Soil Nematodes as a Means of Conservation of Soil Predatory Mites for Biocontrol
Abstract
:1. Introduction
1.1. Trophic Interactions in Soil
1.2. Nematodes in Soil Food Webs
1.3. Mites in Soil Food Webs
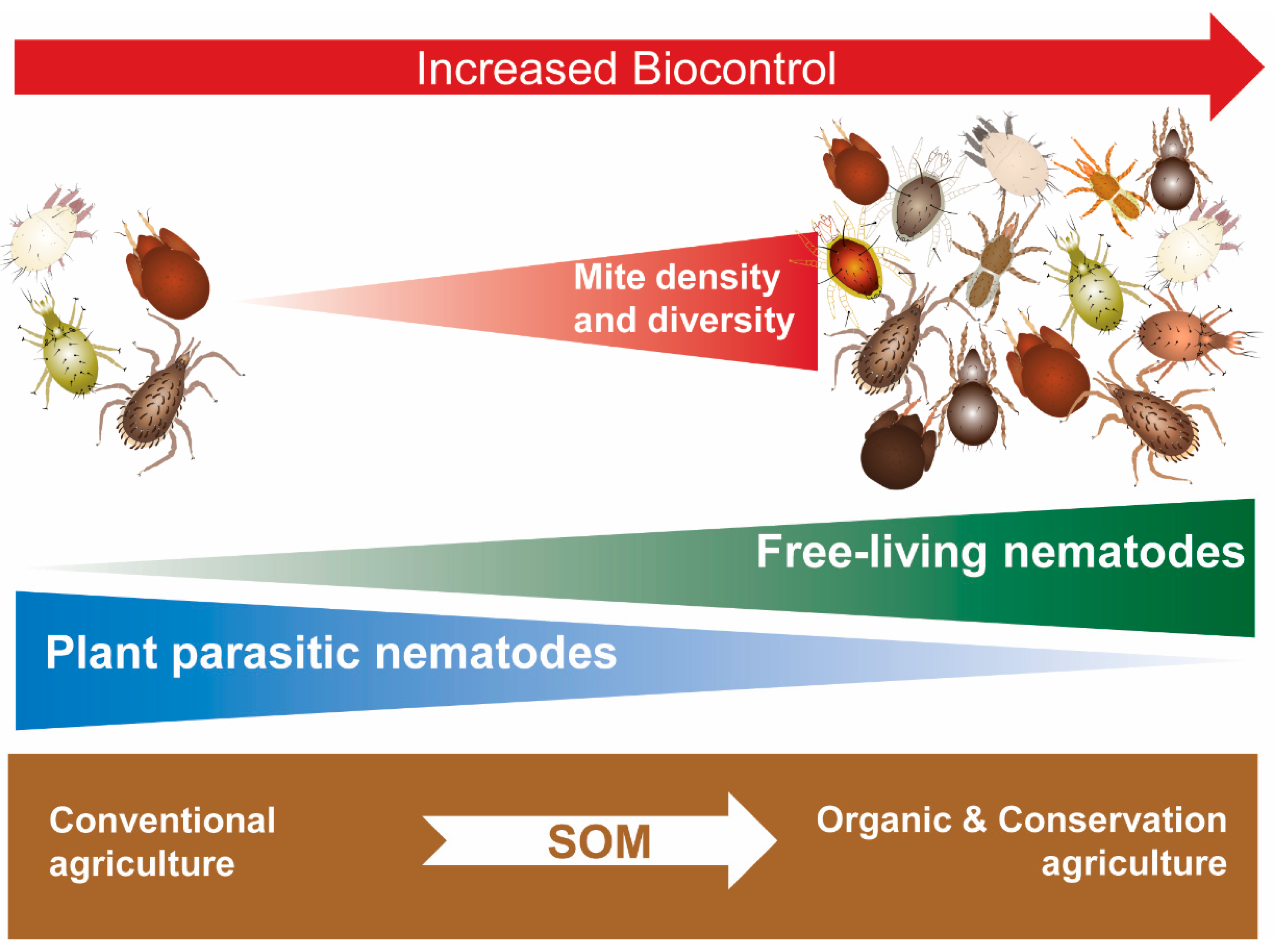
2. What Has Prevented the Broader Use of Soil Predatory Mites and Why Should This Change in the near Future?
3. Nematophagous Mites
3.1. Oribatida (Non-Astigmatina)
3.2. Astigmatina
3.3. Prostigmata
3.4. Endeostigmata
3.5. Mesostigmata
3.5.1. Gamasina
3.5.2. Uropodina
3.5.3. Other Mesostigmata Groups
4. Nematodes as a Food Source for Mites in Ecosystems
5. Variables Important for Trophic Interactions
5.1. Nematodes as an Important Source of Fatty Acids in Soil Food Webs
5.2. Size Matters?
6. Enhancing Conservation and Efficacy of Soil Acarine Biocontrol Agents (ABA)
7. Future Research Needed
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neher, D.A.; Barbercheck, M.E. Soil microarthropods and soil health: Intersection of decomposition and pest suppression in agroecosystems. Insects 2019, 10, 414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, A.; Sabelis, M.W. Alternative food and biological control by generalist predatory mites: The case of Amblyseius swirskii. Exp. Appl. Acarol. 2015, 65, 413–418. [Google Scholar] [CrossRef]
- Muñoz-Cárdenas, K.; Ersin, F.; Pijnakker, J.; van Houten, Y.; Hoogerbrugge, H.; Leman, A.; Pappas, M.L.; Duarte, M.V.A.A.; Messelink, G.J.; Sabelis, M.W.; et al. Supplying high-quality alternative prey in the litter increases control of an above-ground plant pest by a generalist predator. Biological. Control. 2017, 105, 19–26. [Google Scholar] [CrossRef]
- Beretta, G.M.; Deere, J.A.; Messelink, G.J.; Muñoz-Cárdenas, K.; Janssen, A. Review: Predatory soil mites as biocontrol agents of above and below-ground plant pests. Exp. Appl. Acarol. 2022, 87, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Nicol, J.M.; Turner, S.J.; Coyne, D.L.; den Nijs, L.; Hockland, S.; Maafi, Z.T. Current Nematode Threats to World Agriculture. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Jones, J., Gheysen, G., Fenoll, C., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 21–43. ISBN 978-94-007-0434-3. [Google Scholar]
- Walter, D.E.; Hunt, H.W.; Elliott, E.T. Guilds or functional groups? an analysis of predatory arthropods from a shortgrass steppe soil. Pedobiologia 1988, 31, 247–260. [Google Scholar]
- Walter, D.E. Nematophagy by soil arthropods from the shortgrass steppe, Chihuahuan desert and Rocky Mountains of the central United States. Agric. Ecosyst. Environ. 1988, 24, 307–316. [Google Scholar] [CrossRef]
- Castilho, R.C.; Venancio, R.; Narita, J.P.Z. Mesostigmata as biological control agents, with emphasis on Rhodacaroidea and Parasitoidea. In Prospects for Biological Control of Plant Feeding Mites and Other Harmful Organisms; Carrillo, D., de Moraes, G.J., Peña, J.E., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–31. ISBN 978-3-319-15042-0. [Google Scholar]
- Gessner, M.O.; Swan, C.M.; Dang, C.K.; McKie, B.G.; Bardgett, R.D.; Wall, D.H.; Hättenschwiler, S. Diversity meets decomposition. Trends Ecol. Evol. 2010, 25, 372–380. [Google Scholar] [CrossRef]
- de Vries, F.T.; Thébault, E.; Liiri, M.; Birkhofer, K.; Tsiafouli, M.A.; Bjørnlund, L.; Bracht Jørgensen, H.; Brady, M.V.; Christensen, S.; de Ruiter, P.C.; et al. Soil food web properties explain ecosystem services across European land use systems. Proc. Natl. Acad. Sci. USA 2013, 110, 14296–14301. [Google Scholar] [CrossRef] [Green Version]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Bonkowski, M.; Villenave, C.; Griffiths, B. Rhizosphere fauna: The functional and structural diversity of intimate interactions of soil fauna with plant roots. Plant Soil 2009, 321, 213–233. [Google Scholar] [CrossRef]
- Gebremikael, M.T.; Steel, H.; Buchan, D.; Bert, W.; De Neve, S. Nematodes enhance plant growth and nutrient uptake under C and N-rich conditions. Sci. Rep. 2016, 6, 32862. [Google Scholar] [CrossRef] [PubMed]
- Whalen, J.K.; Kernecker, M.L.; Thomas, B.W.; Sachdeva, V.; Ngosong, C. Soil food web controls on nitrogen mineralization are influenced by agricultural practices in humid temperate climates. CABI Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2013, 2013, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Whalen, J.K. Managing Soil Biota-Mediated Decomposition and Nutrient Mineralization in Sustainable Agroecosystems. Adv. Agric. 2014, 2014, 384604. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Roy, M.M.; Jaiswal, A.K.; Baitha, A. Soil Arthropods in Maintaining Soil Health: Thrust Areas for Sugarcane Production Systems. Sugar. Tech. 2018, 20, 376–391. [Google Scholar] [CrossRef]
- Samaddar, S.; Karp, D.S.; Schmidt, R.; Devarajan, N.; McGarvey, J.A.; Pires, A.F.A.; Scow, K. Role of soil in the regulation of human and plant pathogens: Soils’ contributions to people. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20200179. [Google Scholar] [CrossRef]
- Kergunteuil, A.; Bakhtiari, M.; Formenti, L.; Xiao, Z.; Defossez, E.; Rasmann, S. Biological Control beneath the Feet: A Review of Crop Protection against Insect Root Herbivores. Insects 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Wolkovich, E.M. Reticulated channels in soil food webs. Soil Biol. Biochem. 2016, 102, 18–21. [Google Scholar] [CrossRef]
- de Vries, F.T.; Wallenstein, M.D. Below-ground connections underlying above-ground food production: A framework for optimising ecological connections in the rhizosphere. J. Ecol. 2017, 105, 913–920. [Google Scholar] [CrossRef] [Green Version]
- Pausch, J.; Hünninghaus, M.; Kramer, S.; Scharroba, A.; Scheunemann, N.; Butenschoen, O.; Marhan, S.; Bonkowski, M.; Kandeler, E.; Scheu, S.; et al. Carbon budgets of top- and subsoil food webs in an arable system. Pedobiologia 2018, 69, 29–33. [Google Scholar] [CrossRef]
- Rueda-Ramírez, D.; Rios-Malaver, D.; Varela-Ramírez, A.; Moraes, G.J. de Biology and predation capacity of Parasitus bituberosus (Acari: Mesostigmata: Parasitidae) on Frankliniella occidentalis (Thysanoptera: Thripidae) and free-living nematodes as complementary diet. Pest Manag. Sci. 2019, 75, 1819–1830. [Google Scholar] [CrossRef]
- Azevedo, L.H.; Leite, L.G.; Chacon-Orozco, J.G.; Moreira, M.F.P.; Ferreira, M.P.; González-Cano, L.M.; Borges, V.; Rueda-Ramírez, D.; Moraes, G.J.; Palevsky, E. Free living nematodes as alternative prey for soil predatory mites: An interdisciplinary case study of conservation biological control. Biol. Control. 2019, 132, 128–134. [Google Scholar] [CrossRef]
- Azevedo, L.H.; Moreira, M.F.P.; Pereira, G.G.; Borges, V.; de Moraes, G.J.; Inomoto, M.M.; Vicente, M.H.; de Siqueira Pinto, M.; Peres, L.E.P.; Rueda-Ramírez, D.; et al. Combined releases of soil predatory mites and provisioning of free-living nematodes for the biological control of root-knot nematodes on ‘Micro Tom tomato. ’ Biol. Control. 2020, 146, 104280. [Google Scholar] [CrossRef]
- Blaxter, M. Nematodes: The Worm and Its Relatives. PLOS Biol. 2011, 9, e1001050. [Google Scholar] [CrossRef]
- Yeates, G.W. Nematodes as soil indicators: Functional and biodiversity aspects. Biol. Fertil. Soils 2003, 37, 199–210. [Google Scholar] [CrossRef]
- van den Hoogen, J.; Geisen, S.; Routh, D.; Ferris, H.; Traunspurger, W.; Wardle, D.A.; de Goede, R.G.M.; Adams, B.J.; Ahmad, W.; Andriuzzi, W.S.; et al. Soil nematode abundance and functional group composition at a global scale. Nature 2019, 572, 194–198. [Google Scholar] [CrossRef] [Green Version]
- Yeates, G.W.; Bongers, T.; De Goede, R.G.; Freckman, D.W.; Georgieva, S.S. Feeding habits in soil nematode families and genera-an outline for soil ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar]
- Ewald, M.; Glavatska, O.; Ruess, L. Effects of resource manipulation on nematode community structure and metabolic footprints in an arable soil across time and depth. Nematology 2020, 22, 1025–1043. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, T.; Wang, N.; Dou, Z.; Wang, K.; Zuo, Y. A review of soil nematodes as biological indicators for the assessment of soil health. Front. Agr. Sci. Eng. 2020, 7, 275–281. [Google Scholar] [CrossRef] [Green Version]
