Testing the Effect of High pH and Low Nutrient Concentration on Four Leafy Vegetables in Hydroponics
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Location and Growing Conditions
2.2. Experimental Design and Treatments
2.3. Nutrient Solution Menagement
2.4. Plant Biometric Measurements and Determination of Nutrients
2.5. Pigment Analyses
2.6. Statistics
3. Results
3.1. Plant Biomass, Leaf Area and Water Uptake
3.2. Tissue Nutrient Content
3.3. Leaf Pigments
3.4. Overall Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khater, E.S.; Bahnasawy, A.; Abass, W.; Morsy, O.; El-Ghobashy, H.; Shaban, Y.; Egela, M. Production of basil (Ocimum basilicum L.) under different soilless cultures. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Massa, D.; Magán, J.J.; Montesano, F.F.; Tzortzakis, N. Minimizing water and nutrient losses from soilless cropping in southern Europe. Agric. Water Manag. 2020, 241, 106395. [Google Scholar] [CrossRef]
- Maucieri, C.; Nicoletto, C.; Van Os, E.; Anseeuw, D.; Van Havermaet, R.; Junge, R. Hydroponic technologies. In Aquaponics Food Production Systems; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer: Cham, Switzerland, 2019; pp. 77–112. [Google Scholar]
- Sambo, P.; Nicoletto, C.; Giro, A.; Pii, Y.; Valentinuzzi, F.; Mimmo, T.; Lugli, P.; Orzes, G.; Mazzetto, F.; Astolfi, S.; et al. Hydroponic solutions for soilless production systems: Issues and opportunities in a smart agriculture perspective. Front. Plant Sci. 2019, 10, 923. [Google Scholar] [CrossRef] [PubMed]
- FAO. Climate Change and Food Security: Risks and Responses; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016. [Google Scholar]
- Goddek, S.; Delaide, B.; Mankasingh, U.; Ragnarsdottir, K.V.; Jijakli, H.; Thorarinsdottir, R. Challenges of sustainable and commercial aquaponics. Sustainability 2015, 7, 4199–4224. [Google Scholar] [CrossRef]
- Rufí-Salís, M.; Calvo, M.J.; Petit-Boix, A.; Villalba, G.; Gabarrell, X. Exploring nutrient recovery from hydroponics in urban agriculture: An environmental assessment. Resour. Conserv. Recycl. 2020, 155, 104683. [Google Scholar] [CrossRef]
- Klinger, D.; Naylor, R. Searching for solutions in aquaculture: Charting a sustainable course. Annu. Rev. Environ. Resour. 2012, 37, 247–276. [Google Scholar] [CrossRef]
