis juliflora water soluble leaf ethanolic extract as preservation coating material of cucumber. J. Food Process. Preserv. 2022, 46, e16352. [Google Scholar] [CrossRef]
- Palanivel, H.; Tilaye, G.; Belliathan, S.K.; Benor, S.; Abera, S.; Kamaraj, M. Allelochemicals as Natural Herbicides for Sustainable Agriculture to Promote a Cleaner Environment. In Strategies and Tools for Pollutant Mitigation: Avenues to a Cleaner Environment; Aravind, J., Kamaraj, M., Prashanthi Devi, M., Rajakumar, S., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 93–116. [Google Scholar]
- Ullah, R.; Aslam, Z.; Attia, H.; Sultan, K.; Alamer, K.H.; Mansha, M.Z.; Althobaiti, A.T.; Al Kashgry, N.A.T.; Algethami, B.; Zaman, Q.U. Sorghum Allelopathy: Alternative Weed Management Strategy and Its Impact on Mung Bean Productivity and Soil Rhizosphere Properties. Life 2022, 12, 1359. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bibi, S.; Bibi, A.; Al-Ghouti, M.A.; Abu-Dieyeh, M.H. Allelopathic Effects of the Invasive Prosopis juliflora (Sw.) DC. on Native Plants: Perspectives toward Agrosystems. Agronomy 2023, 13, 590. https://doi.org/10.3390/agronomy13020590
Bibi S, Bibi A, Al-Ghouti MA, Abu-Dieyeh MH. Allelopathic Effects of the Invasive Prosopis juliflora (Sw.) DC. on Native Plants: Perspectives toward Agrosystems. Agronomy. 2023; 13(2):590. https://doi.org/10.3390/agronomy13020590
Chicago/Turabian StyleBibi, Shazia, Amina Bibi, Mohammad A. Al-Ghouti, and Mohammed H. Abu-Dieyeh. 2023. "Allelopathic Effects of the Invasive Prosopis juliflora (Sw.) DC. on Native Plants: Perspectives toward Agrosystems" Agronomy 13, no. 2: 590. https://doi.org/10.3390/agronomy13020590





