Metabolomics Analysis Reveals Potential Mechanisms in Bupleurum L. (Apiaceae) Induced by Three Levels of Nitrogen Fertilization
Abstract
:1. Introduction
2. Results
2.1. Comparative Analysis of Quality Traits Parameters among Different Nitrogen Fertilization Groups
2.2. Total Saikosaponins Content Accumulated in Bupleurum Different Tissues under Three Levels of Nitrogen Fertilization
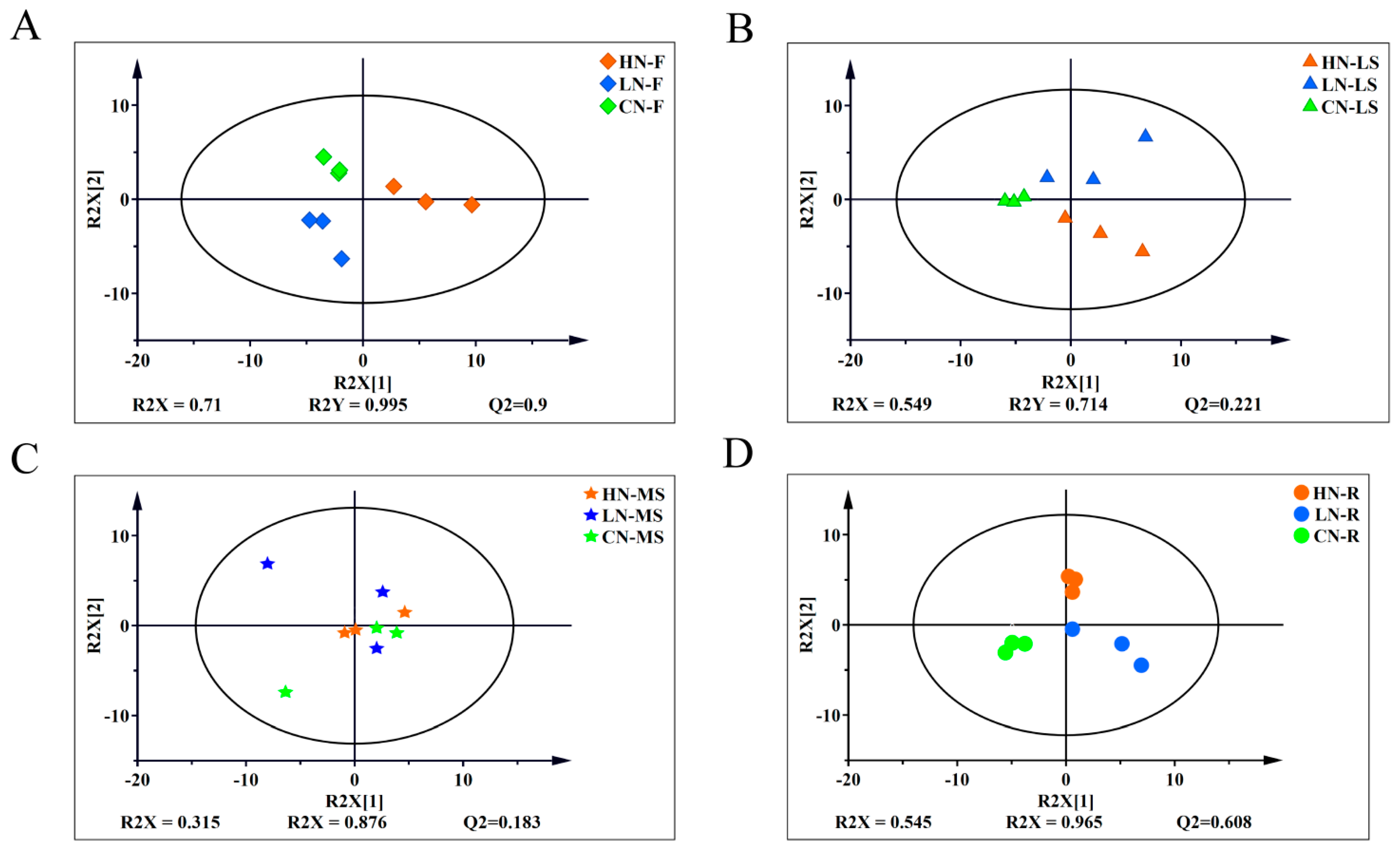
2.3. Overview of the Metabolites Profiles in Response to Three Levels of Nitrogen Fertilization
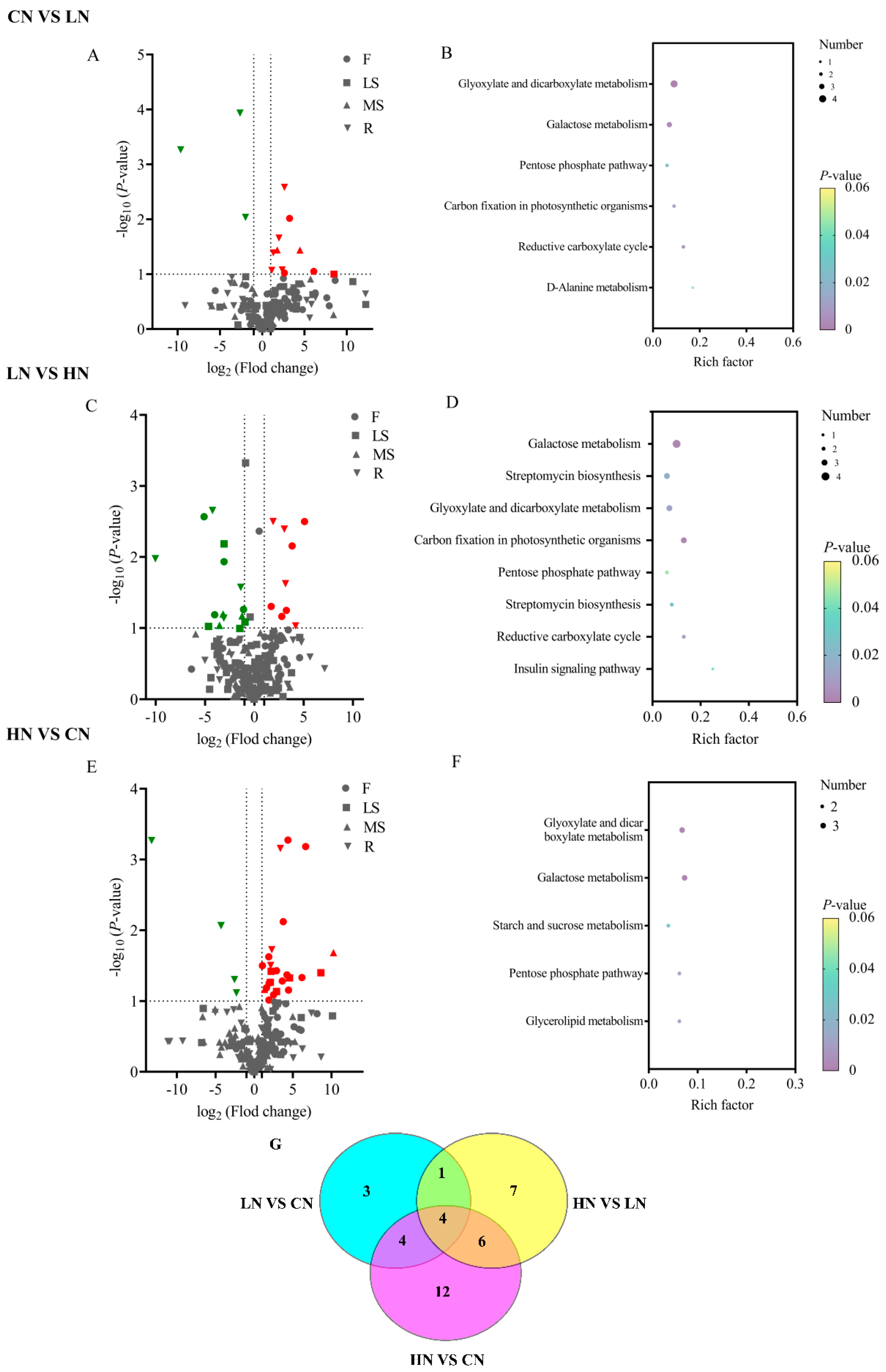
2.4. Identification of Differential Metabolites Relationships under Nitrogen Fertilization
2.5. Metabolite Profiling of Bupleurum under Nitrogen Fertilization in KEGG Enrichment Analysis and Volcanic Map
2.6. Metabolic Network Diagram and Potential Metabolites in Bupleurm under Three Levels of Nitrogen Fertilization
3. Discussion
3.1. Influences of Nitrogen Fertilizer on Bupleurum Quality and Yield
3.2. Metabolic Profiling of Bupleurum Organs under Nitrogen Stress
3.3. KEGG Enrichment Analysis and Comprehensive Metabolic Pathways
4. Conclusions
5. Materials and Methods
5.1. Plant Source and Experimental Design
5.2. Determination of the Saikosaponins Content
5.3. Sample Preparation and Extraction
5.4. GC−MS Analysis
5.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bernstein, N.; Gorelick, J.; Zerahia, R.; Koch, S. Impact of N, P, K, and Humic Acid Supplementation on the Chemical Profile of Medical Cannabis (Cannabis sativa L.). Front. Plant Sci. 2019, 10, 736. [Google Scholar] [CrossRef] [Green Version]
- Mohidin, H.; Hanafi, M.M.; Rafii, Y.M.; Abdullah, S.N.A.; Idris, A.S.; Man, S.; Idris, J.; Sahebi, M. Determination of optimum levels of nitrogen, phosphorus and potassium of oil palm seedlings in solution culture. Bragantia 2015, 74, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Almodares, A.; Taheri, R.; Chung, I.M.; Fathi, M. The effect of nitrogen and potassium fertilizers on growth parameters and carbohydrate contents of sweet sorghum cultivars. J. Environ. Biol. 2008, 29, 849–852. [Google Scholar] [CrossRef]
- Arsova, B.; Kierszniowska, S.; Schulze, W.X. The use of heavy nitrogen in quantitative proteomics experiments in plants. Trends Plant Sci. 2012, 17, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, H.C.; Eriksson, D.; Møller, I.S.; Schjoerring, J.K. Cytosolic glutamine synthetase: A target for improvement of crop nitrogen use efficiency? Trends Plant Sci. 2014, 19, 656–663. [Google Scholar] [CrossRef]