- Ru, D.; Liu, J.; Hu, Z.; Zou, Y.; Jiang, L.; Cheng, X.; Lv, Z. Improvement of aquaponic performance through micro- and macro-nutrient addition. Environ. Sci. Pollut. Res. 2017, 24, 16328–16335. [Google Scholar] [CrossRef]
- Thompson, R.B.; Incrocci, L.; Van Ruijven, J.; Massa, D. Reducing contamination of water bodies from European vegetable production systems. Agric. Water Manag. 2020, 240, 106258. [Google Scholar] [CrossRef]
- Omer, N.H. Water quality parameters. In Water Quality-Science, Assessments and Policy; Summers, J.K., Ed.; IntechOpen: London, UK, 2020; pp. 3–20. [Google Scholar]
- Lennard, W.; Goddek, S. Aquaponics: The basics. In Aquaponics Food Production Systems; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer: Cham, Switzerland, 2019; pp. 113–145. [Google Scholar]
- Van Rijn, J.; Tal, Y.; Schreier, H.J. Denitrification in recirculating systems: Theory and applications. Aquac. Eng. 2006, 34, 364–376. [Google Scholar] [CrossRef]
- Umamaheswari, S.; Preethi, A.; Pravin, E.; Dhanusha, R. Integrating scheduled hydroponic system. In Proceedings of the 2016 IEEE International Conference on Advances in Computer Applications (ICACA), Coimbatore, India, 24 October 2016; pp. 333–337. [Google Scholar]
- Gillespie, D.P.; Kubota, C.; Miller, S.A. Effects of low pH of hydroponic nutrient solution on plant growth, nutrient uptake, and root rot disease incidence of basil (Ocimum basilicum L.). HortScience 2020, 55, 1251–1258. [Google Scholar] [CrossRef]
- Bugbee, B. Nutrient management in recirculating hydroponic culture. In South Pacific Soilless Culture Conference-SPSCC. Acta Hortic. 2003, 648, 99–112. [Google Scholar]
- Nikolic, M.; Pavlovic, J. Plant responses to iron deficiency and toxicity and iron use efficiency in plants. In Plant Micronutrient Use Efficiency; Hossain, M.A., Kamiya, T., Burritt, D.J., Phan Tran, L.S., Fujiwara, T., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 55–69. [Google Scholar]
- Tyson, R.V.; Simonne, E.H.; Treadwell, D.D.; Davis, M.; White, J.M. Effect of water pH on yield and nutritional status of greenhouse cucumber grown in recirculating hydroponics. J. Plant Nutr. 2008, 31, 2018–2030. [Google Scholar] [CrossRef]
- Tyson, R.V.; Simonne, E.H.; White, J.M.; Lamb, E.M. Reconciling water quality parameters impacting nitrification in aquaponics: The pH levels. In Proceedings of the Florida State Horticultural Society, Goldenroad, FL, USA, 6–8 June 2004; Volume 117, pp. 79–83. [Google Scholar]