- Mesa-Valle, C.M.; Garrido-Cardenas, J.A.; Cebrian-Carmona, J.; Talavera, M.; Manzano-Agugliaro, F. Global Research on Plant Nematodes. Agronomy 2020, 10, 1148. [Google Scholar] [CrossRef]
- Ruess, L. Nematode soil faunal analysis of decomposition pathways in different ecosystems. Nematology 2003, 5, 179–181. [Google Scholar] [CrossRef]
- Pausch, J.; Hofmann, S.; Scharroba, A.; Kuzyakov, Y.; Ruess, L. Fluxes of root-derived carbon into the nematode micro-food web of an arable soil. Food Webs 2016, 9, 32–38. [Google Scholar] [CrossRef]
- Nieminen, J.K. Soil animals and ecosystem processes: How much does nutrient cycling explain? Pedobiologia 2008, 51, 367–373. [Google Scholar] [CrossRef]
- Neher, D.A. Ecology of plant and free-living nematodes in Natural and Agricultural Soil. Annu. Rev. Phytopathol. 2010, 48, 371–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Ferris, H.; Bongers, T.; de Goede, R.G.M. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Du Preez, G.; Daneel, M.; De Goede, R.; Du Toit, M.J.; Ferris, H.; Fourie, H.; Geisen, S.; Kakouli-Duarte, T.; Korthals, G.; Sánchez-Moreno, S.; et al. Nematode-based indices in soil ecology: Application, utility, and future directions. Soil Biol. Biochem. 2022, 169, 108640. [Google Scholar] [CrossRef]
- Ferris, H. Form and function: Metabolic footprints of nematodes in the soil food web. Eur. J. Soil Biol. 2010, 46, 97–104. [Google Scholar] [CrossRef]
- Mulder, C.; Maas, R. Unifying the functional diversity in natural and cultivated soils using the overall body-mass distribution of nematodes. BMC Ecol. 2017, 17, 36. [Google Scholar] [CrossRef] [Green Version]
- Melakeberhan, H.; Bonito, G.; Kravchenko, A.N. Application of Nematode Community Analyses-Based Models towards Identifying Sustainable Soil Health Management Outcomes: A Review of the Concepts. Soil Syst. 2021, 5, 32. [Google Scholar] [CrossRef]
- Ferris, H.; Tuomisto, H. Unearthing the role of biological diversity in soil health. Soil Biol. Biochem. 2015, 85, 101–109. [Google Scholar] [CrossRef]
- Lazarova, S.; Coyne, D.G.; Rodríguez, M.; Peteira, B.; Ciancio, A. Functional Diversity of Soil Nematodes in Relation to the Impact of Agriculture—A Review. Diversity 2021, 13, 64. [Google Scholar] [CrossRef]
- Morais, J.W.; Oliveira, F.L.; Braga, R.F.; Korasaki, V. Mesofauna. In O Ecossistema Solo: Componentes, Relações Ecológicas e Efeitos na Produção Vegetal; Moreira, F.M.S., Cares, J.E., Zanetti, R., Stürmer, S.L., Eds.; Universidade Federal de Lavras: Lavras, Brazil, 2013; pp. 183–200. [Google Scholar]
- Coleman, D.C.; Crossley, D.A., Jr.; Hendrix, P.F. Secondary production: Activities of heterotrophic organisms—the soil fauna. In Fundamentals of Soil Ecology; Coleman, D.C., Crossley, D.A., Jr., Hendrix, P.F., Eds.; Academic Press: Burlington, VT, USA, 2004; pp. 79–185. ISBN 978-0-12-179726-3. [Google Scholar]
- Coleman, D.C.; Callaham, M.A.; Crossley, D.A. Chapter 4 - Secondary Production: Activities of Heterotrophic Organisms—The Soil Fauna. In Fundamentals of Soil Ecology, 3rd ed.; Coleman, D.C., Callaham, M.A., Crossley, D.A., Eds.; Academic Press: Burlington, VT, USA, 2018; pp. 77–171. ISBN 978-0-12-805251-8. [Google Scholar]
- Walter, D.E.; Proctor, H.C. Mites: Ecology, Evolution & Behaviour. Life at a microscale, 2nd ed.; Springer: Dordrecht, The Netherlands, 2013; ISBN 978-94-007-7163-5. [Google Scholar]
- Nielsen, U.N. Soil Fauna Assemblages: Global to Local Scales, 1st ed.; Cambridge University Press: Cambridge, UK, 2019; ISBN 978-1-108-12351-8. [Google Scholar]
- Lavelle, P.; Spain, A. Soil Ecology; Dordrecht Kluwer Academic Publishers: Boston, MA, USA, 2001; ISBN 978-0-7923-7123-6. [Google Scholar]
- Sánchez-Moreno, S.; Nicola, N.L.; Ferris, H.; Zalom, F.G. Effects of agricultural management on nematode-mite assemblages: Soil food web indices as predictors of mite community composition. Appl. Soil Ecol. 2009, 41, 107–117. [Google Scholar] [CrossRef]
- Ruf, A.; Beck, L. The use of predatory soil mites in ecological soil classification and assessment concepts, with perspectives for oribatid mites. Ecotoxicol. Environ. Saf. 2005, 62, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Gergócs, V.; Hufnagel, L. Application of oribatid mites as indicators. Appl. Ecol. Environ. Res. 2009, 7, 79–98. [Google Scholar] [CrossRef]
- Ruf, A. A maturity index for predatory soil mites (Mesostigmata: Gamasina) as an indicator of environmental impacts of pollution on forest soils. Appl. Soil Ecol. 1998, 9, 447–452. [Google Scholar] [CrossRef]
- Ruf, A.; Beck, L.; Dreher, P.; Hund-Rinke, K.; Römbke, J.; Spelda, J. A biological classification concept for the assessment of soil quality: “Biological soil classification scheme” (BBSK). Agric. Ecosyst. Environ. 2003, 98, 263–271. [Google Scholar] [CrossRef]
- Bedano, J.C.; Ruf, A. Sensitivity of different taxonomic levels of soil Gamasina to land use and anthropogenic disturbances. Agric. For. Entomol. 2010, 12, 203–212. [Google Scholar] [CrossRef]
- Krantz, G.W.; Ainscough, B.D. Acarina: Mesostigmata (Gamasida). In Soil Biology Guide; Dindal, D.L., Ed.; A Wiley-Interscience Publication: New York, NY, USA, 1990; pp. 583–666. ISBN 0-471-04551-9. [Google Scholar]
- Koehler, H.H. Mesostigmata (Gamasina, Uropodina), efficient predators in agroecosystems. Agric. Ecosyst. Environ. 1997, 62, 105–117. [Google Scholar] [CrossRef]
- Köhler, H.H. Predatory mites (Gamasina, Mesostigmata). Agric. Ecosyst. Environ. 1999, 74, 395–410. [Google Scholar] [CrossRef]
- Walter, D.E.; Ikonen, E.K. Species, guilds and functional groups: Taxonomy and behaviour in nematophagous arthropods. J. Nematol. 1989, 21, 315–327. [Google Scholar]
- Norton, R.A. Acarina: Oribatida. In Soil Biology Guide; Dindal, D.L., Ed.; A Wiley-Interscience Publication: New York, NY, USA, 1990; pp. 779–803. ISBN 0-471-04551-9. [Google Scholar]
- Maraun, M.; Visser, S.; Scheu, S. Oribatid mites enhance the recovery of the microbial community after a strong disturbance. Appl. Soil Ecol. 1998, 9, 175–181. [Google Scholar] [CrossRef]
- Arroyo, J.; Iturrondobeitia, J.C. Differences in the diversity of oribatid mite communities in forests and agrosystems lands. Eur. J. Soil Biol. 2006, 42, 259–269. [Google Scholar] [CrossRef]
- Minor, M.A.; Cianciolo, J.M. Diversity of soil mites (Acari: Oribatida, Mesostigmata) along a gradient of land use types in New York. Appl. Soil Ecol. 2007, 35, 140–153. [Google Scholar] [CrossRef]
- Illig, J.; Schatz, H.; Scheu, S.; Maraun, M. Decomposition and colonization by micro-arthropods of two litter types in a tropical montane rain forest in southern Ecuador. J. Trop. Ecol. 2008, 24, 157–167. [Google Scholar] [CrossRef]
- Kardol, P.; Newton, J.S.; Bezemer, T.M.; Maraun, M.; van der Putten, W.H. Contrasting diversity patterns of soil mites and nematodes in secondary succession. Acta Oecologica 2009, 35, 603–609. [Google Scholar] [CrossRef]
- Nielsen, U.N.; Osler, G.H.R.; Campbell, C.D.; Burslem, D.F.R.P.; van der Wal, R. The influence of vegetation type, soil properties and precipitation on the composition of soil mite and microbial communities at the landscape scale. J. Biogeogr. 2010, 37, 1317–1328. [Google Scholar] [CrossRef]
- Norton, R.A.; Behan-Pelletier, V.M. Order Oribatida. In A Manual of Acarology; Krantz, G.W., Walter, D.E., Eds.; Texas Tech University Press: Lubbock, TX, USA, 2009; pp. 421–564. [Google Scholar]
- Gerson, U.; Smiley, R.L.; Ochoa, R. Mites (Acari) for pest control; Blackwell Science Ltd.: Oxford, UK, 2003; ISBN 0-632-05658-4. [Google Scholar]
- Lindquist, E.E.; Krantz, G.W.; Walter, D.E. Classification. In A Manual of Acarology; Krantz, G.W., Walter, D.E., Eds.; Texas Tech University Press: Lubbock, TX, USA, 2009; pp. 97–103. [Google Scholar]
- Hajihassani, A. Chemical Nematicides for Control of Plant-Parasitic Nematodes in Georgia Vegetable Crops 2018. Available online: https://extension.uga.edu/publications/detail.html?number=B1502#:~:text=The%20most%20common%20fumigants%20used,(dimethyl%20disulfide%20or%20DMDS) (accessed on 5 July 2022).