- Sarwar, M. Effects of potassium fertilization on population build up of rice shoots borers (lepidopteron pests) and rice (Oryza sativa L.) yield. J. Cereals Oilseeds 2012, 3, 6–9. [Google Scholar] [CrossRef]
- Du, X.-Q.; Wang, F.-L.; Li, H.; Jing, S.; Yu, M.; Li, J.; Wu, W.-H.; Kudla, J.; Wang, Y. The Transcription Factor MYB59 Regulates K+/NO3− Translocation in the Arabidopsis Response to Low K+ Stress. Plant Cell 2019, 31, 699–714. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant Acidification in Major Chinese Croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X. Biogeochemistry: A plan for efficient use of nitrogen fertilizers. Nature 2017, 543, 322–323. [Google Scholar] [CrossRef] [PubMed]
- Sheh, M.; Watson, M.F.L. Bupleurum L. In Flora of China; Wu, Z.Y., Raven, P.H., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2005; pp. 60–74. [Google Scholar]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of People’s Republic of China; China Medical Sciences and Technology Press: Beijing, China, 2020; p. 293. (In Chinese) [Google Scholar]
- Ashour, M.L.; Wink, M. Genus Bupleurum: A review of its phytochemistry, pharmacology and modes of action. J. Pharm. Pharmacol. 2011, 63, 305–321. [Google Scholar] [CrossRef]
- Ashour, M.L.; El-Readi, M.; Youns, M.; Mulyaningsih, S.; Sporer, F.; Efferth, T.; Wink, M. Chemical composition and biological activity of the essential oil obtained from Bupleurum marginatum (Apiaceae). J. Pharm. Pharmacol. 2009, 61, 1079–1087. [Google Scholar] [CrossRef]
- Law, B.Y.-K.; Mo, J.-F.; Wong, V.K.-W. Autophagic effects of Chaihu (dried roots of Bupleurum Chinense DC or Bupleurum scorzoneraefolium WILD). Chin. Med. 2014, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhang, G.; Gao, Z.; Sui, C.; Ji, H.; Jiang, J.; Xinwei, G.; Wei, J. Transcriptome profiling of Bupleurum chinense DC. root provides new insights into the continuous inflorescence removal induced improvements to root growth and saikosaponin biosynthesis. Ind. Crop. Prod. 2021, 160, 113085. [Google Scholar] [CrossRef]
- Wei, J.H.; Cheng, H.Z.; Li, K.T.; Ding, W.L.; Xu, Z.X.; Chu, Q.L. Plant growth analysis of Bupleurum chinese. Zhong Yao Cai 2003, 26, 617–619. (In Chinese) [Google Scholar]
- Prieto, J.M.; Ogunsina, M.O.; Novak, A.; Joshi, A.; Kokai, J.; Rocha, I.D.C.; Pardo-De-Santayana, M. Comparative Study of the in vitro Bioactivities of Bupleurum rigidum and B. fruticescens. Nat. Prod. Commun. 2012, 7, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, Z.; Jia, L.; Weng, X. Advances in understanding of the biosynthetic pathway and regulatory mechanism of triterpenoid saponins in plants. Sci. Sin. Vitae 2021, 51, 525–555. [Google Scholar] [CrossRef]