- Goddek, S.; Keesman, K.J. The necessity of desalination technology for designing and sizing multi-loop aquaponics systems. Desalination 2018, 428, 76–85. [Google Scholar] [CrossRef]
- Wortman, S.E. Crop physiological response to nutrient solution electrical conductivity and pH in an ebb-and-flow hydroponic system. Sci. Hortic. 2015, 194, 34–42. [Google Scholar] [CrossRef]
- Arakkal Thaiparambil, N.; Radhakrishnan, V. Challenges in achieving an economically sustainable aquaponic system: A review. Aquacult. Int. 2022, 30, 3035–3066. [Google Scholar] [CrossRef]
- Arnon, D.I.; Hoagland, D.R. Crop production in artificial culture solutions and in soils with special reference to factors influencing yields and absorption of inorganic nutrients. Soil Sci. 1940, 50, 463–485. [Google Scholar]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Maggini, R.; Benvenuti, S.; Leoni, F.; Pardossi, A. Terracrepolo (Reichardia picroides (L.) Roth.): Wild food or new horticultural crop? Sci. Hortic. 2018, 240, 224–231. [Google Scholar] [CrossRef]
- Yavuzcan Yildiz, H.; Robaina, L.; Pirhonen, J.; Mente, E.; Domínguez, D.; Parisi, G. Fish welfare in aquaponic systems: Its relation to water quality with an emphasis on feed and faeces—A review. Water 2017, 9, 13. [Google Scholar] [CrossRef]
- Nozzi, V.; Graber, A.; Schmautz, Z.; Mathis, A.; Junge, R. Nutrient management in aquaponics: Comparison of three approaches for cultivating lettuce, mint and mushroom herb. Agronomy 2018, 8, 27. [Google Scholar] [CrossRef]
- Monsees, H.; Klatt, L.; Kloas, W.; Wuertz, S. Chronic exposure to nitrate significantly reduces growth and affects the health status of juvenile Nile tilapia (Oreochromis niloticus L.) in recirculating aquaculture systems. Aquac. Res. 2017, 48, 3482–3492. [Google Scholar] [CrossRef]
- Yang, X.; Song, X.; Peng, L.; Hallerman, E.; Huang, Z. Effects of nitrate on aquaculture production; blood and histological markers and liver transcriptome of Oplegnathus punctatus. Aquaculture 2019, 501, 387–396. [Google Scholar] [CrossRef]
- Rodgers, D.; Won, E.; Timmons, M.B.; Mattson, N. Complementary nutrients in decoupled aquaponics enhance basil performance. Horticulturae 2022, 8, 111. [Google Scholar] [CrossRef]
- Böhme, M.H.; Dewenter, M.; Gohlke, A. Aquaponics using Asian leafy vegetables—Potential and challenge. Acta Hortic. 2020, 1273, 115–122. [Google Scholar] [CrossRef]