- Panth, M.; Hassler, S.C.; Baysal-Gurel, F. Methods for Management of Soilborne Diseases in Crop Production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Erktan, A.; Or, D.; Scheu, S. The physical structure of soil: Determinant and consequence of trophic interactions. Soil Biol. Biochem. 2020, 148, 107876. [Google Scholar] [CrossRef]
- Hijbeek, R.; Cormont, A.; Hazeu, G.; Bechini, L.; Zavattaro, L.; Janssen, B.; Werner, M.; Schlatter, N.; Guzmán, G.; Bijttebier, J.; et al. Do farmers perceive a deficiency of soil organic matter? A European and farm level analysis. Ecol. Indic. 2017, 83, 390–403. [Google Scholar] [CrossRef]
- Loomans, A.J.M. Every generalist biological control agent requires a special risk assessment. BioControl 2021, 66, 23–35. [Google Scholar] [CrossRef]
- Young, M.R.; Moraza, M.L.; Ueckermann, E.; Heylen, D.; Baardsen, L.F.; Lima-Barbero, J.F.; Gal, S.; Gavish-Regev, E.; Gottlieb, Y.; Roy, L.; et al. Linking morphological and molecular taxonomy for the identification of poultry house, soil, and nest dwelling mites in the Western Palearctic. Sci. Rep. 2019, 9, 5784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrascosa, M.; Sánchez-Moreno, S.; Alonso-Prados, J.L. Relationships between nematode diversity, plant biomass, nutrient cycling and soil suppressiveness in fumigated soils. Eur. J. Soil Biol. 2014, 62, 49–59. [Google Scholar] [CrossRef]
- Gunstone, T.; Cornelisse, T.; Klein, K.; Dubey, A.; Donley, N. Pesticides and Soil Invertebrates: A Hazard Assessment. Front. Environ. Sci. 2021, 9, 122. [Google Scholar] [CrossRef]
- European Food Safety Authority. Administrative guidance on submission of dossiers and assessment reports for the peer-review of pesticide active substances and on the maximum residue level (MRL) application procedure. EFSA J. 2021, 18, EN-6464. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Restrictions and Bans; FAO headquarters, United Nations: Rome, Italy, 2021. [Google Scholar]
- United States Environmental Protection Agency Reregistration and Other Review Programs Predating Pesticide Registration Review 2021. Available online: https://www.epa.gov/pesticide-reevaluation/reregistration-and-other-review-programs-predating-pesticide-registration (accessed on 2 August 2022).
- Mahmood, I.; Imadi, S.R.; Shazadi, K.; Gul, A.; Hakeem, K.R. Effects of Pesticides on Environment. In Plant, Soil and Microbes: Volume 1: Implications in Crop Science; Hakeem, K.R., Akhtar, M.S., Abdullah, S.N.A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 253–269. ISBN 978-3-319-27455-3. [Google Scholar]
- Schaub, S.; Huber, R.; Finger, R. Tracking societal concerns on pesticides – a Google Trends analysis. Environ. Res. Lett. 2020, 15, 084049. [Google Scholar] [CrossRef]
- European Commission; Directorate-General for Research and Innovation; Veerman, C.; Pinto Correia, T.; Bastioli, C.; Biro, B.; Bouma, J.; Cienciala, E.; Emmett, B.; Frison, E.; et al. Caring for Soil Is Caring for Life: Ensure 75% of Soils are Healthy by 2030 for Healthy Food, People, Nature and Climate: Interim Report of the Mission Board for Soil Health and Food; Publications Office: Brussels, Belgium, 2020. [Google Scholar]
- Geisen, S.; Wall, D.H.; van der Putten, W.H. Challenges and Opportunities for Soil Biodiversity in the Anthropocene. Curr. Biol. 2019, 29, R1036–R1044. [Google Scholar] [CrossRef]
- McSorley, R.; Seal, D.R.; Klassen, W. Non-target effects of sunn hemp and marigold cover crops on the soil invertebrate community. Nematropica 2009, 39, 11. [Google Scholar]
- Wang, K.-H.; Radovich, T.; Pant, A.; Cheng, Z. Integration of cover crops and vermicompost tea for soil and plant health management in a short-term vegetable cropping system. Appl. Soil Ecol. 2014, 82, 26–37. [Google Scholar] [CrossRef]
- Leroy, B.L.M.M.; Bommele, L.; Reheul, D.; Moens, M.; De Neve, S. The application of vegetable, fruit and garden waste (VFG) compost in addition to cattle slurry in a silage maize monoculture: Effects on soil fauna and yield. Eur. J. Soil Biol. 2007, 43, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Han, X.; Hu, C.; Chen, J.; Zhang, D.; Steinberger, Y. Changes in the abundance and structure of a soil mite (Acari) community under long-term organic and chemical fertilizer treatments. Appl. Soil Ecol. 2011, 49, 131–138. [Google Scholar] [CrossRef]
- Rueda-Ramírez, D.; Santos, J.C.; Young, M.R.; Mowery, J.; Bauchan, G.; Ochoa, R.; Palevsky, E. In memory of Gary Bauchan: Utilizing an integrated taxonomy approach for the description of a new species of Gamasellodes (Mesostigmata: Ascidae). Syst. Appl. Acarol. 2022, 27, 165–180. [Google Scholar] [CrossRef]
- Rueda-Ramírez, D.; Carta, L.; Mowery, J.; Bauchan, G.; Ochoa, R.; Young, M.; Santos, J.C.; Palevsky, E. In memory of Gary Bauchan: Integrated taxonomy of soil mites in farming systems. Syst. Appl. Acarol. 2022, 27, 181–208. [Google Scholar] [CrossRef]
- van Lenteren, J.C. The state of commercial augmentative biological control: Plenty of natural enemies, but a frustrating lack of uptake. BioControl 2011, 57, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Knapp, M.; van Houten, Y.; van Baal, E.; Groot, T. Use of predatory mites in commercial biocontrol: Current status and future prospects. Acarologia 2018, 58, 7282. [Google Scholar] [CrossRef]
- de Moraes, G.J.; Venancio, R.; dos Santos, V.L.V.; Paschoal, A.D. Potential of Ascidae, Blattisociidae and Melicharidae (Acari: Mesostigmata) as biological control agents of pest organisms. In Prospects for biological control of plant feeding mites and other harmful organisms; Carrillo, D., de Moraes, G.J., Peña, J.E., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 33–75. ISBN 978-3-319-15042-0. [Google Scholar]
- Walter, D.E.; Kaplan, D.T. A guild of thelytokous mites associated with citrus roots in Florida. Environ. Entomol. 1990, 19, 1338–1343. [Google Scholar]
- Rockett, C.L.; Woodring, J.P. Biological investigations on a new species of Ceratozetes and of Pergalumna (Acarina: Cryptostigmata). Acarologia 1966, VIII, 511–520. [Google Scholar]
- Rockett, C.L.; Woodring, J.P. Oribatid mites as predators of soil nematodes. Ann. Entomol. Soc. Am. 1966, 59, 669–671. [Google Scholar] [CrossRef]
- Walter, D.E. Belowground arthropods of semiarid grasslands. In Integrated pest management on rangeland: A shortgrass prairie perspective; Capinera, J.L., Ed.; Westview Press: Boulder, CO, USA, 1987; pp. 271–290. [Google Scholar]
- Walter, D.E. Trophic Behavior of “Mycophagous” Microarthropods. Ecology 1987, 68, 226–229. [Google Scholar] [CrossRef]
- Elmoghazy, M.; El-Kawas, H.; Salman, M. Biological Aspects of Scheloribates laevigatus (Acari: Oribatida) when fed on mixture of the free-living nematode, Eudiplogaster phlagemcaudatus and potato in the Laboratory. Acarines J. Egypt. Soc. Acarol. 2012, 6, 21–24. [Google Scholar] [CrossRef]
- Heidemann, K.; Scheu, S.; Ruess, L.; Maraun, M. Molecular detection of nematode predation and scavenging in oribatid mites: Laboratory and field experiments. Soil Biol. Biochem. 2011, 43, 2229–2236. [Google Scholar] [CrossRef]
- Heidemann, K.; Ruess, L.; Scheu, S.; Maraun, M. Nematode consumption by mite communities varies in different forest microhabitats as indicated by molecular gut content analysis. Exp. Appl. Acarol. 2014, 64, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Heidemann, K.; Hennies, A.; Schakowske, J.; Blumenberg, L.; Ruess, L.; Scheu, S.; Maraun, M. Free-living nematodes as prey for higher trophic levels of forest soil food webs. Oikos 2014, 123, 1199–1211. [Google Scholar] [CrossRef]
- Karg, W. Untersuchungen über die Veränderungen und Wechselbeziehungen der Microarthropoden in Kartoffelnematoden verseuchten Flächen. Nachr. Des Dtsch. Pflanzenschutzd. 1962, 16, 187–195. [Google Scholar]
- Sturhan, D.; Hampel, G. Pflanzenparasitische Nematoden als Beute der Wurzelmilbe Rhizoglyphus echinopus (Acarina Tyroglyphidae). Anz. Schadlingskde. Pflanzenschutz Umweltschutz 1977, 50, 115–118. [Google Scholar] [CrossRef]
- Stirling, G.R. Surrounding the swollen roots of sweetpotato with a decomposing band of an organic amendment enhances nematode-suppressive services and reduces damage caused by root-knot nematode. Australas. Plant Pathol. 2021, 50, 151–168. [Google Scholar] [CrossRef]
- Palevsky, E.; Konopická, J.; Rueda-Ramírez, D.; Zemek, R. A Review of prospective biocontrol agents and sustainable soil practices for bulb mite (Acari: Acaridae) management. Agronomy 2022, 12, 1491. [Google Scholar] [CrossRef]
- Walter, D.E.; Hudgens, R.A.; Freckman, D.W. Consumption of nematodes by fungivorous mites, Tyrophagus spp. (Acarina: Astigmata: Acaridae). Oecologia 1986, 70, 357–361. [Google Scholar] [CrossRef]
- Bilgrami, A.L. Predatory behaviour of a nematode feeding mite Tyrophagus putrescentiae (Sarcoptiformes: Acaridae). Fundam. Appl. Nematol. 1994, 17, 293–297. [Google Scholar]
- Walia, K.K.; Mathur, S. Predatory behavior of two nematophagous mites, Tyrophagus putrescentiae and Hypoaspis calcuttaensis, on root-knot nematode, Meloidogyne javanica. Nematol. Mediterr. 1995, 23, 255–261. [Google Scholar]
- Abou El-Atta, D.A.E.-M.; Osman, M.A. Development and reproductive potential of Tyrophagus putrescentiae (Acari: Acaridae) on plant-parasitic nematodes and artificial diets. Exp. Appl. Acarol. 2016, 68, 477–483. [Google Scholar] [CrossRef]
- Abou El-Atta, D.A.E.-M.; Habashy, M.G.; Mesbah, A.E.; Tawfik, A.A. Life History of Caloglyphus manure, Sancassania (Caloglyphus) berlesei and Tyroghagus putrescentiae (Acari:Acaridae) Feeding on Root-Knot Nematodes, Meloidogyne incognita. J. Plant Prot. Pathol. 2017, 8, 69–72. [Google Scholar] [CrossRef]