- Zhao, Y.-J.; Li, C. Biosynthesis of Plant Triterpenoid Saponins in Microbial Cell Factories. J. Agric. Food Chem. 2018, 66, 12155–12165. [Google Scholar] [CrossRef]
- Thimmappa, R.; Geisler, K.; Louveau, T.; O’Maille, P.; Osbourn, A. Triterpene Biosynthesis in Plants. Annu. Rev. Plant Biol. 2014, 65, 225–257. [Google Scholar] [CrossRef] [PubMed]
- Augustin, J.M.; Kuzina, V.; Andersen, S.B.; Bak, S. Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry 2011, 72, 435–457. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, K.; Iñigo, S.; Kreft, Ł.; Pollier, J.; De Bo, C.; Botzki, A.; Coppens, F.; Bak, S.; Goossens, A. The TriForC database: A comprehensive up-to-date resource of plant triterpene biosynthesis. Nucleic Acids Res. 2018, 46, D586–D594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumner, L.W.; Lei, Z.; Nikolau, B.J.; Saito, K. Modern plant metabolomics: Advanced natural product gene discoveries, improved technologies, and future prospects. Nat. Prod. Rep. 2015, 32, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Jorge, T.; Rodrigues, J.; Caldana, C.; Schmidt, R.; van Dongen, J.; Thomas-Oates, J.; António, C. Mass spectrometry-based plant metabolomics: Metabolite responses to abiotic stress. Mass Spectrom. Rev. 2016, 35, 620–649. [Google Scholar] [CrossRef] [PubMed]
- Omena-Garcia, R.P.; Martins, A.O.; Medeiros, D.B.; Vallarino, J.G.; Ribeiro, D.M.; Fernie, A.R.; Araújo, W.L.; Nunes-Nesi, A. Growth and metabolic adjustments in response to gibberellin deficiency in drought stressed tomato plants. Environ. Exp. Bot. 2019, 159, 95–107. [Google Scholar] [CrossRef]
- Alvarez, S.; Marsh, E.L.; Schroeder, S.G.; Schachtman, D.P. Metabolomic and proteomic changes in the xylem sap of maize under drought. Plant Cell Environ. 2008, 31, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.A.; Behr, J.H.; Erban, A.; Kopka, J.; Zörb, C. Ion-dependent metabolic responses of Vicia faba L. to salt stress. Plant Cell Environ. 2019, 42, 295–309. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, M.R.; Aparicio-Chacón, M.V.; Palma, J.M.; Corpas, F.J. Arsenate disrupts ion balance, sulfur and nitric oxide metabolisms in roots and leaves of pea (Pisum sativum L.) plants. Environ. Exp. Bot. 2019, 161, 143–156. [Google Scholar] [CrossRef]