- Maucieri, C.; Nicoletto, C.; Zanin, G.; Birolo, M.; Trocino, A.; Sambo, P.; Borin, M.; Xiccato, G. Effect of stocking density of fish on water quality and growth performance of European Carp and leafy vegetables in a low-tech aquaponic system. PLoS ONE 2019, 14, e0217561. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Janpen, C.; Kanthawang, N.; Inkham, C.; Tsan, F.Y.; Sommano, S.R. Physiological responses of hydroponically-grown Japanese mint under nutrient deficiency. PeerJ 2019, 7, e7751. [Google Scholar] [CrossRef]
- Nicoletto, C.; Maucieri, C.; Mathis, A.; Schmautz, Z.; Komives, T.; Sambo, P.; Junge, R. Extension of aquaponic water use for NFT baby-leaf production: Mizuna and rocket salad. Agronomy 2018, 8, 75. [Google Scholar] [CrossRef]
- Stathopoulou, P.; Tsoumalakou, E.; Levizou, E.; Vanikiotis, T.; Zaoutsos, S.; Berillis, P. Iron and potassium fertilization improve rocket growth without affecting tilapia growth and histomorphology characteristics in aquaponics. Appl. Sci. 2021, 11, 5681. [Google Scholar] [CrossRef]
- Yang, T.; Samarakoon, U.; Altland, J.; Ling, P. Photosynthesis; biomass production; nutritional quality; and flavor-related phytochemical properties of hydroponic-grown arugula (Eruca sativa Mill.) ‘Standard’ under different electrical conductivities of nutrient solution. Agronomy 2021, 11, 1340. [Google Scholar] [CrossRef]
- Love, D.C.; Fry, J.P.; Li, X.; Hill, E.S.; Genello, L.; Semmens, K.; Thompson, R.E. Commercial aquaponics production and profitability: Findings from an international survey. Aquaculture 2015, 435, 67–74. [Google Scholar] [CrossRef]
- Balliu, A.; Zheng, Y.; Sallaku, G.; Fernández, J.A.; Gruda, N.S.; Tuzel, Y. Environmental and cultivation factors affect the morphology; architecture and performance of root systems in soilless grown plants. Horticulturae 2021, 7, 243. [Google Scholar] [CrossRef]
- Roosta, H.R.; Bagheri, V.; Manzari Tavakkoli, M. Effect of sodium bicarbonate stress on growth and physiological characteristics of lettuce; amaranth and water convolvulus in hydroponic system. Environ. Stresses Crop Sci. 2014, 6, 171–182. [Google Scholar]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Germano, R.P.; Melito, S.; Cacini, S.; Carmassi, G.; Leoni, F.; Maggini, R.; Montesano, F.F.; Pardossi, A.; Massa, D. Sweet basil can be grown hydroponically at low phosphorus and high sodium chloride concentration: Effect on plant and nutrient solution management. Sci. Hortic. 2022, 304, 111324. [Google Scholar] [CrossRef]