- Epsky, N.D.; Walter, D.E.; Capinera, J.L. Potential Role of Nematophagous Microarthropods as Biotic Mortality Factors of Entomogenous Nematodes (Rhabditida: Steinernematidae, Heterorhabditidae). J. Econ. Entomol. 1988, 81, 821–825. [Google Scholar] [CrossRef]
- Karagoz, M.; Gulcu, B.; Cakmak, I.; Kaya, H.K.; Hazir, S. Predation of entomopathogenic nematodes by Sancassania sp. (Acari: Acaridae). Exp. Appl. Acarol. 2007, 43, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Eraky, S.A.; Osman, M.A. Some biological aspects and life table parameters of Caloglyphus manuri Eraky & Osman (Acaridida: Acaridae) fed on different kinds of food. ACARINES J. Egypt. Soc. Acarol. 2008, 2, 45–48. [Google Scholar]
- Muraoka, M.; Ishibashi, N. Nematode-feeding mites and their feeding behavior. Appl. Entomol. Zool. 1976, 11, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Walter, D.E.; Kaplan, D.T. Observations on Coleoscirus simplex (Acarina: Prostigmata), a predatory mite that colonizes greenhouse cultures of rootknot nematode (Meloidogyne spp.), and a review of feeding behavior in the Cunaxidae. Exp. Appl. Acarol. 1991, 12, 47–59. [Google Scholar] [CrossRef]
- Walter, D.E.; Kaplan, D.T.; Davis, E.L. Colonization of greenhouse nematode cultures by nematophagous mites and fungi. Suppl. J. Nematol. 1993, 25, 789–794. [Google Scholar]
- Khalil, A. Biological aspects of the cunaxid mite, Pulaeus pseudominutus when fed on different diets at different temperatures. Egypt. Acad. J. Biol. Sciences. A Entomol. 2016, 9, 49–55. [Google Scholar] [CrossRef]
- Mostafa, M.; Abdel-Rahman, A.; Younis, A.; Yassin, E.; Saber, R. Life History of the Predaceous Mite Cunaxa capreolus (Berlese) (Acari: Prostigmata: Cunaxidae) When Fed on Different Diets at Different Temperatures. Egypt. Acad. J. Biol. Sciences. A Entomol. 2016, 9, 1–6. [Google Scholar] [CrossRef]
- Al-Azzazy, M.M.; Al-Rehiayani, S.M. The soil mite Cunaxa capreolus (Acari: Cunaxidae) as a predator of the root-knot nematode, Meloidogyne incognita and the citrus Nematode, Tylenchulus semipenetrans: Implications for biological control. Acarologia 2022, 62, 174–185. [Google Scholar] [CrossRef]
- Walter, D.E. Predation and mycophagy by endeostigmatid mites (Acariformes: Prostigmata). Exp. Appl. Acarol. 1988, 4, 159–166. [Google Scholar] [CrossRef]
- Amin, A.W.; Mowafe, M.H.; Fatma, S.A. Effect of predaceous mesostigmatid mites in the control of Meloidogyne javanica root-knot nematode on kidney bean. Pak. J. Nematol. 1999, 17, 91–96. [Google Scholar]
- El-Banhawy, E.M.; Osman, H.A.; El-Sawaf, B.M.; Afia, S.I. Interactions of soil predacious mites and citrus nematodes (parasitic and saprophytic), in citrus orchard under different regime of fertilizers. Effect on the population densities and citrus yield. Anz. Schadlingskde. Pflanzenschutz Umweltschutz 1997, 70, 20–23. [Google Scholar] [CrossRef]
- Xu, C.-L.; Chen, Y.-L.; Xu, X.-N.; Wang, D.-W.; Xie, H.; Wang, E.-D.; Li, D.-S.; Zhang, B.-X.; Qin, H.-G. Evaluation of Blattisocius dolichus (Acari: Blattisociidae) for biocontrol of root-knot nematode, Meloidogyne incognita (Tylenchida: Heteroderidae). BioControl 2014, 59, 617–624. [Google Scholar] [CrossRef]
- de Azevedo, L.H.; Emberson, R.M.; de Esteca, F.C.N.; de Moraes, G.J. Macrochelid mites (Mesostigmata: Macrochelidae) as biological control agents. In Prospects for Biological Control of Plant Feeding Mites and Other Harmful Organisms; Carrillo, D., de Moraes, G.J., Peña, J.E., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 103–132. ISBN 978-3-319-15042-0. [Google Scholar]
- McMurtry, J.A.; Sourassou, N.F.; Demite, P.R. The Phytoseiidae (Acari: Mesostigmata) as Biological Control Agents. In Prospects for Biological Control of Plant Feeding Mites and Other Harmful Organisms; Carrillo, D., de Moraes, G.J., Peña, J.E., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 133–149. ISBN 978-3-319-15042-0. [Google Scholar]
- Moreira, G.F.; Moraes, G.J. The potential of free-living laelapid mites (Mesostigmata: Laelapidae) as biological control agents. In Prospects for Biological Control of Plant Feeding Mites and Other Harmful Organisms; Carrillo, D., de Moraes, G.J., Peña, J.E., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 77–102. ISBN 978-3-319-15042-0. [Google Scholar]
- Saber, S.A.; EI-Laithy, A.Y.M.; Korayem, A.M. Biology of the predaceous mite Protogamasellus denticus Nasr (Mesostigmata: Ascidae) feeding on different nematode diets. Int. J. Nematol. 2007, 17, 17–20. [Google Scholar]
- Manwaring, M.; Nahrung, H.F.; Wallace, H. Attack rate and prey preference of Lasioseius subterraneous and Protogamasellus mica on four nematode species. Exp. Appl. Acarol. 2020, 80, 29–41. [Google Scholar] [CrossRef]
- Walter, D.E.; Hunt, H.W.; Elliott, E.T. The influence of prey type on the development and reproduction of some predatory soil mites. Pedobiologia 1987, 30, 419–424. [Google Scholar]
- Binns, E.S. Notes on the biology of Arctoseius cetratus (Sellnick) (Mesostigmata: Ascidae). Acarologia 1974, XVI, 577–582. [Google Scholar]
- Afifi, A.M.; Hassan, M.F.; Nawar, M.S. Notes on the biology feeding habits of Protogamasellus minutus Hafez, El-Badry & Nasr (Acari: Gamasida: Ascidae). Bull. De La Société Entomol. D’égypte 1986, 66, 251–259. [Google Scholar]
- Nawar, M.S.; Nasr, A.K. Biology of the ascid mite Protogamasellus primitivus similis Genis Loots and Ryke with description of immature stages (Acari: Mesostigmata: Ascidae). Bull.-Zool. Soc. Egypt 1989, 68, 85–94. [Google Scholar]
- Walter, D.E.; Lindquist, E.E. The distributions of parthenogenetic ascid mites (Acari: Parasitiformes) do not support the biotic uncertainty hypothesis. Exp. Appl. Acarol. 1995, 19, 423–442. [Google Scholar]
- Stirling, G.R.; Stirling, A.M.; Walter, D.E. The Mesostigmatid mite Protogamasellus mica, an effective predator of free-living and plant-parasitic nematodes. J. Nematol. 2017, 49, 327–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Moraes, G.J.; Moreira, G.F.; Freire, R.A.P.; Beaulieu, F.; Klompen, H.; Halliday, B. Catalogue of the free-living and arthropod-associated Laelapidae Canestrini (Acari: Mesostigmata), with revised generic concepts and a key to genera. Zootaxa 2022, 5184, 001–509. [Google Scholar] [CrossRef]
- Bilgrami, A.L. Evaluation of the predation abilities of the mite Hypoaspis calcuttaensis, predaceous on plant and soil nematodes. Fundam. Appl. Nematol. 1997, 20, 96–98. [Google Scholar]
- Sharma, R.D. Studies of the parasitic nematode Tylenchorhynchus dubius. Meded. Van De Fac. Landbouwwet. 1971, 71, 1–154. [Google Scholar]
- Van de Bund, C.F. Some observations on predatory action of mites on nematodes. Zesz. Probl. Postep. Nauk. Rol. 1972, 129, 103–110. [Google Scholar]
- Salehi, A.; Ostovan, H.; Modarresi, M. Evaluation of the efficiency of Gaeolaelaps aculeifer in control of plant parasitic nematode Tylenchulus semipenetrans under greenhouse conditions. J. Entomol. Nematol. 2014, 6, 150–153. [Google Scholar]
- Yang, S.-H.; Wang, D.; Chen, C.; Xu, C.-L.; Xie, H. Evaluation of Stratiolaelaps scimitus (Acari: Laelapidae) for controlling the root-knot nematode, Meloidogyne incognita (Tylenchida: Heteroderidae). Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Afifi, A.M.; Van Der Geest, L.P.S. Notes on the development and biology of the predaceous soil mite Cosmolaelaps claviger (Berlese 1883) (Gamasida: Laelapidae). In Acarology VI; Griffiths, D.A., Bowman, C.E., Eds.; Ellis Horwood Limited Publishers: West Sussex, UK, 1984; pp. 585–590. [Google Scholar]
- Moreira, G.F.; de Morais, M.R.; Busoli, A.C.; Moraes, G.J. de Life cycle of Cosmolaelaps jaboticabalensis (Acari: Mesostigmata: Laelapidae) on Frankliniella occidentalis (Thysanoptera: Thripidae) and two factitious food sources. Exp. Appl. Acarol. 2015, 65, 219–226. [Google Scholar] [CrossRef]
- Al Rehiayani, S.M.; Fouly, A.H. Cosmolaelaps simplex (Berlese), a Polyphagous Predatory Mite Feeding on Root-knot Nematode Meloidogyne javanica and Citrus Nematode Tylenchulus semipenetrans. Pak. J. Biol. Sci. 2005, 8, 168–174. [Google Scholar] [CrossRef]
- Karg, W. Über die Beziehungen von edaphischen Raubmilben zur Arthropoden- und Nematoden fauna des Bodens. In Tagungsberichte Nr. 45. Bericht über die 9. Wanderversammlung Deutscher Entomologen; Deutche Akademie der Landwirtschaftswissenschaften zu Berlin: Berlin, Germany, 1962; pp. 311–327. [Google Scholar]
- Murphy, P.W.; Sardar, M.A. Resource allocation and utilization contrasts in Hypoaspis aculeifer (Can.) and Alliphis halleri (G. & R. Can.) (Mesostigmata) with emphasis on food source. In The Acari. Reproduction, development and life-history strategies; Schuster, R., Murphy, P.W., Eds.; Chapman & Hall Publications: New York, NY, USA, 1991; pp. 301–311. [Google Scholar]
- Heckmann, L.-H.; Ruf, A.; Nienstedt, K.M.; Krogh, P.H. Reproductive performance of the generalist predator Hypoaspis aculeifer (Acari: Gamasida) when foraging on different invertebrate prey. Appl. Soil Ecol. 2007, 36, 130–135. [Google Scholar] [CrossRef]
- Walter, D.E.; Oliver, J.H. Geolaelaps oreithyiae, n. sp. (Acari, Laelapidae), a thelytokous predator of arthropods and nematodes, and a discussion of clonal reproduction in the Mesostigmata. Acarologia 1989, 30, 293–303. [Google Scholar]
- Berndt, O.; Meyhöfer, R.; Poehling, H.M. Propensity towards cannibalism among Hypoaspis aculeifer and H. miles, two soil-dwelling predatory mite species. Exp. Appl. Acarol. 2003, 31, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Filipponi, A.; Dojmi di Delupis, G. Sul regime dietetico di alcuni macrochelidi (Acari: Mesostigmata), associati in natura a muscidi di interesse sanitario. Riv. Di Parassitol. 1963, XXIV, 277–287. [Google Scholar]