- Chebrolu, K.K.; Fritschi, F.B.; Ye, S.; Krishnan, H.B.; Smith, J.R.; Gillman, J.D. Impact of heat stress during seed development on soybean seed metabolome. Metabolomics 2016, 12, 28. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, W.; Hashiguchi, A.; Nishimura, M.; Tian, J.; Komatsu, S. Metabolic profiles of flooding-tolerant mechanism in early-stage soybean responding to initial stress. Plant Mol. Biol. 2017, 94, 669–685. [Google Scholar] [CrossRef]
- Machado, F.; Dias, M.C.; de Pinho, P.G.; Araújo, A.M.; Pinto, D.; Silva, A.; Correia, C.; Moutinho-Pereira, J.; Santos, C. Photosynthetic performance and volatile organic compounds profile in Eucalyptus globulus after UVB radiation. Environ. Exp. Bot. 2017, 140, 141–149. [Google Scholar] [CrossRef]
- Takshak, S.; Agrawal, S. Defence strategies adopted by the medicinal plant Coleus forskohlii against supplemental ultraviolet-B radiation: Augmentation of secondary metabolites and antioxidants. Plant Physiol. Biochem. 2015, 97, 124–138. [Google Scholar] [CrossRef]
- Contreras, R.A.; Pizarro, M.; Köhler, H.; Zamora, P.; Zúñiga, G.E. UV-B shock induces photoprotective flavonoids but not antioxidant activity in Antarctic Colobanthus quitensis (Kunth) Bartl. Environ. Exp. Bot. 2019, 159, 179–190. [Google Scholar] [CrossRef]
- Van Heerden, P.D.; Viljoen, M.M.; De Villiers, M.F.; Krüger, G.H. Limitation of photosynthetic carbon metabolism by dark chilling in temperate and tropical soybean genotypes. Plant Physiol. Biochem. 2004, 42, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, R.; Ashraf, M.A.; Kamran, S.; Iqbal, M.; Hussain, I. Menadione sodium bisulphite mediated growth, secondary metabolism, nutrient uptake and oxidative defense in okra (Abelmoschus esculentus Moench) under cadmium stress. J. Hazard. Mater. 2018, 360, 604–614. [Google Scholar] [CrossRef]
- Abozeid, A.; Ying, Z.; Lin, Y.; Liu, J.; Zhang, Z.; Tang, Z. Ethylene Improves Root Syshoots Development under Cadmium Stress by Modulating Superoxide Anion Concentration in Arabidopsis thaliana. Front. Plant Sci. 2017, 8, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, A.; Rushton, P.J.; Rohila, J.S. Metabolomic Profiling of Soybeans (Glycine max L.) Reveals the Importance of Sugar and Nitrogen Metabolism under Drought and Heat Stress. Plants 2017, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Singh, S.; Barnaby, J.; Buyer, J.; Reddy, V.; Sicher, R. Effects of growth temperature and carbon dioxide enrichment on soybean seed components at different stages of development. Plant Physiol. Biochem. 