- Niu, Y.F.; Chai, R.S.; Jin, G.L.; Wang, H.; Tang, C.X.; Zhang, Y.S. Responses of root architecture development to low phosphorus availability: A review. Ann. Bot. 2013, 112, 391–408. [Google Scholar] [CrossRef]
- Salehzadeh, H.; Maleki, A.; Rezaee, R.; Shahmoradi, B.; Ponnet, K. The nitrate content of fresh and cooked vegetables and their health-related risks. PLoS ONE 2020, 15, e0227551. [Google Scholar] [CrossRef]
- Pérez-Urrestarazu, L.; Lobillo-Eguíba, J.; Fernández-Cañero, R.; Fernández-Cabanás, V.M. Food safety concerns in urban aquaponic production: Nitrate contents in leafy vegetables. Urban For. Urban Green 2019, 44, 126431. [Google Scholar] [CrossRef]
- Zou, Y.; Hu, Z.; Zhang, J.; Xie, H.; Guimbaud, C.; Fang, Y. Effects of pH on nitrogen transformations in media-based aquaponics. Bioresour. Technol. 2016, 210, 81–87. [Google Scholar] [CrossRef]
- Yang, T.; Kim, H.J. Comparisons of nitrogen and phosphorus mass balance for tomato-, basil-, and lettuce-based aquaponic and hydroponic systems. J. Clean. Prod. 2020, 274, 122619. [Google Scholar] [CrossRef]
- Massa, D.; Lenzi, A.; Montoneri, E.; Ginepro, M.; Prisa, D.; Burchi, G. Plant response to biowaste soluble hydrolysates in hibiscus grown under limiting nutrient availability. J. Plant Nutr. 2018, 41, 396–409. [Google Scholar] [CrossRef]
- Da Silva Cerozi, B.; Fitzsimmons, K. The effect of pH on phosphorus availability and speciation in an aquaponics nutrient solution. Bioresour. Technol. 2016, 219, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qin, J.; Mattson, N.S.; Ao, Y. Effect of potassium application on celery growth and cation uptake under different calcium and magnesium levels in substrate culture. Sci. Hortic. 2013, 158, 33–38. [Google Scholar] [CrossRef]
- Savvas, D.; Gruda, N. Application of soilless culture technologies in the modern greenhouse industry—A review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- Currey, C.J.; Metz, V.C.; Flax, N.J.; Litvin, A.G.; Whipker, B.E. Restricting phosphorous can manage growth and development of containerized sweet basil, dill, parsley, and sage. HortScience 2020, 55, 1722–1729. [Google Scholar] [CrossRef]
