Allelic Complementation in Hybrid Superiority of Popcorn to Multiple Foliar Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Genetic Material
2.2. Experimental Design
2.3. Traits Evaluated
2.3.1. Agronomic
2.3.2. Foliar Diseases
2.3.3. Puccinia polysora
2.3.4. Bipolaris maydis
2.3.5. Exserohilum turcicum
2.4. Statistical and Genetic Analysis of Traits
2.4.1. Variance Analysis
2.4.2. Partial Diallel
2.4.3. GT Biplot
3. Results
3.1. Analysis of Variance
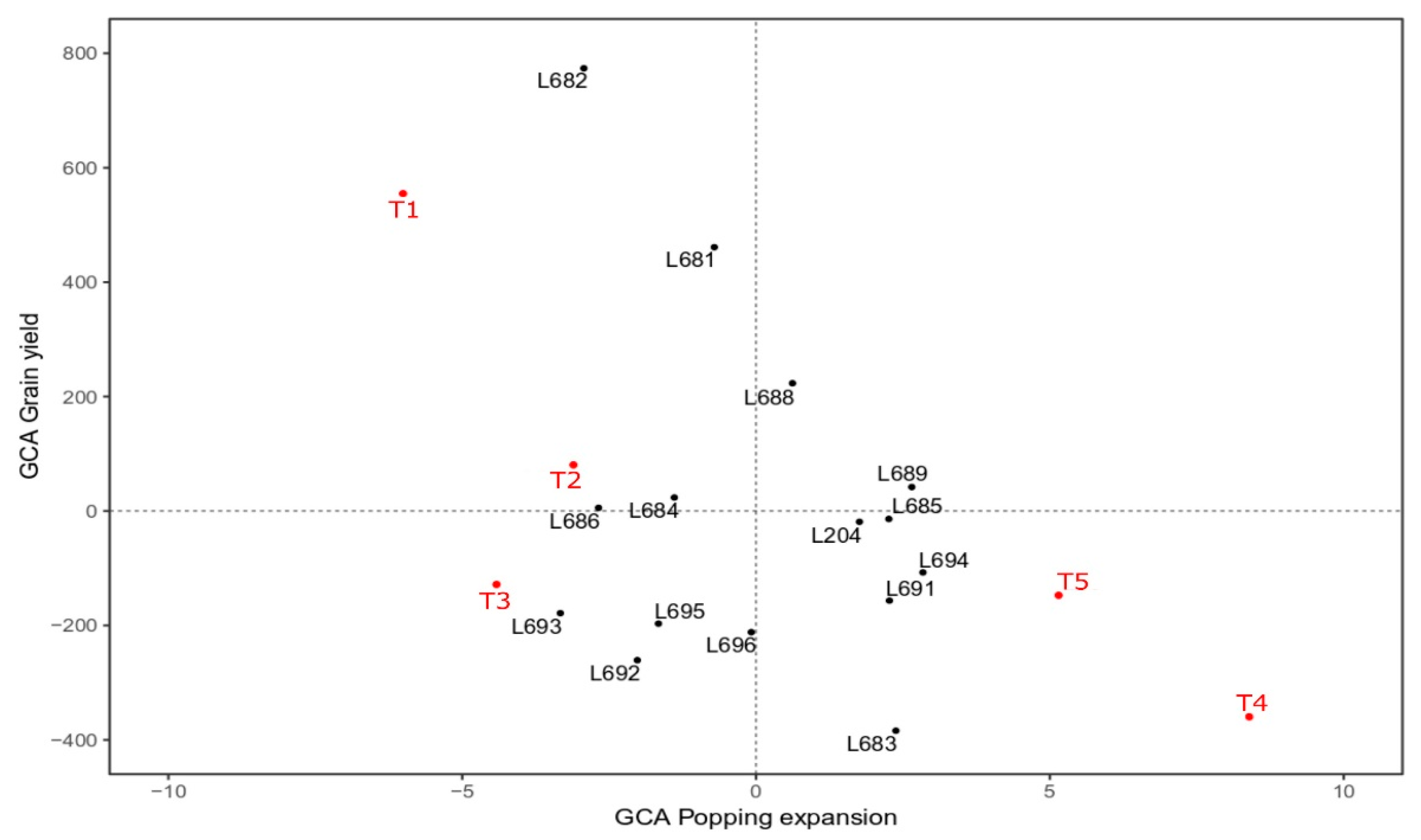
3.2. Estimates of Combinatorial Capabilities
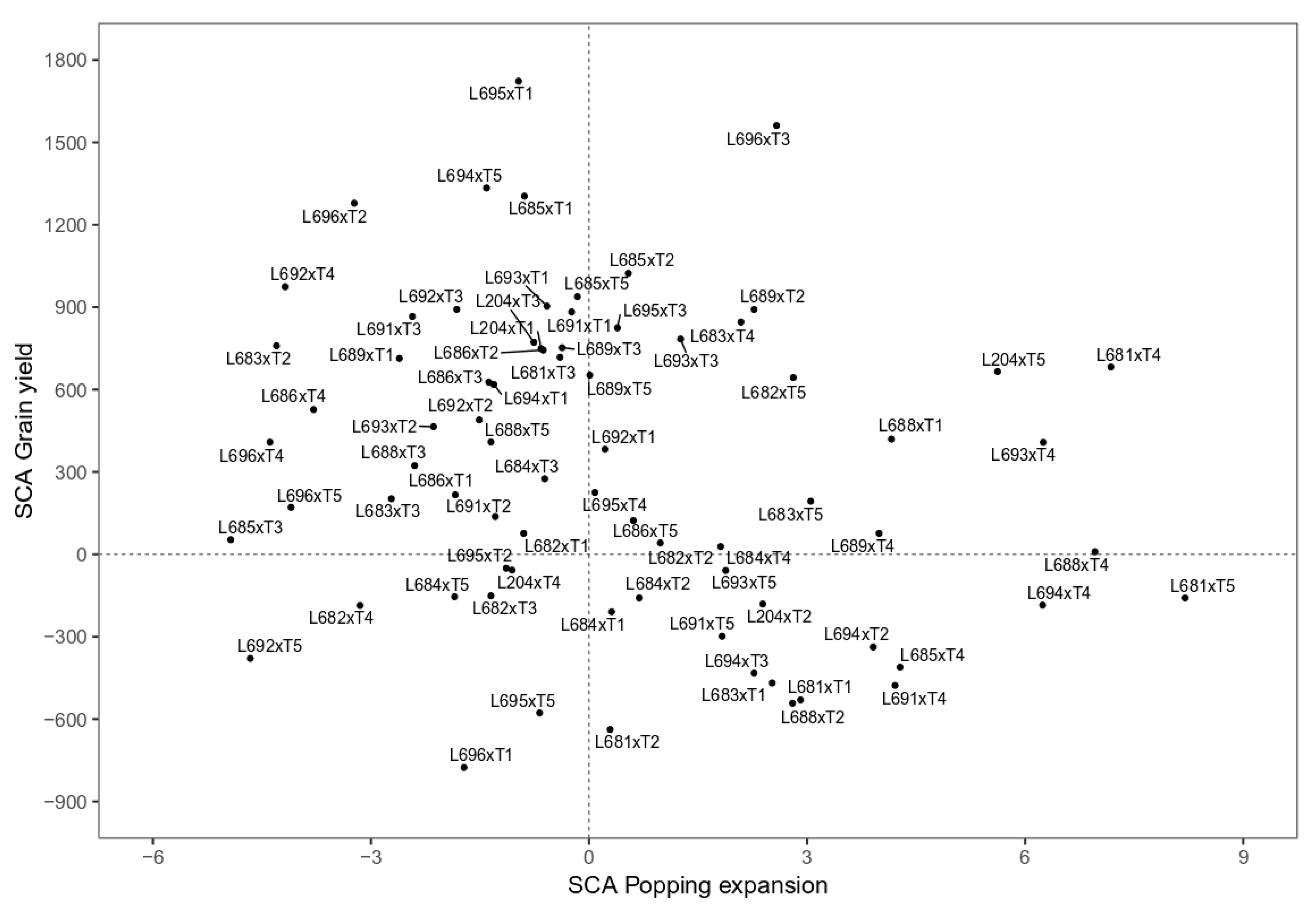
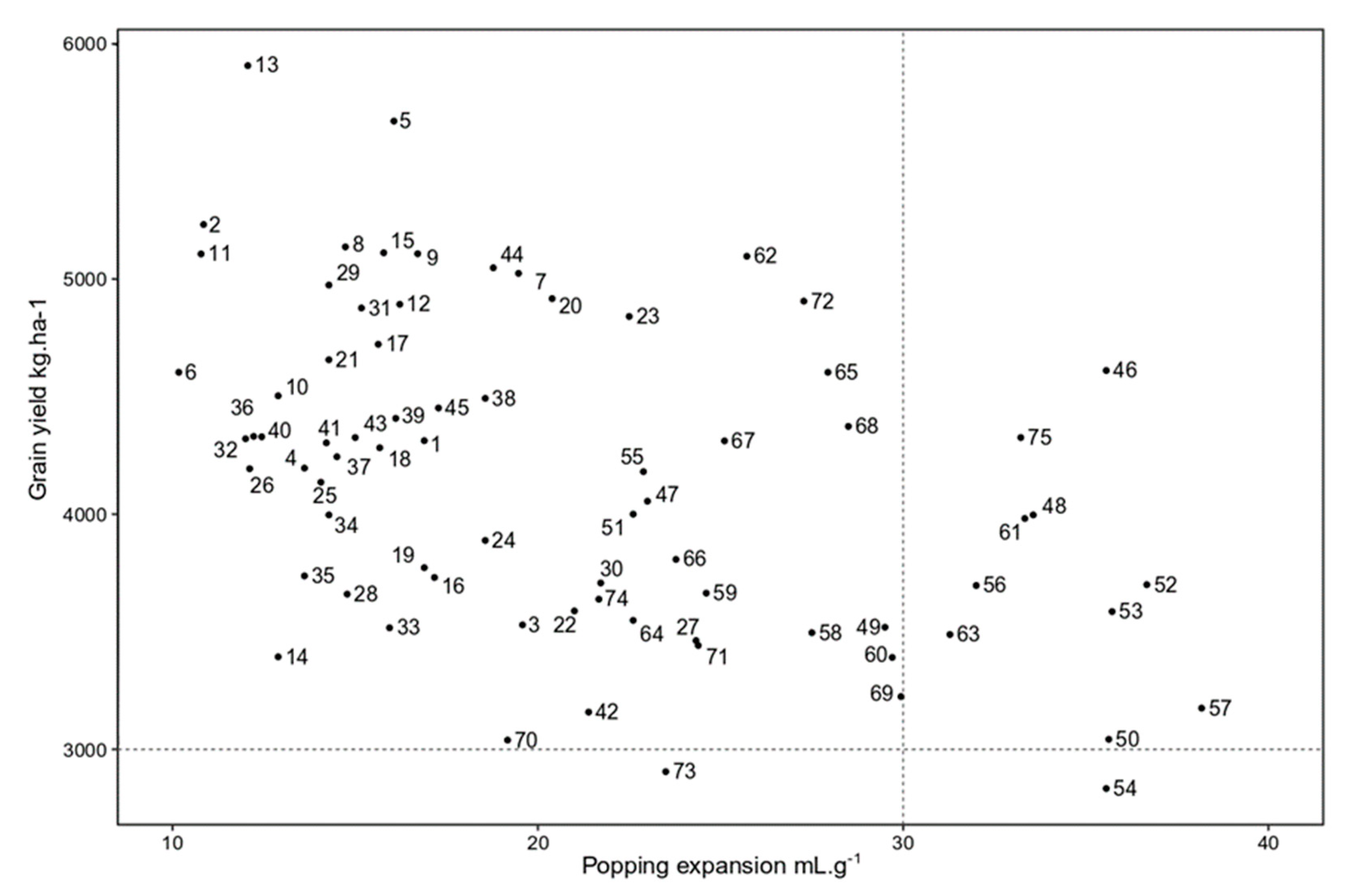
3.3. The Best Hybrids Based on Means of GY and PE
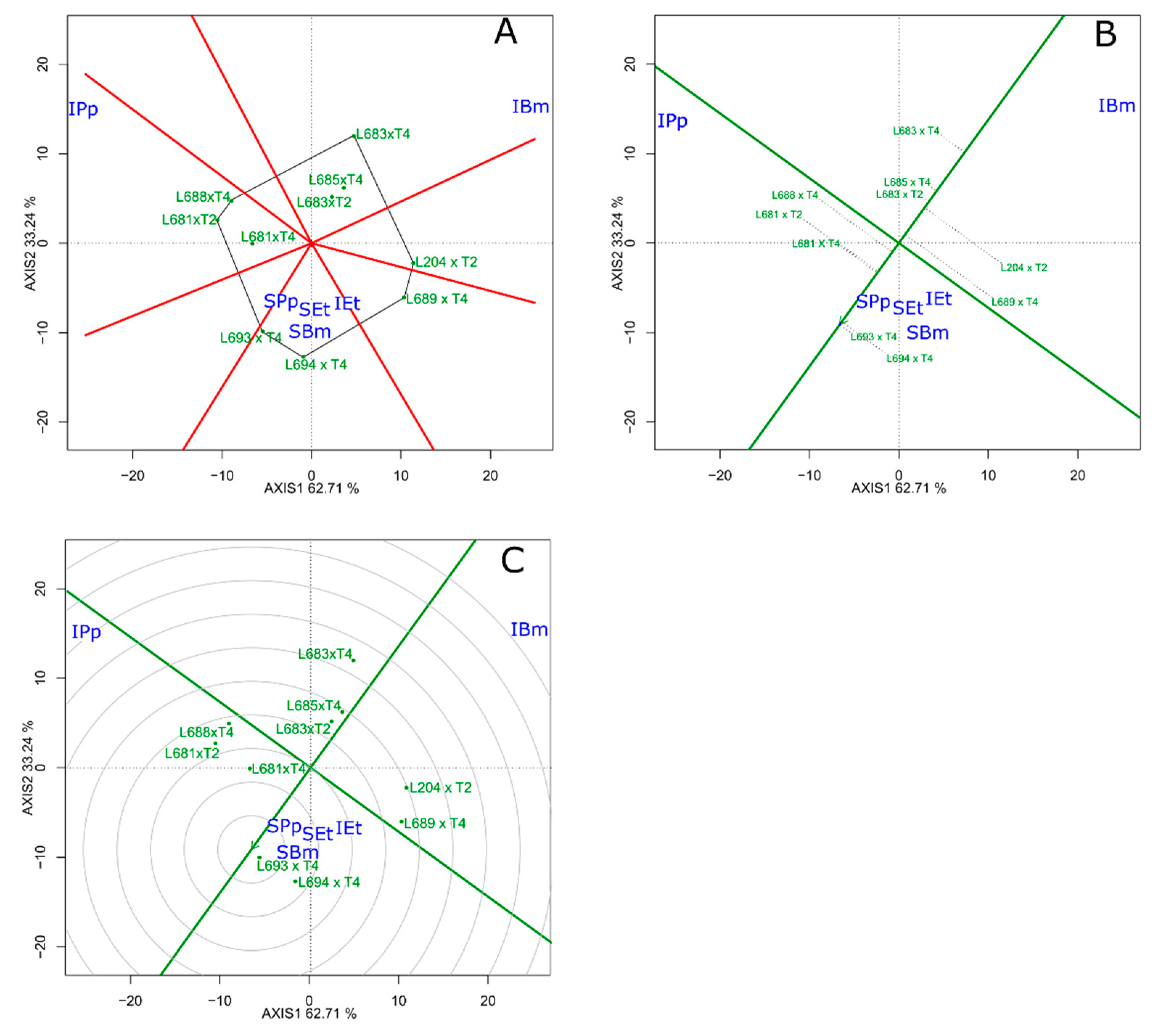
3.4. GT-Biplot Analysis of the Identification of the Superior Genotypes for Multiple Diseases
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ribeiro, R.M.; Amaral Júnior, A.T.; Pena, G.F.; Vivas, M.; Kurosawa, R.N.; Gonçalves, L.S.A. History of Northern Corn Leaf Blight Disease in the Seventh Cycle of Recurrent Selection of an UENF-14 Popcorn Population. Acta Sci. Agron. 2016, 38, 447. [Google Scholar] [CrossRef] [Green Version]
- Kurosawa, R.N.F.; do Amaral Junior, A.T.; Silva, F.H.L.; dos Santos, A.D.; Vivas, M.; Kamphorst, S.H.; Pena, G.F. Multivariate Approach in Popcorn Genotypes Using the Ward-MLM Strategy: Morpho-Agronomic Analysis and Incidence of Fusarium spp. Genet. Mol. Res. 2017, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, R.D.N.F.; Vivas, M.; Amaral, A.T.D.; Ribeiro, R.M.; Miranda, S.B.; Pena, G.F.; Leite, J.T.; Mora, F. Popcorn Germplasm Resistance to Fungal Diseases Caused by Exserohilum Turcicum and Bipolaris Maydis. Bragantia 2017, 77, 36–47. [Google Scholar] [CrossRef] [Green Version]
- Kurosawa, R.D.N.F.; Amaral, A.T.D., Jr.; Vivas, J.M.S.; Guimarães, A.G.; Miranda, S.B.; Dias, V.M.; Scapim, C.A. Potential of Popcorn Germplasm as a Source of Resistance to Ear Rot. Bragantia 2017, 76, 378–385. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.S.; Júnior, A.T.A.; Vivas, M.; Mafra, G.S.; Pena, G.F.; Silva, F.H.L.; Guimarães, A.G. Genetic Control and Combining Ability of Agronomic Attributes and Northern Leaf Blight-Related Attributes in Popcorn. Genet. Mol. Res. 2017, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pena, G.F.; Mafra, G.S.; do Amaral Júnior, A.T.; Alfenas, R.F.; Bhering, L.L.; Santos, J.S.; Kamphorst, S.H.; de Lima, V.J.; de Oliveira Santos, T.; Bispo, R.B.; et al. Mixed Modeling in Genetic Divergence Study of Elite Popcorn Hybrids (Zea Mays Var. Everta). Agriculture 2022, 12, 910. [Google Scholar] [CrossRef]