2016, 108, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Ueno, V.A.; Sawaya, A.C.H.F. Influence of environmental factors on the volatile composition of two Brazilian medicinal plants: Mikania laevigata and Mikania glomerata. Metabolomics 2019, 15, 91. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Kim, J.K.; Shrestha, S.; Seo, K.-H.; Lee, Y.-H.; Noh, H.-J.; Kim, G.-S.; Kim, Y.-B.; Kim, S.-Y.; Baek, N.-I. Quality Evaluation of Panax ginseng Roots Using a Rapid Resolution LC-QTOF/MS-Based Metabolomics Approach. Molecules 2013, 18, 14849–14861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Liu, Y.; Wang, Y.; Abozeid, A.; Zu, Y.-G.; Tang, Z.-H. The integration of GC–MS and LC–MS to assay the metabolomics profiling in Panax ginseng and Panax quinquefolius reveals a tissue-and species-specific connectivity of primary metabolites and ginsenosides accumulation. J. Pharm. Biomed. Anal. 2017, 135, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Wang, Y.; Abozeid, A.; Zu, Y.-G.; Zhang, X.-N.; Tang, Z.-H. GC-MS Metabolomic Analysis to Reveal the Metabolites and Biological Pathways Involved in the Developmental Stages and Tissue Response of Panax ginseng. Molecules 2017, 22, 496. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.L.; Palmer, L.; Roessner, U.; Stangoulis, J. Genotypic Variation in the Root and Shoot Metabolite Profiles of Wheat (Triticum aestivum L.) Indicate Sustained, Preferential Carbon Allocation as a Potential Mechanism in Phosphorus Efficiency. Front. Plant Sci. 2019, 10, 995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafsi, C.; Falleh, H.; Saada, M.; Ksouri, R.; Abdelly, C. Potassium deficiency alters growth, photosynthetic performance, secondary metabolites content, and related antioxidant capacity in Sulla carnosa grown under moderate salinity. Plant Physiol. Biochem. 2017, 118, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Ding, C.; Li, W.; Wang, D.; Cui, D. Applications of metabolomics in the research of soybean plant under abiotic stress. Food Chem. 2020, 310, 125914. [Google Scholar] [CrossRef]
- Villate, A.; Nicolas, M.S.; Gallastegi, M.; Aulas, P.-A.; Olivares, M.; Usobiaga, A.; Etxebarria, N.; Aizpurua-Olaizola, O. Review: Metabolomics as a prediction tool for plants performance under environmental stress. Plant Sci. 2021, 303, 110789. [Google Scholar] [CrossRef] [PubMed]