- Alexopoulos, A.A.; Marandos, E.; Assimakopoulou, A.; Vidalis, N.; Petropoulos, S.A.; Karapanos, I.C. Effect of nutrient solution pH on the growth, yield and quality of Taraxacum officinale and Reichardia picroides in a floating hydroponic system. Agronomy 2021, 11, 1118. [Google Scholar] [CrossRef]
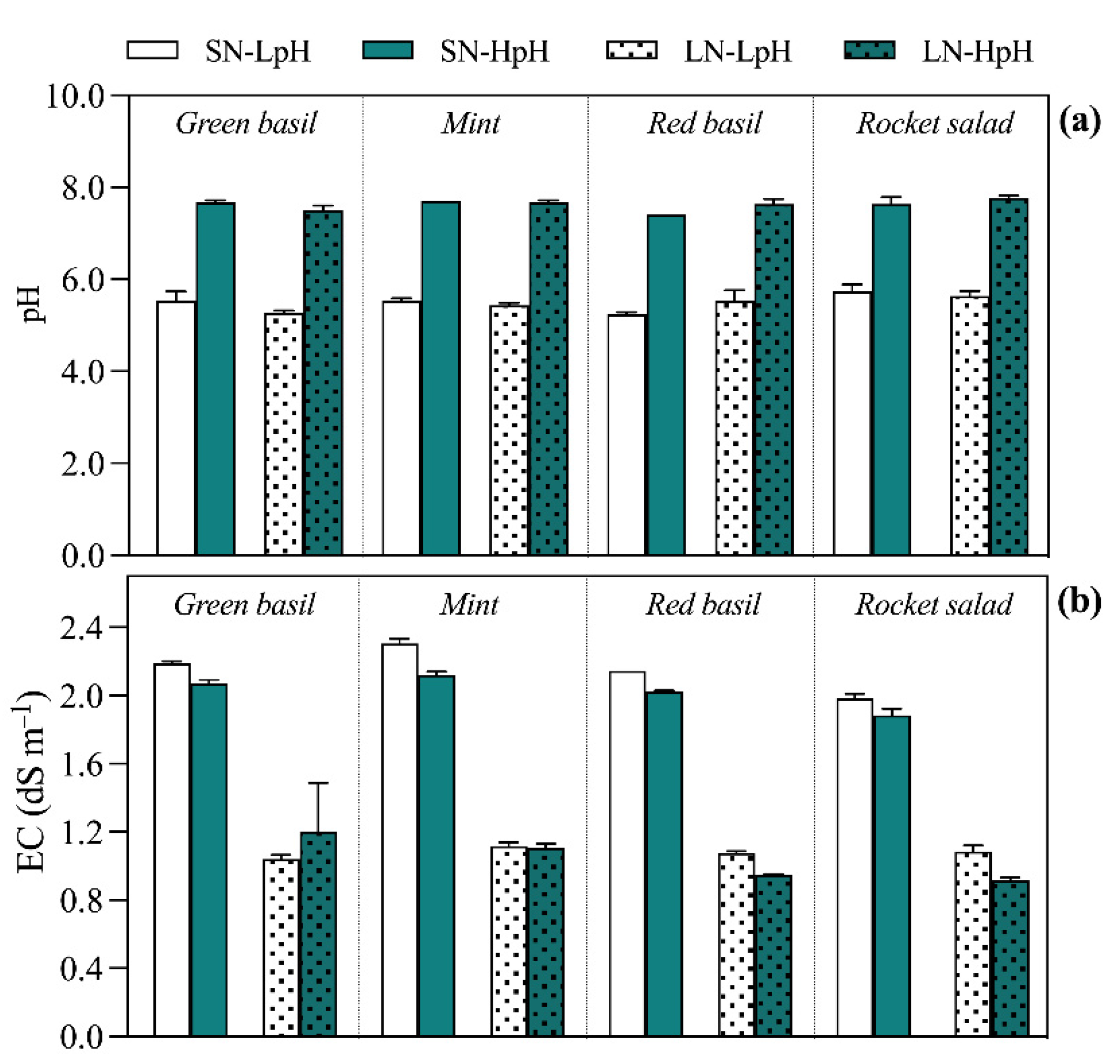
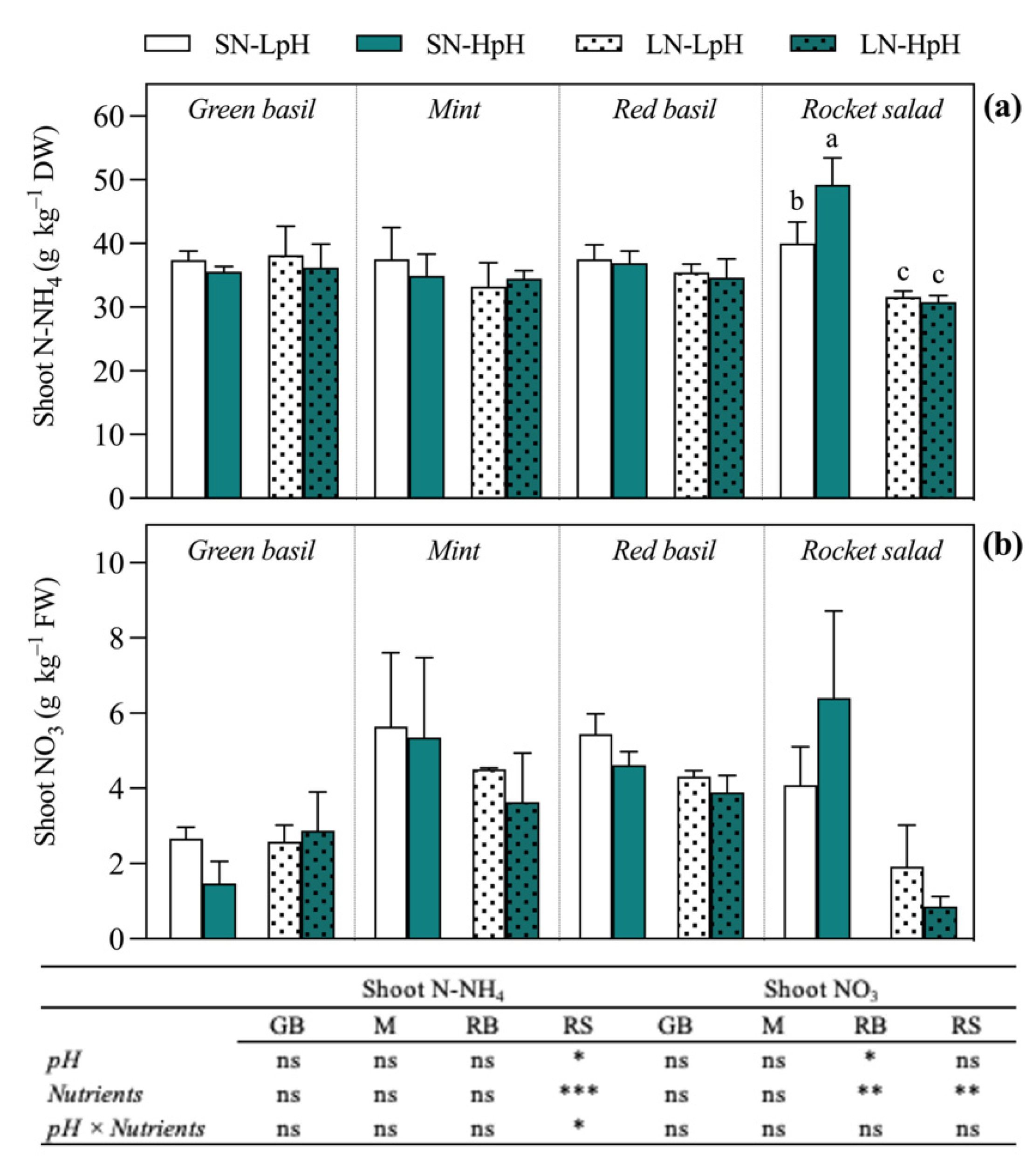
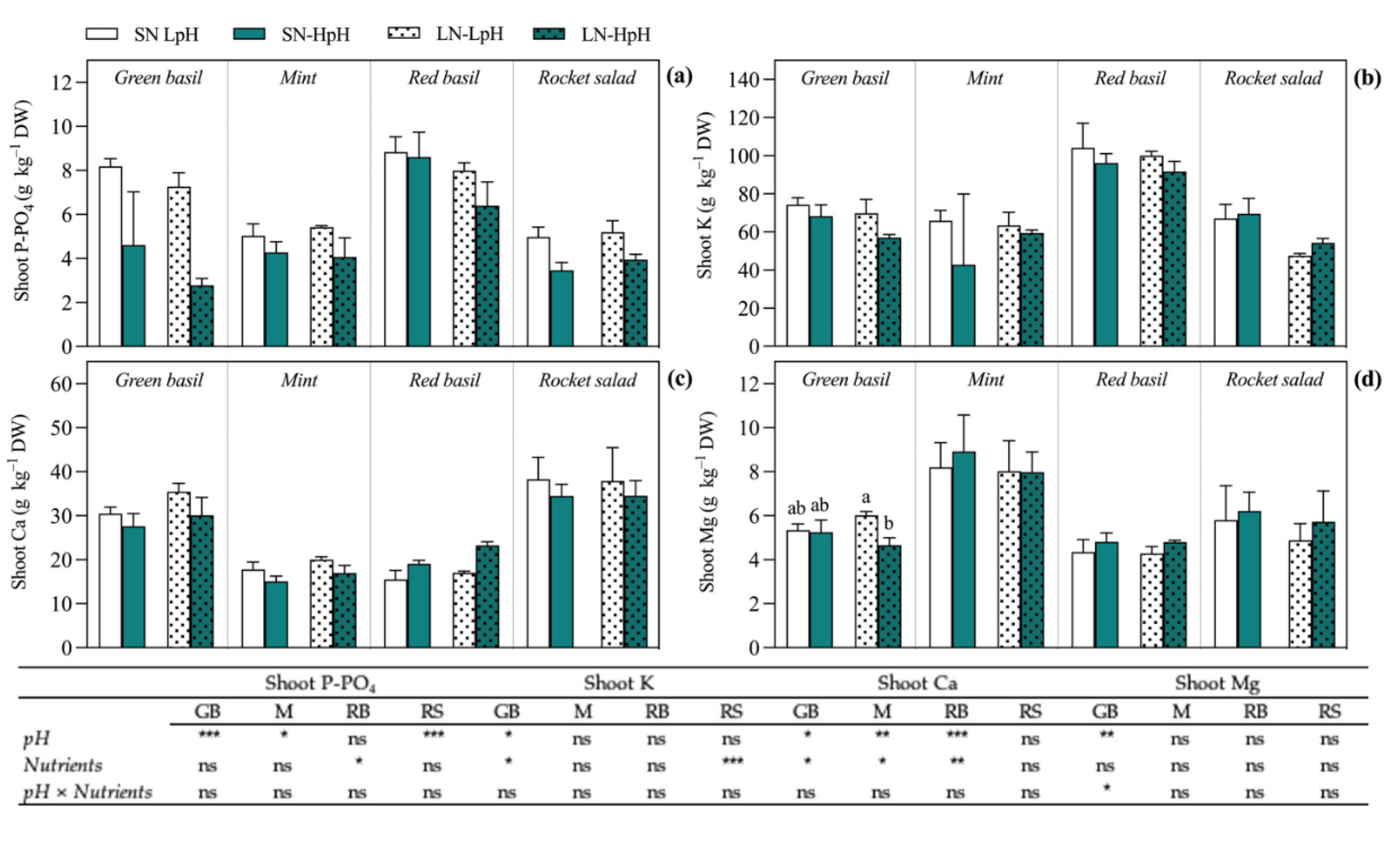


| EC | N-NO3 | N-NH4 | P | K | Ca | Mg | Na | S-SO4 | Cl | Fe | B | Cu | Zn | Mn | Mo | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dS m−1 | mmol L−1 | µmol L−1 | ||||||||||||||
| Tap water | 0.62 | 0.5 | 0.0 | 0.0 | 0.3 | 1.0 | 0.3 | 1.5 | 0.2 | 1.3 | 3.0 | 5.1 | 0.1 | 7.3 | 0.1 | 0.0 |
| SN solution | 2.14 | 14.0 | 1.0 | 1.00 | 6.0 | 4.0 | 2.0 | 1.5 | 2.0 | 1.3 | 45.0 | 45.1 | 1.0 | 7.3 | 10.0 | 1.0 |
| LN solution | 1.00 | 3.9 | 0.3 | 0.25 | 1.7 | 1.8 | 0.7 | 1.5 | 0.6 | 1.3 | 13.5 | 15.1 | 0.3 | 7.3 | 2.6 | 0.2 |
| Shoot FW (g plant−1) | Root FW (g plant−1) | Shoot DW (g plant−1) | Root DW (g plant−1) | Shoot %DW | Root %DW | Root/Shoot | Leaf Area (cm2 plant−1) | Plant ET (mL plant−1) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Green basil | SN-LpH | 54.1 ± 10.32 | 20.7 ± 2.81 | 5.0 ± 1.30 | 0.94 ± 0.299 | 9.2 ± 1.26 | 4.5 ± 0.88 | 0.19 ± 0.035 | 938 ± 176.8 | 507 ± 98.7 |
| SN-HpH | 52.9 ± 12.14 | 24.1 ± 8.59 | 4.5 ± 0.79 | 0.76 ± 0.085 | 8.6 ± 1.09 | 3.3 ± 0.72 | 0.17 ± 0.017 | 944 ± 208.2 | 531 ± 123.9 | |
| LN-LpH | 43.1 ± 4.60 | 24.3 ± 3.14 | 3.7 ± 0.60 | 0.89 ± 0.140 | 8.5 ± 0.50 | 3.7 ± 0.12 | 0.24 ± 0.001 | 802 ± 66.1 | 454 ± 56.2 | |
| LN-HpH | 47.8 ± 7.87 | 30.6 ± 4.01 | 4.7 ± 1.05 | 0.86 ± 0.110 | 9.8 ± 0.82 | 2.8 ± 0.52 | 0.18 ± 0.015 | 938 ± 281.3 | 493 ± 100.0 | |
| pH | ns | ns | ns | ns | ns | * | * | ns | ns | |
| Nutrients | ns | ns | ns | ns | ns | ns | * | ns | ns | |
| pH × Nutrients | ns | ns | ns | ns | ns | ns | ns | ns | ns | |
| Mint | SN-LpH | 55.0 ± 9.67 | 19.9 ± 4.55 | 6.1 ± 1.69 | 0.84 ± 0.101 | 11.0 ± 1.33 | 4.3 ± 0.65 | 0.14 ± 0.017 | 3587 ± 782.7 | 621 ± 189.0 |
| SN-HpH | 46.8 ± 8.72 | 16.5 ± 1.14 | 4.7 ± 1.32 | 0.89 ± 0.265 | 10.0 ± 1.80 | 5.3 ± 1.22 | 0.19 ± 0.030 | 3087 ± 404.9 | 523 ± 70.4 | |
| LN-LpH | 43.6 ± 2.92 | 20.9 ± 3.93 | 4.7 ± 0.62 | 0.82 ± 0.134 | 10.7 ± 1.00 | 3.9 ± 0.27 | 0.17 ± 0.025 | 2862 ± 157.6 | 523 ± 22.7 | |
| LN-HpH | 35.2 ± 2.16 | 14.3 ± 2.83 | 3.9 ± 0.19 | 1.00 ± 0.123 | 11.0 ± 0.93 | 7.3 ± 2.34 | 0.26 ± 0.026 | 2249 ± 302.0 | 457 ± 29.0 | |