- Filipponi, A.; Petrelli, M.G. Autoecologia e capacita moltiplicativa di Macrocheles muscadomesticae (Scopoli) (Acari: Mesostigmata). Riv. Di Parassitol. 1967, XXVIII, 129–156. [Google Scholar]
- Karg, W. Ökologische Untersuchungen an Milben aus Komposterden im Freiland und unter Glas besonders im Hinblick auf die Uroobovella marginata C.L. Koch. Arch. Für Pflanzenschutz 1968, 4, 93–122. [Google Scholar] [CrossRef]
- Afifi, A.M. Laboratory studies on the biology and feeding habits of three Macrochelid mites (Acari: Gamasida: Macrochelidae). Bull. De La Société Entomol. D’égypte 1989, 68, 169–174. [Google Scholar]
- Ito, Y. The effects of nematode feeding on the predatory efficiency for house fly eggs and reproduction rate of Macrocheles muscaedomesticae (Acarina: Mesostigmata). Jpn. Soc. Med. Entomol. Zool. 1973, 23, 209–213. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, L.H.; Ferreira, M.P.; de Castilho, R.C.; Cançado, P.H.D.; de Moraes, G.J. Potential of Macrocheles species (Acari: Mesostigmata: Macrochelidae) as control agents of harmful flies (Diptera) and biology of Macrocheles embersoni Azevedo, Castilho and Berto on Stomoxys calcitrans (L.) and Musca domestica. Biol. Control. 2018, 123, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, J.G.; Wade, C.F.; Wells, C.N. Nematodes as a natural food for Macrocheles muscaedomesticae (Acarina: Macrochelidae), a predator of the house fly egg. Ann. Entomol. Soc. Am. 1962, 55, 507–511. [Google Scholar] [CrossRef]
- Axtell, R.C. Macrochelidae (Acarina: Mesostigmata) as Biological Control Agents for Synanthropic Flies. In Proceedings of the 2nd International Congress of Acarology, Sutton Bonington, UK, 19–25 July 1969; pp. 401–416. [Google Scholar]
- Ito, Y. Predatory activity of mesostigmatid mites (Acarina: Mesostigmata) for house fly eggs and larvae under feeding of nematodes. Med. Entomol. Zool. 1977, 28, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.C. Rhodacaridae (Acari: Mesostigmata) from near Adelaide, Australia iii. Behaviour and development. Acarologia 1974, XVI, 21–44. [Google Scholar]
- Beaulieu, F.; Walter, D.E. Predation in suspended and forest floor soils: Observations on Australian mesostigmatic mites. Acarologia 2007, XLVII, 43–54. [Google Scholar]
- Mowafi, M.H. Effect of food type on development and reproduction of mite species Lasioseius africanus Nasr (Acari: Ascidae). Egypt. J. Biol. Pest Control. 2005, 15, 93–95. [Google Scholar]
- Britto, E.P.J.; Gago, E.; de Moraes, G.J. How promising is Lasioseius floridensis as a control agent of Polyphagotarsonemus latus? Exp. Appl. Acarol. 2012, 56, 221–231. [Google Scholar] [CrossRef]
- Abou-Awad, B.A.; Korayem, A.M.; Hassan, M.F.; Abou-Elela, M.A. Life history of the predatory mite Lasioseius athiasae (Acari, Ascidae) on various kinds of food substances: A polypeptide analysis of prey consideration. J. Appl. Entomol. 2001, 125, 125–130. [Google Scholar] [CrossRef]
- Tawfik, A.A.; Mesbah, A.E.; Abou El-Atta, D.A.; Abou Zaid, W.R. Effect of food type on life tables and feeding behavior of the ascid mite, Lasioseius athiasae (Acari: Ascidae). J. Plant Prot. Pathol. 2017, 8, 65–68. [Google Scholar] [CrossRef]
- Chen, Y.L.; Xu, C.L.; Xu, X.N.; Xie, H.; Zhang, B.X.; Qin, H.G.; Zhou, W.Q.; Li, D.S. Evaluation of predation abilities of Blattisocius dolichus (Acari: Blattisociidae) on a plant-parasitic nematode, Radopholus similis (Tylenchida: Pratylenchidae). Exp. Appl. Acarol. 2013, 60, 289–298. [Google Scholar] [CrossRef]
- Szafranek, P.; Lewandowski, M.; Kozak, M. Prey preference and life tables of the predatory mite Parasitus bituberosus (Acari: Parasitidae) when offered various prey combinations. Exp. Appl. Acarol. 2013, 61, 53–67. [Google Scholar] [CrossRef]
- Afifi, A.M.; Nasr, A.K. Biology and feeding habits of the predatory mite, Porrhostaspis lunulata Muller (Acari-Gamsida-Parasitidae). Bull. Fac. Agric. Univ. Cairo 1984, 35, 1741–1746. [Google Scholar]
- Rossini, L.A.C.J.; Prado, T.J.; Ferreira, R.J.; Soares, P.L.M.; de Moraes, G.J.; Castilho, R.C. Suitability of the soybean cyst nematode as prey to Protogamasellopsis zaheri (Acari: Mesostigmata: Rhodacaridae). Biol. Control. 2022, 170, 104905. [Google Scholar] [CrossRef]
- Beaulieu, F.; Dowling, A.P.G.; Klompen, H.; de Moraes, G.J.; Walter, D.E. Superorder Parasitiformes Reuter, 1909. In: Zhang, Z.-Q. (Ed.) Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa 2011, 3148, 123–128. [Google Scholar] [CrossRef]
- Sardar, M.A.; Murphy, P.W. Feeding tests of grassland soil-inhabiting gamasine predators. Acarologia 1987, XXVIII, 117–121. [Google Scholar]
- Royce, L.A.; Krantz, G.W. A new rearing method for nematophagous mites. In Modern Acarology; Dusbábek, F., Bukva, V., Eds.; Academia and SPB Academic Publishing BV: Prague, Czech Republic; The Hague, The Netherlands, 1991; Volume 2, pp. 619–622. [Google Scholar]
- Karg, W. Ökologische Untersuchungen von edaphischen Gamasiden (Acarina, Parasitiformes). Pedobiologia 1961, 1, 77–98. [Google Scholar] [CrossRef]
- Habersaat, U. Die Bedeutung der Bodenraubmilben als Prädatoren von landwirtschaftlichen Schädlingen am Beispiel von Hypoaspis angusta KARG, 1965 (Acari: Gamasina). Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 1989; p. 87S. [Google Scholar]
- Martikainen, E.; Huhta, V. Interactions between nematodes and predatory mites in raw humus soil: A microcosm experiment. Rev. D’écologie Et De Biol. Du Sol 1990, 27, 13–20. [Google Scholar]
- Ito, Y. Predation by manure-inhabiting mesostigmatids (Acarina: Mesostigmata) on some free-living nematodes. Appl. Entomol. Zool. 1971, 6, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Willis, R.R.; Axtell, R.C. Mite predators of the house fly: A comparison of Fuscuropoda vegetans and Macrocheles muscaedomesticae. J. Econ. Entomol. 1968, 61, 1669–1674. [Google Scholar] [CrossRef]
- Jalil, M.; Rodriguez, J.G. Biology of and Odor Perception by Fuscuropoda vegetans (Acarina: Uropodidae), a Predator of the House Fly. Ann. Entomol. Soc. Am. 1970, 63, 935–938. [Google Scholar] [CrossRef]
- Yeates, G.W. Nematodes in Ecological Webs. In Encyclopedia of Life Science Chichester; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; pp. 1–10. ISBN 978-0-470-01590-2. [Google Scholar]
- Lee, Q.; Widden, P. Folsomia candida, a “fungivorous” collembolan, feeds preferentially on nematodes rather than soil fungi. Soil Biol. Biochem. 1996, 28, 689–690. [Google Scholar] [CrossRef]
- Zaitsev, A.S.; Birkhofer, K.; Ekschmitt, K.; Wolters, V. Belowground Tritrophic Food Chain Modulates Soil Respiration in Grasslands. Pedosphere 2018, 28, 114–123. [Google Scholar] [CrossRef]
- Moore, J.C.; McCann, K.; de Ruiter, P.C. Modeling trophic pathways, nutrient cycling, and dynamic stability in soils. Pedobiologia 2005, 49, 499–510. [Google Scholar] [CrossRef]
- Perdomo, G.; Evans, A.; Maraun, M.; Sunnucks, P.; Thompson, R. Mouthpart morphology and trophic position of microarthropods from soils and mosses are strongly correlated. Soil Biol. Biochem. 2012, 53, 56–63. [Google Scholar] [CrossRef]
- Bowman, C.E. Feeding design in free-living mesostigmatid chelicerae (Acari: Anactinotrichida). Exp. Appl. Acarol. 2021, 84, 1–119. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.; Migge, S.; Norton, R.A.; Scheu, S.; Langel, R.; Reineking, A.; Maraun, M. Trophic niche differentiation in soil microarthropods (Oribatida, Acari): Evidence from stable isotope ratios (15N/14N). Soil Biol. Biochem. 2004, 36, 1769–1774. [Google Scholar] [CrossRef]
- Díaz-Aguilar, I.; Quideau, S.A. Trophic ecology of mesostigmatan and oribatid mites in harvested and control coniferous and deciduous stands of the boreal mixedwood forest determined using 15N stable isotopes. Soil Biol. Biochem. 2013, 67, 147–154. [Google Scholar] [CrossRef]
- Klarner, B.; Maraun, M.; Scheu, S. Trophic diversity and niche partitioning in a species rich predator guild – Natural variations in stable isotope ratios (13C/12C, 15N/14N) of mesostigmatid mites (Acari, Mesostigmata) from Central European beech forests. Soil Biol. Biochem. 2013, 57, 327–333. [Google Scholar] [CrossRef]
- Traugott, M.; Kamenova, S.; Ruess, L.; Seeber, J.; Plantegenest, M. Chapter Three - Empirically Characterising Trophic Networks: What Emerging DNA-Based Methods, Stable Isotope and Fatty Acid Analyses Can Offer. In Advances in Ecological Research; Woodward, G., Bohan, D.A., Eds.; Academic Press: Burlington, VT, USA, 2013; Volume 49, pp. 177–224. ISBN 0065-2504. [Google Scholar]
- Mcsorley, R.; Wang, K.-H.; Kokalis-Burelle, N.; Church, G. Effects of soil type and steam on nematode biological control potential of the rhizosphere community. Nematropica 2006, 36, 197–214. [Google Scholar]
- Laakso, J.; Setala, H. Sensitivity of Primary Production to Changes in the Architecture of Belowground Food Webs. Oikos 1999, 87, 57. [Google Scholar] [CrossRef]
- Kühn, J.; Ruess, L. Effects of resource quality on the fitness of collembola fed single and mixed diets from the green and brown food chain. Soil Biol. Biochem. 2021, 154, 108156. [Google Scholar] [CrossRef]
- Łukasiewicz, A. Juvenile diet quality and intensity of sexual conflict in the mite Sancassania berlesei. BMC Evol. Biol. 2020, 20, 35. [Google Scholar] [CrossRef]
- Brückner, A.; Schuster, R.; Wehner, K.; Heethoff, M. Nutritional quality modulates trait variability. Front. Zool. 2018, 15, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brückner, A.; Schuster, R.; Wehner, K.; Heethoff, M. Effects of nutritional quality on the reproductive biology of Archegozetes longisetosus (Actinotrichida, Oribatida, Trhypochthoniidae). Soil Org. 2018, 90, 1–12. [Google Scholar]