- Sui, C.; Chen, M.; Xu, J.; Wei, J.; Jin, Y.; Xu, Y.; Sun, J.; Gao, K.; Yang, C.; Zhang, Z.; et al. Comparison of root transcriptomes and expressions of genes involved in main medicinal secondary metabolites from Bupleurum chinense and Bupleurum scorzonerifolium, the two Chinese official Radix bupleuri source species. Physiol. Plant. 2014, 153, 230–242. [Google Scholar] [CrossRef]
- Allwood, J.W.; Xu, Y.; Martinez-Martin, P.; Palau, R.; Cowan, A.; Goodacre, R.; Marshall, A.; Stewart, D.; Howarth, C. Rapid UHPLC-MS metabolite profiling and phenotypic assays reveal genotypic impacts of nitrogen supplementation in oats. Metabolomics 2019, 15, 42. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.G.; Wei, F.; Wan, R.; Wang, Y.J.; An, W.; Qin, K. Effect of different fertilizeation levels on N, P, K contents and resistance of “0909” wolfberry leaves. North. Hortic. 2016, 21, 165–170. [Google Scholar] [CrossRef]
- Sun, C.-H.; Yu, J.-Q.; Hu, D.-G. Nitrate: A Crucial Signal during Lateral Roots Development. Front. Plant Sci. 2017, 8, 485. [Google Scholar] [CrossRef]
- Shi, Z.; Wei, F.; Wan, R.; Li, Y.; Wang, Y.; An, W.; Qin, K.; Dai, G.; Cao, Y.; Feng, J. Impact of Nitrogen Fertilizer Levels on Metabolite Profiling of the Lycium barbarum L. Fruit. Molecules 2019, 24, 3879. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Wang, X.; Zhao, C.; Wang, J.; Li, P.; Dou, Y.; Bi, Y. Alternative pathway is involved in the tolerance of highland barley to the low-nitrogen stress by maintaining the cellular redox homeostasis. Plant Cell Rep. 2015, 35, 317–328. [Google Scholar] [CrossRef]
- Jin, J.; Wang, X.; Wang, J.; Li, K.; Wang, S.; Zhang, W.; Zhang, G.; Bi, Y. Glucose-6-Phosphate Dehydrogenase Is Involved in the Tolerance of Soybean Seedlings to Low Nitrogen Stress. Agronomy 2021, 11, 637. [Google Scholar] [CrossRef]
- Zhen, S.; Zhou, J.; Deng, X.; Zhu, G.; Cao, H.; Wang, Z.; Yan, Y. Metabolite profiling of the response to high-nitrogen fertilizer during grain development of bread wheat (Triticum aestivum L.). J. Cereal Sci. 2016, 69, 85–94. [Google Scholar] [CrossRef]
- Lerner, S.; Seghezzo, M.; Molfese, E.; Ponzio, N.; Cogliatti, M.; Rogers, W. N- and S-fertiliser effects on grain composition, industrial quality and end-use in durum wheat. J. Cereal Sci. 2006, 44, 2–11. [Google Scholar] [CrossRef]
- Ottaiano, L.; Di Mola, I.; Cozzolino, E.; El-Nakhel, C.; Rouphael, Y.; Mori, M. Biostimulant Application under Different Nitrogen Fertilization Levels: Assessment of Yield, Leaf Quality, and Nitrogen Metabolism of Tunnel-Grown Lettuce. Agronomy 2021, 11, 1613. [Google Scholar] [CrossRef]