- Schwantes, I.A.I.A.; do Amaral Júnior, A.T.; Gerhardt, I.F.S.I.F.S.; Vivas, M.; de Lima e Silva, F.H.F.H.; Kamphorst, S.H.S.H. Diallel Analysis of Resistance to Fusarium Ear Rot in Brazilian Popcorn Genotypes. Trop. Plant Pathol. 2017, 42, 70–75. [Google Scholar] [CrossRef]
- Schwantes, I.A.; Amaral, A.T.D., Jr.; Vivas, M.; de Almeida Filho, J.E.; Kamphorst, S.H.; Guimarães, A.G.; Khan, S. Inheritance of Resistance to Fusarium Ear Rot in Popcorn. Crop Breed. Appl. Biotechnol. 2018, 18, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Amaral, A.T.D., Jr.; de Poltronieri, T.P.S.; dos Santos, P.H.D.; Vivas, M.; Gerhardt, I.F.S.; Carvalho, B.M.; da Freitas, C.S.; da Silveira, S.F. Reaction of Popcorn Lines (S7) Cultivated in Distinct Phosphorus Levels to Bipolaris Maydis Infection. Summa Phytopathol. 2019, 45, 18–22. [Google Scholar] [CrossRef]
- Santos, J.S.; de Souza, M.V.Y.P.; Amaral, A.T.D., Jr.; de Almeida, R.N.; Saluci, J.C.G.; Mafra, G.S.; de Oliveira, F.T.; Khan, S.; Vivas, J.M.S. Resistance of Popcorn Hybrid (Zea Mays) to Multiple Diseases and Correlation between Leaf Disease Intensity and Agronomic Traits. Aust. J. Crop Sci. 2020, 11, 1800–1809. [Google Scholar] [CrossRef]
- Carlos, M.C.; Vivas, M.; Costa, A.C.; Vasconcelos, L.C.; de Lima, W.L.; de Almeida, R.N.; Valadares, F.V. Resistance to Multiple Leaf Diseases in Popcorn Lines with Potential for Baby Corn Production. Acta Sci. Agron. 2022, 44, e55857. [Google Scholar] [CrossRef]
- Santos, J.S.; Souza, Y.P.; Vivas, M.; Amaral, A.T.D., Jr.; Almeida Filho, J.E.; Mafra, G.S.; Viana, A.P.; Gravina, G.A.; Ferreira, F.R.A.; Saltires Santos, J.; et al. Genetic Merit of Popcorn Lines and Hybrids for Multiple Foliar Diseases and Agronomic Properties. Funct. Plant Breed. J. 2020, 2, 33–47. [Google Scholar] [CrossRef]
- Do Kurosawa, R.N.F.; Amaral, A.T.D., Jr.; Vivas, M.; Almeida, R.N.; Vivas, J.M.S.; Lima, V.J.; da Silveira, S.F. Diallel Analysis for Resistance to Northern Leaf Blight in Popcorn under Contrasting Nitrogen Availability. Agron. J. 2021, 113, 1029–1038. [Google Scholar] [CrossRef]