| pH | ns | ns | ns | ns | ns | * | ** | ns | ns | |
| Nutrients | * | ns | ns | ns | ns | ns | ** | * | ns | |
| pH× Nutrients | ns | ns | ns | ns | ns | ns | ns | ns | ns | |
| Red basil | SN-LpH | 24.1 ± 4.45 | 5.3 ± 0.50 a | 1.6 ± 0.30 | 0.19 ± 0.049 | 6.8 ± 0.29 | 3.6 ± 0.56 | 0.12 ± 0.01 a | 663 ± 148.3 | 451 ± 54.4 |
| SN-HpH | 20.0 ± 3.16 | 3.5 ± 0.78 b | 1.4 ± 0.23 | 0.11 ± 0.025 | 7.0 ± 0.28 | 3.3 ± 2.99 | 0.08 ± 0.00 b | 566 ± 76.5 | 414 ± 52.9 | |
| LN-LpH | 18.1 ± 2.10 | 3.2 ± 0.65 b | 1.2 ± 0.17 | 0.12 ± 0.015 | 6.4 ± 0.19 | 3.7 ± 0.29 | 0.10 ± 0.01 ab | 444 ± 79.8 | 393 ± 27.6 | |
| LN-HpH | 15.6 ± 2.48 | 3.2 ± 0.06 b | 1.0 ± 0.22 | 0.11 ± 0.006 | 6.6 ± 0.39 | 3.3 ± 0.22 | 0.11 ± 0.02 ab | 482 ± 68.8 | 375 ± 19.2 | |
| pH | ns | * | ns | * | ns | ns | ns | ns | ns | |
| Nutrients | * | ** | * | * | * | ns | ns | * | ns | |
| pH × Nutrients | ns | * | ns | ns | ns | ns | * | ns | ns | |
| Rocket salad | SN-LpH | 129.3 ± 6.48 | 26.9 ± 2.38 | 10.1 ± 1.48 | 1.63 ± 0.308 | 7.8 ± 0.91 | 6.0 ± 0.65 | 0.16 ± 0.012 | 2833 ± 227.7 | 1681 ± 159.6 |
| SN-HpH | 94.1 ± 20.65 | 17.6 ± 1.87 | 7.7 ± 0.33 | 1.14 ± 0.167 | 8.5 ± 1.95 | 6.5 ± 0.31 | 0.15 ± 0.015 | 1960 ± 454.7 | 1209 ± 170.2 | |
| LN-LpH | 61.9 ± 16.12 | 15.5 ± 5.66 | 5.9 ± 1.57 | 0.96 ± 0.300 | 9.7 ± 1.93 | 6.3 ± 0.85 | 0.16 ± 0.010 | 1172 ± 340.7 | 1000 ± 271.5 | |
| LN-HpH | 56.2 ± 12.18 | 12.6 ± 2.78 | 6.0 ± 1.65 | 0.83 ± 0.151 | 10.6 ± 0.74 | 6.7 ± 0.61 | 0.14 ± 0.015 | 984 ± 180.0 | 996 ± 228.8 | |
| pH | * | * | ns | ns | ns | ns | ns | * | ns | |
| Nutrients | *** | ** | ** | ** | * | ns | ns | *** | ** | |
| pH × Nutrients | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Chl a + b (mg g−1 FW) | Carotenoids (mg g−1 FW) | Total Phenols (A320 g−1 FW) | Chl a/Chl b | Greenness | ||
|---|---|---|---|---|---|---|
| Green basil | SN-LpH | 1.5 ± 0.30 | 0.24 ± 0.03 | 6.4 ± 0.75 | 3.6 ± 0.25 | 6.2 ± 0.52 |
| SN-HpH | 1.5 ± 0.19 | 0.25 ± 0.02 | 6.4 ± 0.57 | 3.8 ± 0.35 | 6.1 ± 0.47 | |
| LN-LpH | 1.4 ± 0.24 | 0.23 ± 0.03 | 6.1 ± 1.13 | 3.5 ± 0.25 | 6.2 ± 0.32 | |
| LN-HpH | 1.4 ± 0.11 | 0.24 ± 0.01 | 6.0 ± 0.33 | 3.8 ± 0.33 | 6.1 ± 0.71 | |
| pH | ns | ns | ns | ns | ns | |
| Nutrients | ns | ns | ns | ns | ns | |
| pH × Nutrients | ns | ns | ns | ns | ns | |
| Mint | SN-LpH | 2.3 ± 0.07 a | 0.40 ± 0.02 a | 10.5 ± 0.57 | 3.2 ± 0.16 | 5.7 ± 0.09 |