- Liang, Z.; Oh, K.; Wang, Y.; Yi, T.; Chen, H.; Zhao, Z. Cell type-specific qualitative and quantitative analysis of saikosaponins in three Bupleurum species using laser microdissection and liquid chromatography–quadrupole/time of flight-mass spectrometry. J. Pharm. Biomed. Anal. 2014, 97, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Liang, Z.; Han, R.; Dong, J. Growth and saikosaponin production of the medicinal herb Bupleurum chinense DC. under different levels of nitrogen and phosphorus. Ind. Crop. Prod. 2009, 29, 96–101. [Google Scholar] [CrossRef]
- Kim, M.S.; Park, G.C.; Chung, B.J.; Park, T.D.; Kim, H.K.; Kim, H.W.; Park, I.J.; Kim, C.C.; Sim, J.H. Effect of organic fertilizers application on root yield and total saikosaponin contents in Bupleurum falcatum L. Korean J. Plant. Res. 1997, 10, 175–182. [Google Scholar]
- Zhu, Z.B.; Liang, Z.S.; Wei, X.R.; Mao, J.C. Study on nitrogen, phosphorus and organic manure fertilization recommendation of Bupleurum chinense DC. Plant Nutr. Fert. Sci. 2007, 13, 167–170. [Google Scholar] [CrossRef]
- Deep, K.; Prasad, V.M. Effect of Nitrogen, Potash and Boron on Plant Growth, Flowering, Fruit Drop and Fruit Setting of Pomegranate (Punica granatum) cv. Bhagwa. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 2181–2185. [Google Scholar] [CrossRef]
- Cheng, Y.; Min, X.U.; Xiong, R.; Lin, J.; Yun, R.; Ren, T. Effects of different nitrogen treatments on pitaya fruit growth and quality under drip irrigation. J. Trop. Biol. 2020, 11, 25–30. [Google Scholar] [CrossRef]
- Gyuga, P.; Demagante, A.L.; Paulsen, G.M. Photosynthesis and grain growth of wheat under extreme nitrogen nutrition regimes during maturation. J. Plant Nutr. 2002, 25, 1281–1290. [Google Scholar] [CrossRef]
- Qu, H.; Cheng, L.; Chen, J.F.; Lu, X.Y.; Shen, Y.X. Effects of nitrogen on agronomic traits and dry matter yield and their relationship in sweet sorghum. Acta Prataculturae Sin. 2016, 25, 13–25. [Google Scholar] [CrossRef]
- Nagappan, S.; Devendran, S.; Tsai, P.-C.; Jayaraman, H.; Alagarsamy, V.; Pugazhendhi, A.; Ponnusamy, V.K. Metabolomics integrated with transcriptomics and proteomics: Evaluation of systems reaction to nitrogen deficiency stress in microalgae. Process. Biochem. 2019, 91, 1–14. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Naylor, R.; Crews, T.; David, M.B.; Drinkwater, L.E.; Holland, E.; Johnes, P.J.; Katzenberger, J.; Martinelli, L.A.; Matson, P.A.; et al. Nutrient Imbalances in Agricultural Development. Science 2009, 324, 1519–1520. [Google Scholar] [CrossRef]