- Vieira, R.A.; Scapim, C.A.; Moterle, L.M.; Tessmann, D.J.; do Amaral, A.T., Jr.; Gonçalves, L.S.A. The Breeding Possibilities and Genetic Parameters of Maize Resistance to Foliar Diseases. Euphytica 2012, 185, 325–336. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, Z.X.; Wang, F.G.; Xiao, Y.N.; Zhao, J.R.; Zheng, Y.L.; Qiu, F.Z. Mapping of HtNB, a Gene Conferring Non-Lesion Resistance before Heading to Exserohilum Turcicum (Pass.), in a Maize Inbred Line Derived from the Indonesian Variety Bramadi. Genet. Mol. Res. 2012, 11, 2523–2533. [Google Scholar] [CrossRef]
- Hurni, S.; Scheuermann, D.; Krattinger, S.G.; Kessel, B.; Wicker, T.; Herren, G.; Fitze, M.N.; Breen, J.; Presterl, T.; Ouzunova, M.; et al. The Maize Disease Resistance Gene Htn1 against Northern Corn Leaf Blight Encodes a Wall-Associated Receptor-like Kinase. Proc. Natl. Acad. Sci. USA 2015, 112, 8780–8785. [Google Scholar] [CrossRef] [Green Version]
- Galiano-Carneiro, A.L.; Miedaner, T. Genetics of Resistance and Pathogenicity in the Maize/Setosphaeria Turcica Pathosystem and Implications for Breeding. Front. Plant Sci. 2017, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Atanda, S.A.; Olsen, M.; Burgueño, J.; Crossa, J.; Dzidzienyo, D.; Beyene, Y.; Gowda, M.; Dreher, K.; Zhang, X.; Prasanna, B.M.; et al. Maximizing efficiency of genomic selection in CIMMYT’s tropical maize breeding program. Theor. Appl. Genet. 2021, 134, 279–294. [Google Scholar] [CrossRef]
- Abdelsalam, N.R.; Balbaa, M.G.; Osman, H.T.; Ghareeb, R.Y.; Desoky, E.-S.M.; Elshehawi, A.M.; Aljuaid, B.S.; Elnahal, A.S.M. Inheritance of Resistance against Northern Leaf Blight of Maize Using Conventional Breeding Methods. Saudi J. Biol. Sci. 2022, 29, 1747–1759. [Google Scholar] [CrossRef]
- Ma, W.; Gao, X.; Han, T.; Mohammed, M.T.; Yang, J.; Ding, J.; Zhao, W.; Peng, Y.-L.; Bhadauria, V. Molecular Genetics of Anthracnose Resistance in Maize. J. Fungi 2022, 8, 540. [Google Scholar] [CrossRef] [PubMed]
- Pena, G.F.; Amaral, A.T.D., Jr.; Gonçalves, L.S.A.; Vivas, M.; Ribeiro, R.M.; Mafra, G.S.; dos Santos, A.; Scapim, C.A. Comparison of Testers in the Selection of S3 Families Obtained from the UENF-14 Variety of Popcorn. Bragantia 2016, 75, 135–144. [Google Scholar] [CrossRef] [Green Version]
- De Almeida, R.N.; Vivas, M.; dos Santos Junior, D.R.; Saluci, J.C.G.; Carlos, M.C.; Santos, J.S.; Amaral, A.T.D., Jr.; Scapim, C.A. Combining Abilities Analysis for Ear Rot Resistance in Popcorn Hybrids Development. Rev. Ceres 2021, 68, 61–70. [Google Scholar] [CrossRef]