| SN-HpH | 2.1 ± 0.21 b | 0.37 ± 0.02 b | 9.1 ± 1.18 | 3.2 ± 0.13 | 5.6 ± 0.33 | |
| LN-LpH | 2.4 ± 0.08 a | 0.38 ± 0.02 ab | 11.1 ± 0.16 | 3.3 ± 0.14 | 6.4 ± 0.42 | |
| LN-HpH | 2.5 ± 0.07 a | 0.41 ± 0.01 a | 11.4 ± 0.32 | 3.3 ± 0.04 | 6.1 ± 0.14 | |
| pH | ns | ns | ns | ns | ns | |
| Nutrients | ** | ns | ** | ns | ** | |
| pH × Nutrients | * | * | ns | ns | ns | |
| Red basil | SN-LpH | 1.2 ± 0.31 b | 0.24 ± 0.04 b | 7.8 ± 1.65 ab | 3.1 ± 0.27 | 5.2 ± 0.61 |
| SN-HpH | 1.7 ± 0.21 a | 0.30 ± 0.03 a | 9.3 ± 0.79 a | 3.1 ± 0.08 | 5.7 ± 0.50 | |
| LN-LpH | 1.5 ± 0.15 ab | 0.27 ± 0.03 ab | 8.2 ± 1.03 ab | 3.2 ± 0.09 | 5.7 ± 0.31 | |
| LN-HpH | 1.4 ± 0.24 ab | 0.23 ± 0.04 b | 6.9 ± 1.28 b | 3.2 ± 0.16 | 6.0 ± 0.22 | |
| pH | ns | ns | ns | ns | ns | |
| Nutrients | ns | ns | * | ns | ns | |
| pH × Nutrients | * | ** | * | ns | ns | |
| Rocket salad | SN-LpH | 1.0 ± 0.25 | 0.16 ± 0.03 | 6.6 ± 0.69 | 3.3 ± 0.28 | 6.2 ± 0.20 |
| SN-HpH | 1.1 ± 0.10 | 0.18 ± 0.03 | 8.0 ± 0.77 | 3.1 ± 0.47 | 6.5 ± 0.41 | |
| LN-LpH | 1.0 ± 0.28 | 0.18 ± 0.04 | 8.3 ± 2.48 | 3.0 ± 0.03 | 6.2 ± 0.54 | |
| LN-HpH | 1.1 ± 0.09 | 0.17 ± 0.02 | 7.4 ± 0.89 | 3.2 ± 0.16 | 6.1 ± 0.30 | |
| pH | ns | ns | ns | ns | ns | |
| Nutrients | ns | ns | ns | ns | ns | |
| pH × Nutrients | ns | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fimbres-Acedo, Y.E.; Traversari, S.; Cacini, S.; Costamagna, G.; Ginepro, M.; Massa, D. Testing the Effect of High pH and Low Nutrient Concentration on Four Leafy Vegetables in Hydroponics. Agronomy 2023, 13, 41. https://doi.org/10.3390/agronomy13010041
Fimbres-Acedo YE, Traversari S, Cacini S, Costamagna G, Ginepro M, Massa D. Testing the Effect of High pH and Low Nutrient Concentration on Four Leafy Vegetables in Hydroponics. Agronomy. 2023; 13(1):41. https://doi.org/10.3390/agronomy13010041
Chicago/Turabian StyleFimbres-Acedo, Yenitze Elizabeth, Silvia Traversari, Sonia Cacini, Giulia Costamagna, Marco Ginepro, and Daniele Massa. 2023. "Testing the Effect of High pH and Low Nutrient Concentration on Four Leafy Vegetables in Hydroponics" Agronomy 13, no. 1: 41. https://doi.org/10.3390/agronomy13010041
APA StyleFimbres-Acedo, Y. E., Traversari, S., Cacini, S., Costamagna, G., Ginepro, M., & Massa, D. (2023). Testing the Effect of High pH and Low Nutrient Concentration on Four Leafy Vegetables in Hydroponics. Agronomy, 13(1), 41. https://doi.org/10.3390/agronomy13010041