- Marques, R.V.; Sarmento, R.A.; Lemos, F.; Pedro-Neto, M.; Sabelis, M.W.; Venzon, M.; Pallini, A.; Janssen, A. Active prey mixing as an explanation for polyphagy in predatory arthropods: Synergistic dietary effects on egg production despite a behavioural cost. Funct. Ecol. 2015, 29, 1317–1324. [Google Scholar] [CrossRef]
- Hubert, J.; Nesvorná, M.; Palevsky, E.; Smrz, J. Diseases of Prey Mites Used for Mass Rearing Predatory Mites. In Proceedings of the Acta Horticulturae; International Society for Horticultural Science (ISHS), Leuven, Belgium, 10 July 2014; pp. 177–185. [Google Scholar]
- Delisle, J.F.; Brodeur, J.; Shipp, L. Evaluation of various types of supplemental food for two species of predatory mites, Amblyseius swirskii and Neoseiulus cucumeris (Acari: Phytoseiidae). Exp. Appl. Acarol. 2015, 65, 483–494. [Google Scholar] [CrossRef]
- Scheu, S.; Folger, M. Single and mixed diets in Collembola: Effects on reproduction and stable isotope fractionation. Funct. Ecol. 2004, 18, 94–102. [Google Scholar] [CrossRef]
- Haubert, D.; Langel, R.; Scheu, S.; Ruess, L. Effects of food quality, starvation and life stage on stable isotope fractionation in Collembola. Pedobiologia 2005, 49, 229–237. [Google Scholar] [CrossRef]
- Ruess, L.; Müller-Navarra, D.C. Essential Biomolecules in Food Webs. Front. Ecol. Evol. 2019, 7, 269. [Google Scholar] [CrossRef] [Green Version]
- Gladyshev, M.I.; Sushchik, N.N.; Makhutova, O.N. Production of EPA and DHA in aquatic ecosystems and their transfer to the land. Prostaglandins Other Lipid Mediat. 2013, 107, 117–126. [Google Scholar] [CrossRef]
- Rothstein, M.; Götz, P. Biosynthesis of fatty acids in the free-living nematode, Turbatrix aceti. Arch. Biochem. Biophys. 1968, 126, 131–140. [Google Scholar] [CrossRef]
- Watts, J.L.; Browse, J. Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2002, 99, 5854–5859. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, C.L.; Krusberg, L.R. Investigation of some lipids from Turbatrix aceti. Comp. Biochem. Physiol. Part B Comp. Biochem. 1973, 45, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Menzel, R.; von Chrzanowski, H.; Tonat, T.; van Riswyck, K.; Schliesser, P.; Ruess, L. Presence or absence? Primary structure, regioselectivity and evolution of Δ12/ω3 fatty acid desaturases in nematodes. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2019, 1864, 1194–1205. [Google Scholar] [CrossRef]
- Malcicka, M.; Visser, B.; Ellers, J. An evolutionary perspective on linoleic acid synthesis in animals. Evol. Biol. 2018, 45, 15–26. [Google Scholar] [CrossRef] [Green Version]
- Brückner, A.; Heethoff, M. Fatty acid metabolism in an oribatid mite: De novo biosynthesis and the effect of starvation. Exp. Appl. Acarol. 2020, 81, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, N.; Naito, M.; Mori, N.; Kuwahara, Y. De novo biosynthesis of linoleic acid and its conversion to the hydrocarbon (Z,Z)-6,9-heptadecadiene in the astigmatid mite, Carpoglyphus lactis: Incorporation experiments with 13C-labeled glucose. Insect Biochem. Mol. Biol. 2014, 45, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Raspotnig, G.; Krisper, G. Fatty acids as cuticular surface components in oribatid mites (Acari: Oribatida). Arthropod Biology: Contributions to Morphology, Ecology and Systematics. Biosyst. Ecol. Ser. 1998, 14, 215–243. [Google Scholar]
- Raspotnig, G.; Schuster, R.; Krisper, G.; Fauler, G.; Leis, H.-J. Chemistry of the oil gland secretion of Collohmannia gigantea (Acari: Oribatida). Exp. Appl. Acarol. 2001, 25, 933–946. [Google Scholar] [CrossRef]
- Dmitryjuk, M.; Zalewski, K.; Raczkowski, M.; Żółtowska, K. Composition of fatty acids in the Varroa destructor mites and their hosts, Apis mellifera drone-prepupae. Ann. Parasitol. 2015, 61, 21–26. [Google Scholar]
- Menzel, R.; Geweiler, D.; Sass, A.; Simsek, D.; Ruess, L. Nematodes as important source for Omega-3 Long-Chain fatty acids in the soil food web and the impact in nutrition for higher trophic levels. Front. Ecol. Evol. 2018, 6, 96. [Google Scholar] [CrossRef] [Green Version]
- Stirling, G.R. Biological control of plant-parasitic nematodes: Soil ecosystem management in sustainable agriculture, 2nd ed.; CABI Pub.: Croydon, UK, 2014; ISBN 978-1-78064-415-8. [Google Scholar]
- Eilenberg, J.; Hajek, A.; Lomer, C. Suggestions for unifying the terminology in biological control. Bio. Control. 2001, 46, 387–400. [Google Scholar]
- Barbercheck, M.E. Effect of soil physical factors on biological control agents of soil insect pests. Fla. Entomol. 1992, 75, 539–548. [Google Scholar] [CrossRef]
- Yadav, S.; Patil, J.; Kanwar, R.S. The role of free living nematode population in the organic matter recycling. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2726–2734. [Google Scholar] [CrossRef]
- Treonis, A.M.; Sutton, K.A.; Unangst, S.K.; Wren, J.E.; Dragan, E.S.; McQueen, J.P. Soil organic matter determines the distribution and abundance of nematodes on alluvial fans in Death Valley, California. Ecosphere 2019, 10, e02659. [Google Scholar] [CrossRef]
- FAO Conservation Agriculture 2022. Available online: https://www.fao.org/publications/card/en/c/CB8350EN/ (accessed on 10 September 2022).
- Venter, Z.S.; Jacobs, K.; Hawkins, H.-J. The impact of crop rotation on soil microbial diversity: A meta-analysis. Pedobiologia 2016, 59, 215–223. [Google Scholar] [CrossRef]
- Abán, C.L.; Verdenelli, R.; Gil, S.V.; Huidobro, D.J.; Meriles, J.M.; Brandan, C.P. Service crops improve a degraded monoculture system by changing common bean rhizospheric soil microbiota and reducing soil-borne fungal diseases. FEMS Microbiol. Ecol. 2021, 97, fiaa258. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moreno, S.; Cano, M.; López-Pérez, A.; Rey Benayas, J.M. Microfaunal soil food webs in Mediterranean semi-arid agroecosystems. Does organic management improve soil health? Appl. Soil Ecol. 2018, 125, 138–147. [Google Scholar] [CrossRef]
- Wang, K.-H.; Hooks, C.R.R.; Marahatta, S.P. Can using a strip-tilled cover cropping system followed by surface mulch practice enhance organisms higher up in the soil food web hierarchy? Appl. Soil Ecol. 2011, 49, 107–117. [Google Scholar] [CrossRef]
- Zhang, X.; Ferris, H.; Mitchell, J.; Liang, W. Ecosystem services of the soil food web after long-term application of agricultural management practices. Soil Biol. Biochem. 2017, 111, 36–43. [Google Scholar] [CrossRef]
- Udalova, Z.; Ushakova, N.; Butorina, N.; Zinovieva, S. Influence of insectocomposts through Hermetia illucens larvae on nematodes of various ecological-trophic groups. Res. Crops 2021, 22, 150–157. [Google Scholar] [CrossRef]
- Messelink, G.J.; De Kogel, W.J. Impact of chrysanthemum cultivar, fertilization and soil-dwelling predatory mites on Frankliniella occidentalis. Proc. Neth. Entomol. Soc. Meet. 2005, 16, 101–107. [Google Scholar]
- Grosman, A.H.; Messelink, G.J.; Groot, E.B. de Combined use of a mulch layer and the soil-dwelling predatory mite Macrocheles robustulus (Berlese) enhance the biological control of sciarids in potted plants. IOBC/WPRS Bull. 2011, 68, 51–54. [Google Scholar]
- de Esteca, F.C.N.; Rodrigues, L.R.; de Moraes, G.J.; Júnior, I.D.; Klingen, I. Mulching with coffee husk and pulp in strawberry affects edaphic predatory mite and spider mite densities. Exp. Appl. Acarol. 2018, 76, 161–183. [Google Scholar] [CrossRef] [PubMed]
- Young, M.R.; Hebert, P.D.N. Unearthing soil arthropod diversity through DNA metabarcoding. Peer. J. 2022, 10, e12845. [Google Scholar] [CrossRef] [PubMed]
- Schenk, J.; Geisen, S.; Kleinboelting, N.; Traunspurger, W. Metabarcoding data allow for reliable biomass estimates in the most abundant animals on earth. MBMG 2019, 3, e46704. [Google Scholar] [CrossRef]
- Santos, P.F.; Whitford, W.G. The effects of microarthropods on litter decomposition in a Chihuahuan desert ecosystem. Ecology 1981, 62, 654–663. [Google Scholar] [CrossRef]
- Santos, P.F.; Phillips, J.; Whitford, W.G. The role of mites and nematodes in early stages of buried litter decomposition in a desert. Ecology 1981, 62, 664–669. [Google Scholar] [CrossRef]
- Elkins, N.Z.; Whitford, W.G. The role of microarthropods and nematodes in decomposition in a semi-arid ecosystem. Oecologia 1982, 55, 303–310. [Google Scholar] [CrossRef]
- Parker, L.W.; Santos, P.F.; Phillips, J.; Whitford, W.G. Carbon and nitrogen dynamics during the decomposition of litter and roots of a Chihuahuan desert annual, Lepidium lasiocarpum. Ecol. Monogr. 1984, 54, 339–360. [Google Scholar] [CrossRef]
- Hyvönen, R.; Persson, T. Effects of fungivorous and predatory arthropods on nematodes and tardigrades in microcosms with coniferous forest soil. Biol Fertil Soils 1996, 21, 121–127. [Google Scholar] [CrossRef]
- Mcsorley, R.; Wang, K.-H. Possibilities for biological control of root-knot nematodes by natural predators in Florida soils. Proc. Fla. State Hortic. Soc. 2009, 122, 421–425. [Google Scholar]
- Stirling, G.R.; Mankau, R. Biological control of nematode parasited of citrus by natural enemies. Proc. Int. Soc. Citric. 1977, 3, 843–847. [Google Scholar]
- Walter, D.E.; Proctor, H.C. Predatory mites in Tropical Australia: Local species richness complementarity. Biotropica 1998, 30, 72–81. [Google Scholar] [CrossRef]
- El-Banhawy, E.M.; Nasr, A.K.; Afia, S.I. Survey of predacious soil mites (Acari: Mesostigmata) in citrus orchards of the Nile Delta and Middle Egypt with notes on the abundance of the citrus parasitic nematode Tylenchulus semipenetrans (Tylenchida: Tylenchulidae). JTI 2006, 26. [Google Scholar] [CrossRef]