- Amaral, A.T.D., Jr.; Gonçalves, L.S.A.; de Freitas Júnior, S.P.; Candido, L.S.; Vittorazzi, C.; Pena, G.F.; Ribeiro, R.M.; de Silva, T.R.C.; Pereira, M.G.; Scapim, C.A.; et al. UENF 14: A New Popcorn Cultivar. Crop. Breed. Appl. Biotechnol. 2013, 13, 218–220. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, A.G.; Amaral, A.T.D., Jr.; de Lima, V.J.; Leite, J.T.; Scapim, C.A.; Vivas, M. Genetic Gains and Selection Advances Of The Uenf-14 Popcorn Population. Rev. Caatinga 2018, 31, 271–278. [Google Scholar] [CrossRef]
- Guimarães, A.G.; Amaral, A.T.D., Jr.; Pena, G.F.; de Almeida Filho, J.E.; Pereira, M.G.; Santos, P.H.A.D. Genetic Gains in the Popcorn Population Uenf-14: Developing the Ninth Generation of Intrapopulation Recurrent Selection. Rev. Caatinga 2019, 32, 625–633. [Google Scholar] [CrossRef]
- Vittorazzi, C.; Júnior, A.T.A.; Guimarães, A.G.; Silva, F.H.L.; Pena, G.F.; Daher, R.F.; Gerhardt, I.F.S.; Oliveira, G.H.F.; Santos, P.H.A.D.; Souza, Y.P.; et al. Evaluation of Genetic Variability to Form Heterotic Groups in Popcorn. Genet. Mol. Res. 2018, 17, 1–17. [Google Scholar] [CrossRef]
- Mafra, G.S.; Amaral, A.T.D., Jr.; Vivas, M.; dos Santos, J.S.; Silva, F.H.D.L.E.; Guimarães, A.G.; Pena, G.F. The Combining Ability of Popcorn S7 Lines for Puccinia Polysora Resistance Purposes. Bragantia 2018, 77, 519–526. [Google Scholar] [CrossRef]
- Santos, J.S.; Vivas, M.; Amaral, A.T.D., Jr.; Ribeiro, R.M.; Mafra, G.S.; Pena, G.F. Gene Effects from Bipolaris Maydis Incidence and Severity on Popcorn. Rev. Bras. De Ciênc. Agrár.—Braz. J. Agric. Sci. 2019, 14, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Agroceres. Guia Agroceres de Sanidade; Sementes Agroceres: São Paulo, Brazil, 1996; Volume 2, pp. 1–72. [Google Scholar]
- Chester, K.S. Plant Disease Losses: Their Appraisal and Interpretation; Forgotten Books: London, UK, 1950; Volume 1, pp. 1–193. [Google Scholar]
- James, W.C. Manual of Assessment Keys for Plant Diseases; American Phytopathological Society: Saint Paul, MN, USA, 1971; ISBN 0890540810. [Google Scholar]
- Rodriguez, R.N. Statistical Model Building for Large, Complex Data: Five New Directions in SAS/STAT® Software. In Proceedings of the SAS Global Forum Conference, Las Vegas, NV, USA, 18–21 April 2016; Available online: http://support.sas.com/resources/papers/proceedings16/SAS4900-2016.pdf (accessed on 8 July 2022).