- Lindquist, E.E.; Walter, D.E. Antennoseius ( Vitzthumia ) janus n.sp. (Acari: Ascidae), a mesostigmatic mite exhibiting adult female dimorphism. Can. J. Zool. 1989, 67, 1291–1310. [Google Scholar] [CrossRef]
- Halliday, R.B.; Walter, D.E.; Lindquist, E.E. Revision of the Australian Ascidae (Acarina: Mesostigmata). Invert. Syst. 1998, 12, 1–54. [Google Scholar] [CrossRef]
- Binns, E.S. Arctoseius cetratus (Sellnick) (Acarina: Ascidae) phoretic on mushroom sciarid flies. Acarologia 1972, XIV, 350–356. [Google Scholar]
- Binns, E.S. Three predator mites from mushroom beds. Mushroom J. 1973, 7, 319–320. [Google Scholar]
- Binns, E.S. Predatory mites - neglected allies? Mushroom J. 1973, 12, 540–544. [Google Scholar]
- Karg, W. Acari (Acarina), Milben. Unterordnung Anactinochaeta (Parasitiformes). Die freilebenden Gamasina (Gamasides), Raubmilben. Die Tierwelt Dtschl. 1971, 59, 1–475. [Google Scholar]
- Chaires-Grijalva, M.P.; Estrada-Venegas, E.G.; Equihua-Martinez, A.; Moser, J.C.; Blomquist, S.R. Trophic habits of mesostigmatid mites associated with bark beetles in Mexico. J. Acarol. Soc. Jpn. 2016, 25, S161–S167. [Google Scholar] [CrossRef] [Green Version]
- Rudzińska, M. Life history of the phoretic predatory mite Arctoseius semiscissus (Acari: Ascidae) on a diet of sciarid fly eggs. Exp. Appl. Acarol. 1998, 22, 643–648. [Google Scholar] [CrossRef]
- Walter, D.E. The genus Gamasellodes (Acari: Mesostigmata: Ascidae): New Australian and North American species. Syst. Appl. Acarol. Spec. Publ. 2003, 15, 1–10. [Google Scholar] [CrossRef]
- Walter, D.E. Life history, trophic behavior, and description of Gamasellodes vermivorax n. sp. (Mesostigmata: Ascidae), a predator of nematodes and arthropods in semiarid grassland soils. Can. J. Zool. 1987, 65, 1689–1695. [Google Scholar] [CrossRef]
- Walter, D.E.; Lindquist, E.E. Arrhenoseius gloriosus n. g., n. sp. (Acari: Mesostigmata: Ascidae), an arrhenotokous mite from rainforests in Queensland, Australia. Acarologia 2000, 41, 53–68. [Google Scholar]
- Walter, D.E.; Lindquist, E.E. Life history and behavior of mites in the genus Lasioseius (Acari: Mesostigmata: Ascidae) from grassland soils in Colorado, with taxonomic notes and description of a new species. Can. J. Zool. 1989, 67, 2797–2813. [Google Scholar] [CrossRef]
- Walter, D.E.; Lindquist, E.E. Australian species of Lasioseius (Acari: Mesostigmata: Ascidae): The porulosus group and other species from rainforest canopies. Invert. Syst. 1997, 11, 525. [Google Scholar] [CrossRef]
- Mankau, R.; Imbriani, J.L. Studies on the mite Lasioseius scapulatusa predator on soil nematodes. Nematropica 1978, 8, 15. [Google Scholar]
- Imbriani, J.L.; Mankau, R. Studies on Lasioseius scapulatus, a Mesostigmatid mite predaceous on nematodes. J. Nematol. 1983, 15, 523–528. [Google Scholar]
- Fouly, A.H. Influence of different nourishment on the biology of Lasioseius dentatus (Fox), a new record from Egypt (Acari: Gamasida: Ascidae). Egypt. J. Biol. Pest Control. 1997, 7, 1–6. [Google Scholar]
- Van de Bund, C.F. Mesofauna of arable soil. In Integrated control of pests in the Netherlands; Minks, A.K., Gruys, P., Eds.; Centre for Agricultural Publishing and Documentation: Pudoc, Wageningen, 1980; pp. 87–92. [Google Scholar]
- Aguilar-Marcelino, L.; Quintero-Martínez, M.T.; Mendoza de Gives, P.; López-Arellano, M.E.; Liébano-Hernández, E.; Torres-Hernández, G.; González-Camacho, J.M.; Cid del Prado, I. Evaluation of predation of the mite Lasioseius penicilliger (Aracnida: Mesostigmata) on Haemonchus contortus and bacteria-feeding nematodes. J. Helminthol. 2014, 88, 20–23. [Google Scholar] [CrossRef]
- Nasr, A.K.; Nawar, M.S.; Mowafi, M.H.A. Biological studies and feeding habits of Lastoseius [sic] athiasae Nawar and Nasr (Acari: Mesostigmata: Ascidae) in Egypt. Bull.-Zool. Soc. Egypt 1990, 39. [Google Scholar]
- Cayrol, J.C. Action des autres composants de la biocénose du champignon de couche sur le nématode mycophage, Ditylenchus myceliophagus J. B. Goodey, 1958, et étude de son anabiose: Forme de survie en conditions défavorables. Rev. D’écologie Et De Biol. Du Sol 1970, VII, 409–440. [Google Scholar]
- Karg, W. Verbreitung und Bedeutung Von Raubmilben der Cohors Gamasina als Antagonisten von Nematoden. Pedobiologia 1983, 25, 419–432. [Google Scholar]
- Binns, E.S. Digamasellus fallax Leitner (Mesostigmata: Digamasellidae) phoretic on mushroom sciarid flies. Acarologia 1973, 15, 10–17. [Google Scholar] [PubMed]
- Kinn, D.N. Mutualism Between Dendrolaelaps neodisetus and Dendroctonus frontalis1. Environ. Entomol. 1980, 9, 756–758. [Google Scholar] [CrossRef]
- Abou-El-Soud, A.B.; Shoeib, A.A. Root-knot nematode Meloidogyne javanica and acarid mite, Acarus siro L. predation by digamasellid mite, Dendrolaelaps rasmii Nasr and Mersal. Al-Azhar J. Agric. Res. 2000, 31, 45–50. [Google Scholar]
- Krantz, G.W.; Poinar, G.O., Jr. Mites, nematodes and the multimillion dollar weevil. J. Nat. Hist. 2004, 38, 135–141. [Google Scholar] [CrossRef]
- Kinn, D.N. Notes on the life cycle and habitats of Digamasellus quadrisetus (Mesostigmata: Digamasellidae). Ann. Entomol. Soc. Am. 1967, 60, 862–865. [Google Scholar] [CrossRef]
- Hirschmann, W.; Rühm, W. Ein „Haustier" des Buchdruckers? Mikrokosmos 1955, 44, 234–236. [Google Scholar]
- Enda, N.; Tamura, H. Nematophagous mites found on adults of the Japanese pine sawyer. Shinrin Boecki 1970, 26, 188–190. [Google Scholar]
- Enda, N.; Tamura, H. Nematode feeding mites found on adults of the Japanese pine sawyer. Shinrin Boecki 1980, 29, 17–20. [Google Scholar]
- Walter, D.E. Heatherellidae — a new family of Mesostigmata (Acari: Parasitiformes) based on two new species from rainforest litter in Australia. Int. J. Acarol. 1997, 23, 167–175. [Google Scholar] [CrossRef]
- Inserra, R.N.; Davis, D.W. Hypoaspis nr. aculeifer: A mite predacious on Root-knot and Cyst nematodes. J. Nematol. 1983, 15, 324–325. [Google Scholar] [PubMed]
- Walter, D.E.; Campbell, N.J.H. Exotic vs endemic biocontrol agents: Would the real Stratiolaelaps miles (Berlese) (Acari: Mesostigmata: Laelapidae), please stand up? Biol. Control. 2003, 26, 253–269. [Google Scholar] [CrossRef]
- Brodsgaard, H.F.; Sardar, M.A.; Enkegaard, A. Prey preference of Hypoaspis miles (Berlese) (Acarina: Hypoaspididae): Non-interference with other beneficials in glasshouse crops. IOBC/WPRS Bull. 1996, 19, 23–26. [Google Scholar]
- Singer, G.; Krantz, G.W. The use of nematodes and oligochaetes for rearing predatory mites. Acarologia 1967, IX, 485–487. [Google Scholar]
- Krantz, G.W.; Royce, L.A. Observations on the biology and behavior of Macrocheles mycotrupetes Krantz and Mellott (Acari: Macrochelidae). Int. J. Acarol. 1994, 20, 115–121. [Google Scholar] [CrossRef]
- Manning, M.J.; Halliday, R.B. Biology and reproduction of some australian species of Macrochelidae (Acarina). Aust. Entomol. 1994, 21, 89–94. [Google Scholar]
- Krantz, G.W. A new species of Macrocheles (Acarina: Macrochelidae) associated with bark beetles of the genera Ips and Dendroctonus. J. Kans. Entomol. Soc. 1965, 38, 145–153. [Google Scholar]
- Kinn, D.N.; Witcosky, J.J. The life cycle and behaviour of Macrocheles boudreauxi Krantz. Z. Für Angew. Entomol. 1977, 84, 136–144. [Google Scholar] [CrossRef]
- Cicolani, B. The influence of temperature on the speed of development, from egg to adult, of Macrocheles matrius (Acarina: Mesostigmata). Acarologia 1977, XIX, 384–394. [Google Scholar]
- Filipponi, A.; Cicolani, B. Influenza della temperatura sulla fecondita’ longevita’ e capacita’ moltiplicativa nello intervallo ottimale di Macrocheles matrius (Acarina, Mesostigmata). Riv. Di Parassitol. 1974, XXXV, 291–306. [Google Scholar]
- Singh, P.; Rodriguez, J.G. Food for macrochelid mites (Acarina) by an improved method for mass rearing of a nematode, Rhabditella leptura. Acarologia 1966, VIII, 549–550. [Google Scholar]
- Filipponi, A.; Francaviglia, G. Larviparita Facoltativa in alguni Macrochelidi (Acari: Mesostigmata) associati a muscidi di interesse sanitario. Parassitologia 1964, VI, 291–301. [Google Scholar]
- Geden, C.J.; Axtell, R.C. Predation by Carcinops pumilio (Coleoptera: Histeridae) and Macrocheles muscaedomesticae (Acarina: Macrochelidae) on the house fly (Diptera: Muscidae): Functional response, effects of temperature, and availability of alternative prey. Environ. Entomol. 1988, 17, 739–744. [Google Scholar] [CrossRef] [Green Version]
- Heikal, H.M. Parasitus fimetorum and Macrocheles muscaedomesticae (Acarina:Parasitidae, Macrochelidae) as Natural Predators of the Root Knot Nematode, Meloidogyne javanica Treub. Egypt J. Biol. Pest Control. 2020, 30, 33. [Google Scholar] [CrossRef]
- Ho, C.-C.; Cromroy, H.L.; Patterson, R.S. Mass production of the predaceous mite, Macrocheles muscaedomesticae (Scopoli) (Acarina: Macrochelidae), a predator of the house fly. In Biocontrol of arthropods affecting livestock and poultry; Rutz, D.A., Patterson, R.S., Eds.; Westview Press: Boulder, CO, USA, 1990; pp. 201–213. [Google Scholar]
- Filipponi, A. Influence of Temperature on Population Increase of Four Dung-Living Macrochelid Mites within Optimum Range. In Proceedings of the 3rd International Congress of Acarology, Prague; Rosický B, D.M., Ed.; S. Junk, The Hague and Academia: Prague, Czech Republic, 1971; pp. 775–779. [Google Scholar]
- Filipponi, A.; Mosna, B.; Petrelli, G. L’ottimo di temperatura di Macrocheles muscaedomesticae (Scopoli), come attributo di popolazione. Riv. Di Parassitol. 1971, 32, 193–218. [Google Scholar]
- Costa, M. Notes on macrochelids associated with manure and coprid beetles in Israel. II. Three new species of the Macrocheles pisentii complex, with notes on their biology. Acarologia 1967, IX, 304–329. [Google Scholar]