- Griffing, B. Concept of General and Specific Combining Ability in Relation to Diallel Crossing Systems. Aust. J. Biol. Sci. 1956, 9, 462–493. [Google Scholar] [CrossRef] [Green Version]
- Cruz, C.D. GENES—A Software Package for Analysis in Experimental Statistics and Quantitative Genetics. Acta Sci. Agron. 2013, 35, 271–276. [Google Scholar] [CrossRef]
- Yan, W.; Rajcan, I. Biplot Analysis of Test Sites and Trait Relations of Soybean in Ontario. Crop. Sci. 2002, 42, 11–20. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: Integrated Development for R. R Core Team, 2018. [Google Scholar]
- Hallauer, A.R.; Carena, M.J.; Miranda Filho, J.B. Quantitative Genetics in Maize Breeding; Springer: New York, NY, USA, 2010; ISBN 978-1-4419-0765-3. [Google Scholar]
- Barreta, D.; Nardino, M.; Konflanz, V.A.; de Pelegrin, A.J.; Ferrari, M.; Szareski, V.J.; Carvalho, I.R.; de Souza, V.Q.; de Oliveira, A.C.; da Maia, L.C. Diallelic Analysis of Endogamic Maize Lines with Emphasis on Agronomic Traits of Tassel in Different Environments. J. Crop. Sci. Biotechnol. 2019, 22, 101–111. [Google Scholar] [CrossRef]
- De Lima, V.J.; do Amaral Junior, A.T.; Kamphorst, S.H.; Pena, G.F.; Leite, J.T.; Schmitt, K.F.M.; Vittorazzi, C.; de Almeida Filho, J.E.; Mora, F. Combining Ability of S3 Progenies for Key Agronomic Traits in Popcorn: Comparison of Testers in Top-Crosses. Genet. Mol. Res. 2016, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.J.B.; Kvitschal, M.V.; Scapim, C.A.; Fracaro, M.; Bignotto, L.S.; Souza Neto, I.L. Análise Dialélica Parcial de Linhagens de Milho-Pipoca. Rev. Bras. Milho Sorgo 2007, 6, 325–337. [Google Scholar] [CrossRef]
- Daros, M.; Amaral, A.T.D., Jr.; Pereira, M.G.; Santos, F.S.; Gabriel, A.P.C.; Scapim, C.A.; de Paiva Freitas, S., Jr.; Silvério, L. Recurrent Selection in Inbred Popcorn Families. Sci. Agric. 2004, 61, 609–614. [Google Scholar] [CrossRef]
- Kamphorst, S.H.; Amaral, A.T.D., Jr.; de Lima, V.J.; Carena, M.J.; Azeredo, V.C.; Mafra, G.S.; Santos, P.H.A.D.; Leite, J.T.; Schmitt, K.F.M.; dos Santos Junior, D.R.; et al. Driving Sustainable Popcorn Breeding for Drought Tolerance in Brazil. Front. Plant Sci. 2021, 12, 1–20. [Google Scholar] [CrossRef]
- Amaral, A.T.D., Jr.; dos Santos, A.; Gerhardt, I.F.S.; Kurosawa, R.N.F.; Moreira, N.F.; Pereira, M.G.; Gravina, G.d.A.; Silva, F.H.d.L. Proposal of a Super Trait for the Optimum Selection of Popcorn Progenies Based on Path Analysis. Genet. Mol. Res. 2016, 15, 1–9. [Google Scholar] [CrossRef]
- Schwantes, I.A.; do Amaral Júnior, A.T.; Almeida Filho, J.E.; Vivas, M.; Silva Cabral, P.D.; Gonçalves Guimarães, A.; Lima e Silva, F.H.; Araújo Diniz Santos, P.H.; Gonzaga Pereira, M.; Pio Viana, A.; et al. Genomic Selection Helps Accelerate Popcorn Population Breeding. Crop Sci. 2020, 60, 1373–1385. [Google Scholar] [CrossRef]
- Simon, G.A.; Scapim, C.A.; Pacheco, C.A.P.; Pinto, R.J.B.; Braccini, A.D.L.E.; Tonet, A. Depressão Por Endogamia Em Populações de Milho-Pipoca. Bragantia 2004, 63, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Gerhardt, I.F.S.; Teixeira do Amaral Junior, A.; Ferreira Pena, G.; Moreira Guimarães, L.J.; de Lima, V.J.; Vivas, M.; Araújo Diniz Santos, P.H.; Alves Ferreira, F.R.; Mendonça Freitas, M.S.; Kamphorst, S.H. Genetic Effects on the Efficiency and Responsiveness to Phosphorus Use in Popcorn as Estimated by Diallel Analysis. PLoS ONE 2019, 14, e0216980. [Google Scholar] [CrossRef]
- Mafra, G.S.; de Almeida Filho, J.E.; do Amaral Junior, A.T.; Maldonado, C.; Kamphorst, S.H.; de Lima, V.J.; dos Santos Junior, D.R.; Leite, J.T.; Santos, P.H.A.D.; de Oliveira Santos, T.; et al. Regional Heritability Mapping of Quantitative Trait Loci Controlling Traits Related to Growth and Productivity in Popcorn (Zea mays L.). Plants 2021, 10, 1845. [Google Scholar] [CrossRef] [PubMed]
- Pinho, R.G.V.; Ramalho, M.A.P.; Silva, H.P.; Resende, I.C.; Pozar, G. Danos Causados Pelas Ferrugens Polissora e Tropical Do Milho. Fitopatol. Bras. 1999, 24, 400–409. [Google Scholar]
- Costa, D.F.; Vieira, B.S.; Lopes, E.A.; Moreira, L.C.B. Application of Fungicides in the Control of Foliar Diseases in Maize Crop. Rev. Bras. Milho Sorgo 2012, 11, 108–115. [Google Scholar]
- Ramirez-Cabral, N.Y.Z.; Kumar, L.; Shabani, F. Global Risk Levels for Corn Rusts (Puccinia Sorghi and Puccinia Polysora) under Climate Change Projections. J. Phytopathol. 2017, 165, 563–574. [Google Scholar] [CrossRef]
- Vieira, R.A.; Scapim, C.A.; Tessmann, D.J.; Hata, F.T. Diallel Analysis of Yield, Popping Expansion, and Southern Rust Resistance in Popcorn Lines. Rev. Ciênc. Agron. 2011, 42, 774–780. [Google Scholar] [CrossRef] [Green Version]
- Akcura, M.; Kokten, K. Variations in Grain Mineral Concentrations of Turkish Wheat Landraces Germplasm. Qual. Assur. Saf. Crops Foods 2017, 9, 153–159. [Google Scholar] [CrossRef]
- Kaplan, M.; Baran, O.; Unlukara, A.; Kale, H.; Arslan, M.; Kara, K.; Beyzi, S.B.; Konca, Y.; Ulas, A. The Effects Of Different Nitrogen Doses and Irrigation Levels on Yield, Nutritive Value, Fermentation and Gas Production of Corn Silage. Turk. J. Field Crops 2016, 21, 100. [Google Scholar] [CrossRef]
- Yang, L.; Fountain, J.C.; Ji, P.; Ni, X.; Chen, S.; Lee, R.D.; Kemerait, R.C.; Guo, B. Deciphering Drought-Induced Metabolic Responses and Regulation in Developing Maize Kernels. Plant Biotechnol. J. 2018, 16, 1616–1628. [Google Scholar] [CrossRef]
| Male Parents | |||||
|---|---|---|---|---|---|
| Genotypes | Type | Obtainer | Reaction to | ||
| E. turcicum | B. maydis | P. polissora | |||
| T1 (PARA 172) | Population | CIMMYT | Resistant | Resistant | Resistant |
| T2 (L270) | Inbred line | UENF | Unknown | Unknown | Unknown |
| T3 (L651) | Inbred line | UENF | Unknown | Unknown | Unknown |
| T4 (P1) | Inbred line | UEM | Intermediary | Susceptible | Intermediary |
| T5 (L70) | Inbred line | UEM | Susceptible | Resistant | Intermediary |
| Female parents | |||||
| L204 | Inbred line | CIMMYT | Unknown | Unknown | Unknown |
| L681 | Inbred line | UENF 14 | Unknown | Unknown | Unknown |
| L682 | Inbred line | UENF 14 | Unknown | Unknown | Unknown |
| L683 | Inbred line | UENF 14 | Unknown | Unknown | Unknown |
| L684 | Inbred line | UENF 14 | Unknown | Unknown | Unknown |
| L685 | Inbred line | UENF 14 | Unknown | Unknown | Unknown |
| L686 | Inbred line | UENF 14 | Unknown | Unknown | Unknown |
| L688 | Inbred line | UENF 14 | Unknown | Unknown | Unknown |
| L689 | Inbred line | UENF 14 | Unknown | Unknown | Unknown |
| L691 | Inbred line | UENF 14 | Unknown | Unknown | Unknown |
| L692 | Inbred line | UENF 14 | Unknown | Unknown | Unknown |
| L693 | Inbred line | UENF 14 | Unknown | Unknown | Unknown |
| L694 | Inbred line | UENF 14 | Unknown | Unknown | Unknown |
| L695 | Inbred line | UENF 14 | Unknown | Unknown | Unknown |
| L696 | Inbred line | UENF 14 | Unknown | Unknown | Unknown |
| SV | DF | MS | ||
|---|---|---|---|---|
| Agronomic Traits | ||||
| GY | PE | |||
| Treatments | 94 | 4,544,342.76 ** | 155.49 ** | |
| Group I vs. Group II | 1 | 129,010,910.71 ** | 60.61 ** | |
| GCA (Group I) | 14 | 2,374,624.59 ** | 136.23 ** | |
| GCA (Group II) | 4 | 6,863,585.00 ** | 2310.54 ** | |
| SCA I × II | 75 | 3,166,109.61 ** | 45.40 ** | |
| Residue | 188 | 311,266.00 | 7.71 | |
| Disease incidence | ||||
| IPp | IBm | IEt | ||
| Treatments | 94 | 425.02 ** | 809.35 ** | 66.16 ** |
| Group I vs. Group II | 1 | 4500.17 ** | 10,505.13 ** | 0.84 ns |
| GCA (Group I) | 14 | 871.43 ** | 1725.05 ** | 149.31 ** |
| GCA (Group II) | 4 | 529.47 * | 3666.27 ** | 272.75 ** |
| SCA I × II | 75 | 281.78 * | 356.78 ** | 40.49 ns |
| Residue | 188 | 184.68 | 133.6 | 31.81 |
| Disease severity | ||||
| SPp | SBm | SEt | ||
| Treatments | 94 | 27.45 ** | 60.72 ns | 19.31 ** |
| Group I vs. Group II | 1 | 1.57 ns | 131.74 ns | 67.64 * |