- Krantz, G.W. Nature of the association between pisentii group mites (Acari, Macrochelidae, Macrocheles) and dung beetles of the genus Scarabaeus (Coleoptera, Scarabaeidae) in Southern France. Acarologia 1991, XXXII, 3–11. [Google Scholar]
- Costa, M. Notes on Macrochelids associated with manure and coprid beetles in Israel. I Macrocheles robustulus (Berlese, 1904). Development and biology. Acarologia 1966, VIII, 532–548. [Google Scholar]
- Nasr, A.K.; Nawar, M.S.; Mowafi, M.H.A. Biological studies on Proctolaelaps bickleyi Bram (Acari: Gamasida: Ascidae). Bull.-Zool. Soc. Egypt 1990, 39, 89–100. [Google Scholar]
- Nawar, M.S. Life tables of Proctolaelaps deleoni Nawar, Childers and Abou-Setta (Gamasida: Ascidae) at different temperatures. Exp. Appl. Acarol. 1992, 13, 281–285. [Google Scholar] [CrossRef]
- Kinn, D.N. The life cycle of Proctolaelaps dendroctoni lindquist and hunter (acari: Ascidae): A mite associated with pine bark beetles. Int. J. Acarol. 1983, 9, 205–210. [Google Scholar] [CrossRef]
- Evans, G.O. A revision of the British Aceosejinae (Acarina: Mesostigmata). Proc. Zool. Soc. Lond. 1958, 131, 177–229. [Google Scholar] [CrossRef]
- Hirschmann, W.; Rühm, W. Milben und Fadenwürmer als Symphonsten und Parasiten des Buchdruckers (Ips typographus). Nachr. Des Nat. Mus. Der Stadt Aschaffenbg. 1954, 43, 41–50. [Google Scholar]
- Kinn, D.N. The life cycle and behaviour of Cercoleipus coleonotus (Acarina: Mesostigmata) including a survey of phoretic mite associates of California Scolytidae. University of California Publications in Entomology; University of California Press: Berkeley, CA, USA, 1971; Volume 65. [Google Scholar]
- Lindquist, E.E.; Hunter, P.E. Some Mites of the Genus Proctolaelaps Berlese (Acarina: Blattisociidae) Associated with Forest Insect Pests. Can. Entomol. 1965, 97, 15–32. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Mowafi, M.H. Life tables of Proctolaelaps pygmaeus (Muller) at different temperatures. Al-Azhar J. Agric. Res. 1998, 27, 338–348. [Google Scholar]
- Metwalli, A.M.; Abbasy, M.R.A.; Montaser, S.A. Life history of the ascid mite Proctolaelaps pygmaeus Muller when fed on two different preys. Al-Azhar J. Agric. Res. 1991, 13, 237–246. [Google Scholar]
- Ibrahim, G.A.; Abdel-Samedi, M.A.; El-Gazzar, H.F. Biological aspects of predator mite Proctolaelaps subcorticalis Lindquist (Ascidae: Mesostigmata) with description to its immature stages. Minufiya J. Agric. Res. 1992, 17, 2015–2023. [Google Scholar]
- Pugh, P.J.A.; King, P.E. Feeding in intertidal Acari. J. Exp. Mar. Biol. Ecol. 1985, 94, 269–280. [Google Scholar] [CrossRef]
- Costa, M. Descriptions of the juvenile stages of Pachylaelaps hispani Berlese (Acari: Mesostigmata). Acarologia 1966, VIII, 9–22. [Google Scholar]
- Ishikawa, K. Taxonomic and ecological studies in the family Parholaspidae from Japan (Part 4). Rep. Res. Matsuyama Shinonome Jr. Col 1980, 11, 183–190. [Google Scholar]
- Lindquist, E.E.; Krantz, G.W.; Walter, D.E. Order Mesostigmata. In A Manual of Acarology; Krantz, G.W., Walter, D.E., Eds.; Texas Tech University Press: Lubbock, TX, USA, 2009; pp. 124–232. [Google Scholar]
- Hse, S.H.H. Biological Studies of Mites Associated with Bark Beetles. M.S. Thesis, Lousiana State University, Baton Rouge, LA, USA, 1964. [Google Scholar]
- Karg, W. Zur Kenntnis der Gattung Parasitus Latreille, 1795 (Acarina, Parasitiformes) aus Komposterden und Gurkenkulturen. Dtsch. Entomol. Z. 1972, 19, 55–63. [Google Scholar] [CrossRef]
- Al-Amidi, A.H.K.; Downes, M.J. Parasitus bituberosus (Acari: Parasitidae), a possible agent for biological control of Heteropeza pygmaea (Diptera: Cecidomyiidae) in mushroom compost. Exp. Appl. Acarol. 1990, 8, 13–25. [Google Scholar] [CrossRef]
- Costa, M. Descriptions of the hitherto unknown stages of Parasitus copridis Costa (Acari: Mesostigmata) with notes on its biology. Zool. J. Linn. Soc. 1964, 45, 209–222. [Google Scholar] [CrossRef]
- Muma, M.H.; Denmark, H.A. Biological Studies on Macroseius biscutatus (Acarina: Phytoseiidae). Fla. Entomol. 1967, 50, 249–255. [Google Scholar] [CrossRef]
- Taha, H.A.; Shereef, G.M.; Soliman, Z.R.; Wafaa, O.G. Effect of different prey types and temperature on biological aspects of predatory mite Protogamasellus discorus (Acari: Gamasida: Ascidae) with special references of chemical analysis of prey. Egypt. J. Plant Prot. Res. Inst. 2019, 2, 480–487. [Google Scholar]
- Castilho, R.C.; de Moraes, G.J.; Silva, E.S.; Silva, L.O. Predation potential and biology of Protogamasellopsis posnaniensis Wisniewski & Hirschmann (Acari: Rhodacaridae). Biol. Control. 2009, 48, 164–167. [Google Scholar] [CrossRef]
- Bunt, J.A. Effect and mode of action of some systemic nematicides; Mededelingen Landbouwhogeschool Wageningen Nederland: Wageningen, The Netherlands, 1975; Volume 75-10. [Google Scholar]
- Walter, D.E.; Proctor, H.C. Feeding behavior and phylogeny: Observations on early derivative Acari. Exp. Appl. Acarol. 1998, 22, 39–50. [Google Scholar] [CrossRef]
- El-Banhawy, E.M.; El-Borolossy, M.A.; El-Sawaf, B.M.; Afia, S.I. Biological aspects and feeding behaviour of the predacious soil mite Nenteria hypotrichus (Uropodina: Uropodidae). Acarologia 1997, XXXVIII, 357–360. [Google Scholar]
- Walter, D.E. From the subantarctic to the subtropics: A revision of the Davacaridae Kethley, 1977 (Acari: Trigynaspida: Mesostigmata) with the description of a new genus and three new species. J. Nat. Hist. 2004, 38, 2033–2049. [Google Scholar] [CrossRef]
- Seeman, O.D. Life cycle, development, feeding and immature life stages of the Fedrizziidae (Mesostigmata: Fedrizzioidea). Acarologia 2000, 41, 39–52. [Google Scholar]
- Butler, L.; Hunter, P.E. Redescription of Megisthanus floridanus with observations on its biology (Acarina: Megisthanidae). Fla. Entomol. 1968, 51, 187. [Google Scholar] [CrossRef]
- Walter, D.E. A jumping mesostigmatan mite, Saltiseius hunteri n. g., n. sp. (Acari: Mesostigmata: Trigynaspida: Saltiseiidae, n. fam.) from Australia. Int. J. Acarol. 2000, 26, 25–31. [Google Scholar] [CrossRef]
- Seeman, O.D.; Walter, D.E. A new species of Triplogyniidae (Mesostigmata: Celaenopsoidea) from Australian rainforests. Int. J. Acarol. 1997, 23, 49–59. [Google Scholar] [CrossRef]
- Rockett, C.L. Nematode predation by oribatid mites (Acari: Oribatida). Int. J. Acarol. 1980, 6, 219–224. [Google Scholar] [CrossRef]
- Oliveira, A.R.; De Moraes, G.J.; Ferraz, L.C.C.B. Consumption rate of phytonematodes by Pergalumna sp. (Acari: Oribatida: Galumnidae) under laboratory conditions determined by a new method. Exp. Appl. Acarol. 2007, 41, 183–189. [Google Scholar] [CrossRef]
- Abou El-Atta, D.A.E.-M.; Ghazy, N.A.; Osman, M.A. Effects of temperature on the life-history traits of Sancassania (Caloglyphus) berlesei (Acari: Astigmatina: Acaridae) feeding on root-knot nematodes, Meloidogyne spp. (Nematoda: Meloidogynidae). Exp. Appl. Acarol. 2014, 64, 299–307. [Google Scholar] [CrossRef]
- Sell, P. Caloglyphus sp. (Acarina: Acaridae), an effective nematophagous mite on root-knot nematodes (Meloidogyne spp.). Nematol 1988, 34, 246–248. [Google Scholar] [CrossRef]
- Walter, D.E.; Kaplan, D.T. Feeding observations on two astigmatic mites, Schwiebea rocketti (Acaridae) and Histiostoma bakeri (Histiostomatidae) associated with Citrus feeder roots. Pedobiologia 1990, 34, 281–286. [Google Scholar]
- Taha, H.A.; El-Naggar, M.E.E.; Abou-El-Ngaga, M.M.; Soliman, S.M. Effect of different prey species on the development and fecundity of the predacious mite, Neocunaxoides andrei Baker and Hoff. (Acari: Cunaxidae). Agric. Res. Rev. 1988, 66, 129–135. [Google Scholar]
- Hernandes, F.A.; de Castro, T.M.M.G.; Venancio, R. Prostigmata (Acari: Trombidiformes) as Biological Control Agents. In Prospects for Biological Control of Plant Feeding Mites and Other Harmful Organisms; Carrillo, D., de Moraes, G.J., Peña, J.E., Eds.; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-15042-0. [Google Scholar]
- Fan, Q.-H.; Walter, D.E. Genus Caligohomus Habeeb (Acari: Prostigmata: Stigmaeidae). Syst. Appl. Acarol. 2004, 9, 77–88. [Google Scholar] [CrossRef]




| Species | Target | Region of Application | First Commercial Use |
|---|---|---|---|
| Androlaelaps casalis (Berlese, 1887) | Dermanyssus | Europe | 2012 |
| Stratiolaelaps miles (Berlese, 1882) | Sciaridae | Europe, North America, Asia, Aus/NZ | 1995 |
| Stratiolaelaps scimitus (Womersley, 1956) | Sciaridae, thrips | Europe, Latin America | 1990 |
| Gaeolaelaps aculeifer (Canestrini, 1884) | Diptera, thrips, mites | Europe, North Africa, North America, Asia, Aus/NZ | 1995 |
| Gaeolaelaps gillespiei Beaulieu | Sciaridae, thrips | North America (Canada) | 2009 |
| Macrocheles robustulus (Berlese, 1904) | Diptera, thrips, Lepidoptera? | Europe | 2010 |
| Pergamasus quisquiliarum (Canestrini, 1882) | Symphyla | Europe | 2000 |
| Cheyletus eruditus (Schrank, 1781) | Dermanyssus, pests of stored products | Europe | 1985 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rueda-Ramírez, D.; Palevsky, E.; Ruess, L. Soil Nematodes as a Means of Conservation of Soil Predatory Mites for Biocontrol. Agronomy 2023, 13, 32. https://doi.org/10.3390/agronomy13010032
Rueda-Ramírez D, Palevsky E, Ruess L. Soil Nematodes as a Means of Conservation of Soil Predatory Mites for Biocontrol. Agronomy. 2023; 13(1):32. https://doi.org/10.3390/agronomy13010032
Chicago/Turabian StyleRueda-Ramírez, Diana, Eric Palevsky, and Liliane Ruess. 2023. "Soil Nematodes as a Means of Conservation of Soil Predatory Mites for Biocontrol" Agronomy 13, no. 1: 32. https://doi.org/10.3390/agronomy13010032