| GCA (Group I) | 14 | 63.43 ** | 52.09 ns | 20.80 * |
| GCA (Group II) | 4 | 97.07 ** | 17.77 ns | 82.36 ** |
| SCA I × II | 75 | 17.37 ** | 63.68 ns | 15.03 * |
| Residue | 188 | 8.54 | 63.94 | 10.37 |
| Effect of GCA Associated with Group I—Progenies S7 | ||||||
|---|---|---|---|---|---|---|
| S7 | IPp | IBm | IEt | SPp | SBm | SEt |
| L681 | −5.15 | 8.26 | 0.11 | −0.29 | −1.15 | −0.11 |
| L682 | −7.1 | 7.14 | 1.4 | −1.67 | 2.7 | 0.7 |
| L683 | 1.67 | −9.67 | −1.36 | 0.37 | −1.59 | −0.35 |
| L684 | −3.13 | −7.49 | −2.89 | −0.84 | −0.77 | −0.65 |
| L685 | −6.26 | −9.15 | −1.8 | −2.02 | −2.56 | −0.75 |
| L686 | −2.78 | 6.77 | −0.62 | 1.04 | 0.68 | 1.07 |
| L688 | −2.62 | 3.44 | −0.93 | −1.26 | 0.02 | −0.53 |
| L689 | 5.83 | 5.66 | −0.39 | 1.29 | 1.24 | −0.56 |
| L691 | −0.71 | −8.41 | −1.63 | −0.78 | −0.09 | −0.73 |
| L692 | −2.02 | 5.29 | 5.83 | 0.57 | −0.98 | 1.59 |
| L693 | 6.64 | 9 | 1.4 | 0.33 | −0.68 | 0.83 |
| L694 | 8.63 | 7.29 | 4.18 | 1.75 | 1.13 | 1.52 |
| L695 | 10.7 | 1.59 | −1.84 | 1.35 | 1.23 | −0.61 |
| L696 | −5.73 | −13.19 | −0.56 | −2.64 | −0.64 | −0.68 |
| L204 | 2.03 | −6.54 | −0.89 | 2.81 | 1.45 | −0.74 |
| Effect of GCA Associated with Group II—Testers | ||||||
| Testers | IPp | IBm | IEt | SPp | SBm | SEt |
| T1 (PARA172) | −0.14 | 7.40 | −1.28 | −0.89 | −0.5 | −1.03 |
| T2 (L270) | −5.10 | −5.32 | 3.50 | −1.83 | 0.90 | 2.00 |
| T3(L651) | 2.48 | 11.94 | −2.29 | 0.75 | −0.41 | −0.76 |
| T4 (P1) | 0.61 | 4.34 | −0.03 | 1.25 | −0.09 | −0.33 |
| T5 (L70) | 2.14 | −3.57 | 0.09 | 0.72 | 0.1 | 0.12 |
| Cruzamentos | IPp | IBm | IEt | SPp | SBm | SEt |
|---|---|---|---|---|---|---|
| L681 × T1 (1) | −5.81 | 3.17 | −1.58 | −0.53 | 1.65 | 0.07 |
| L682 × T1 (2) | −3.20 | 4.28 | −2.88 | 0.01 | 4.19 | −0.45 |
| L683 × T1 (3) | −4.47 | −2.24 | −0.12 | −0.64 | 0.37 | 0.59 |
| L684 × T1 (4) | 3.84 | −1.09 | −0.25 | 2.52 | 1.00 | 0.45 |
| L685 × T1 (5) | −3.20 | 3.91 | 0.32 | −0.75 | 1.28 | 0.72 |
| L686 × T1 (6) | 8.32 | −8.69 | −0.86 | 0.09 | 4.87 | −1.38 |
| L688 × T1 (7) | 14.82 | −5.35 | −0.55 | 1.94 | −4.29 | 0.33 |
| L689 × T1 (8) | 6.38 | 2.43 | 0.42 | −0.72 | −7.13 | 0.36 |
| L691 × T1 (9) | −0.42 | −1.83 | −1.34 | −0.59 | −5.07 | 0.42 |
| L692 × T1 (10) | −4.10 | −10.54 | 0.86 | 1.94 | 2.59 | −0.85 |
| L693 × T1 (11) | −1.10 | −0.91 | 3.45 | 0.8 | 6.9 | 0.20 |
| L694 × T1 (12) | 0.25 | 4.13 | −3.99 | −2.29 | −4.24 | −1.61 |
| L695 × T1 (13) | 8.17 | −6.83 | 0.36 | 2.83 | −1.62 | 0.30 |
| L696 × T1 (14) | 1.27 | −3.39 | −0.92 | 0.15 | −2.14 | 0.37 |
| L204 × T1 (15) | −7.99 | −5.20 | −2.08 | −4.35 | 5.05 | 0.43 |
| L681 × T2 (16) | 0.82 | 1.09 | 1.80 | 1.52 | −4.47 | 0.65 |
| L682 × T2 (17) | −5.23 | 15.53 | 2.01 | 0.51 | 1.62 | 1.24 |
| L683 × T2 (18) | 10.5 | −2.65 | −1.73 | −1.64 | −1.81 | −2.89 |
| L684 × T2 (19) | −4.69 | 0.16 | −4.87 | 4.3 | −3.35 | −2.3 |
| L685 × T2 (20) | −2.9 | −8.17 | −5.96 | −0.92 | −3.17 | −2.59 |
| L686 × T2 (21) | −4.88 | −0.76 | −4.14 | −0.64 | −2.69 | −3.58 |
| L688 × T2 (22) | −6.71 | −14.1 | −5.33 | 0.83 | 8.53 | −2.59 |
| L689 × T2 (23) | −5.16 | 3.68 | −4.20 | −0.61 | 2.75 | −2.56 |
| L691 × T2 (24) | 1.38 | −2.25 | −6.29 | 0.63 | 7.64 | −2.22 |
| L692 × T2 (25) | −3.8 | 0.72 | 9.25 | −1.34 | −3.25 | 0.51 |
| L693 × T2 (26) | −5.97 | −6.32 | −1.33 | 2.57 | 6.73 | 5.83 |
| L694 × T2 (27) | 21.88 | −7.95 | 10.89 | 1.98 | 3.53 | 4.97 |
| L695 × T2 (28) | 13.14 | −8.91 | −5.92 | 0.72 | −8.85 | −2.51 |
| L696 × T2 (29) | −0.43 | −2.47 | 5.80 | 0.26 | −5.04 | −2.27 |
| L204 × T2 (30) | 5.31 | −10.78 | −0.37 | −3.52 | 2.71 | −2.60 |
| L681 × T3 (31) | −5.09 | −6.18 | −0.58 | 1.16 | −2.16 | −0.47 |
| L682 × T3 (32) | 3.36 | −8.4 | −1.87 | 4.20 | −5.29 | 2.73 |
| L683 × T3 (33) | −8.75 | 5.09 | 2.39 | −1.45 | 0.89 | 0.61 |
| L694 × T3 (34) | 9.39 | 6.23 | 0.92 | 2.27 | −2.92 | 0.08 |
| L685 × T3 (35) | 2.52 | −2.1 | −0.17 | 0.39 | 1.58 | 0.18 |
| L686 × T3 (36) | 5.71 | 5.31 | 0.15 | 0.39 | −2.66 | −1.64 |
| L688 × T3 (37) | 8.88 | −14.69 | 0.46 | −0.09 | 4.62 | 0.18 |
| L689 × T3 (38) | 10.43 | −6.92 | 3.09 | −0.69 | 4.73 | 0.10 |
| L691 × T3 (39) | 3.80 | −2.84 | −0.34 | −0.29 | 3.01 | 0.16 |
| L692 × T3 (40) | 1.62 | 0.12 | −4.63 | 6.69 | −1.32 | −0.89 |
| L693 × T3 (41) | −3.55 | 6.42 | −0.37 | 1.65 | −3.68 | 0.27 |
| L694 × T3 (42) | −0.70 | −17.21 | −2.98 | −0.88 | 11.34 | −1.82 |
| L695 × T3 (43) | 15.56 | −9.51 | −0.13 | 1.19 | −5.42 | 0.03 |
| L696 × T3 (44) | −1.01 | 1.94 | −1.41 | 0.18 | 9.61 | 0.11 |
| L204 × T3 (45) | −2.27 | −4.71 | −1.08 | −4.72 | −6.37 | 0.28 |
| L681 × T4 (46) | −3.06 | −8.58 | −2.84 | −0.18 | 1.3 | 0.71 |
| L682 × T4 (47) | 5.39 | 9.20 | 0.70 | −0.75 | −3.82 | −0.65 |
| L683 × T4 (48) | −10.96 | −7.01 | −0.22 | −0.73 | 3.46 | 1.47 |
| L684 × T4 (49) | 11.42 | −7.84 | −1.34 | −1.01 | 0.71 | 0.47 |
| L685 × T4 (50) | 1.22 | −11.17 | −2.43 | −2.34 | 0.05 | −0.25 |
| L686 × T4 (51) | −0.59 | 2.90 | 4.38 | −0.39 | 3.97 | −1.85 |
| L688 × T4 (52) | −12.59 | −7.10 | 3.03 | −1.70 | −1.47 | −0.48 |
| L689 × T4 (53) | 12.29 | −15.99 | −2.34 | 5.47 | 1.25 | 0.78 |
| L691 × T4 (54) | −1.17 | −16.91 | 0.40 | 0.59 | −2.42 | −0.17 |
| L692 × T4 (55) | 10.15 | 4.39 | −7.06 | −1.59 | 1.80 | −2.49 |
| L693 × T4 (56) | −5.19 | −2.65 | −2.63 | −1.07 | −4.00 | −1.00 |
| L694 × T4 (57) | 6.16 | 2.39 | −0.41 | 1.67 | 0.80 | 4.19 |
| L695 × T4 (58) | 10.76 | −5.24 | −0.89 | 2.91 | 3.48 | −0.4 |
| L696 × T4 (59) | −7.65 | −3.8 | −2.17 | −0.33 | 4.35 | −0.11 |
| L204 × T4 (60) | −7.08 | −3.78 | 2.99 | −1.33 | −7.24 | 0.01 |
| L681 × T5 (61) | −9.75 | 2.67 | 0.05 | 1.75 | 4.11 | −0.9 |
| L682 × T5 (62) | −10.98 | −6.22 | −2.58 | −1.32 | −3.85 | 0.41 |
| L683 × T5 (63) | −8.25 | 0.60 | −2.99 | −0.30 | −1.45 | −0.66 |
| L684 × T5 (64) | −15.11 | 11.74 | 0.05 | −0.48 | −1.60 | −0.80 |
| L685 × T5 (65) | −8.64 | 0.07 | 0.45 | −0.69 | −1.26 | −0.59 |
| L686 × T5 (66) | −5.62 | 0.81 | −2.23 | −0.41 | 9.50 | −1.80 |
| L688 × T5 (67) | 2.54 | −5.85 | −1.92 | 0.50 | 0.72 | −0.93 |
| L689 × T5 (68) | −5.90 | −8.07 | −2.45 | −0.11 | 0.06 | −0.67 |
| L691 × T5 (69) | 10.63 | −4.00 | 0.45 | 0.85 | 0.28 | −0.61 |
| L692 × T5 (70) | 5.45 | −7.70 | 7.66 | −0.22 | 3.28 | 8.34 |
| L693 × T5 (71) | 6.78 | −8.07 | 2.25 | 0.02 | 4.36 | −2.06 |
| L694 × T5 (72) | 17.97 | −6.37 | −7.03 | −0.29 | −5.00 | −2.70 |
| L695 × T5 (73) | 9.23 | −10.67 | −1.01 | 1.50 | −0.76 | −0.46 |
| L696 × T5 (74) | −4.35 | −5.89 | 2.55 | −0.40 | −5.18 | −0.77 |
| L204 × T5 (75) | 11.23 | −2.54 | −0.29 | −2.19 | 1.01 | −0.60 |
| Agronomic Traits Evaluated | |||
|---|---|---|---|
| SV | DF | GY | PE |
| Blocks | 2 | 416.521.028 | 29.326 |
| Hybrids (H) | 74 | 1,374,847.550 ** | 172.859 ** |
| Residue | 148 | 338.768.973 | 6.390 |
| Means | 4.152.489 | 20.983 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos Junior, D.R.; Ribeiro, R.M.; do Amaral Junior, A.T.; Vivas, M.; Saluce, J.C.G.; Leite, J.T.; Bispo, R.B.; de Lima, V.J.; Lamego, D.L.; Xavier, K.B.; et al. Allelic Complementation in Hybrid Superiority of Popcorn to Multiple Foliar Diseases. Agronomy 2022, 12, 3103. https://doi.org/10.3390/agronomy12123103
dos Santos Junior DR, Ribeiro RM, do Amaral Junior AT, Vivas M, Saluce JCG, Leite JT, Bispo RB, de Lima VJ, Lamego DL, Xavier KB, et al. Allelic Complementation in Hybrid Superiority of Popcorn to Multiple Foliar Diseases. Agronomy. 2022; 12(12):3103. https://doi.org/10.3390/agronomy12123103
Chicago/Turabian Styledos Santos Junior, Divino Rosa, Rodrigo Moreira Ribeiro, Antônio Teixeira do Amaral Junior, Marcelo Vivas, Julio Cesar Gadice Saluce, Jhean Torres Leite, Rosimeire Barboza Bispo, Valter Jário de Lima, Danielle Leal Lamego, Kevelin Barbosa Xavier, and et al. 2022. "Allelic Complementation in Hybrid Superiority of Popcorn to Multiple Foliar Diseases" Agronomy 12, no. 12: 3103. https://doi.org/10.3390/agronomy12123103