- Du, X.; Zhang, X.; Kong, L.; Xi, M. Split Application under Reduced Nitrogen Rate Favors High Yield by Altering Endogenous Hormones and C/N Ratio in Sweet Potato. Agronomy 2020, 10, 1243. [Google Scholar] [CrossRef]
- Schlüter, U.; Colmsee, C.; Scholz, U.; Bräutigam, A.; Weber, A.P.; Zellerhoff, N.; Bucher, M.; Fahnenstich, H.; Sonnewald, U. Adaptation of maize source leaf metabolism to stress related disturbances in carbon, nitrogen and phosphorus balance. BMC Genom. 2013, 14, 442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalhais, L.C.; Dennis, P.G.; Fedoseyenko, D.; Hajirezaei, M.-R.; Borriss, R.; von Wirén, N. Erratum: Root exudation of sugars, amino acids, and organic acids by maize as affected by nitrogen, phosphorus, potassium, and iron deficiency. J. Plant Nutr. Soil Sci. 2013, 176, 641. [Google Scholar] [CrossRef]
- Zhou, W.; Liang, X.; Li, K.; Dai, P.; Li, J.; Liang, B.; Sun, C.; Lin, X. Metabolomics analysis reveals potential mechanisms of phenolic accumulation in lettuce (Lactuca sativa L.) induced by low nitrogen supply. Plant Physiol. Biochem. 2021, 158, 446–453. [Google Scholar] [CrossRef]
- Yang, L.; Fountain, J.C.; Ji, P.; Ni, X.; Chen, S.; Lee, R.D.; Kemerait, R.C.; Guo, B. Deciphering drought-induced metabolic responses and regulation in developing maize kernels. Plant Biotechnol. J. 2018, 16, 1616–1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, A.J.; Chapman, W.; Jenkins, G.I.; Graham, I.; Martin, T.; Crozier, A. The effect of nitrogen and phosphorus deficiency on flavonol accumulation in plant tissues. Plant Cell Environ. 2002, 24, 1189–1197. [Google Scholar] [CrossRef]
- Larbat, R.; Olsen, K.M.; Slimestad, R.; Løvdal, T.; Bénard, C.; Verheul, M.; Bourgaud, F.; Robin, C.; Lillo, C. Influence of repeated short-term nitrogen limitations on leaf phenolics metabolism in tomato. Phytochemistry 2012, 77, 119–128. [Google Scholar] [CrossRef]
- Palumbo, M.J.; Putz, F.E.; Talcott, S.T. Nitrogen fertilizer and gender effects on the secondary metabolism of yaupon, a caffeine-containing North American holly. Oecologia 2006, 151, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; An, L.W.; Li, J.C.; Qi, W.T.; Liu, C.L.; Li, L. Advances in bioculture technology of Bupleuri Radix and biosynthesis pathways of saikosaponins. Chin. J. Exp. Tradit. Med. Formulae 2019, 25, 228–234. [Google Scholar] [CrossRef]
- Eom, J.-S.; Choi, S.-B.; Ward, J.; Jeon, J.-S. The mechanism of phloem loading in rice (Oryza sativa). Mol. Cells 2012, 33, 431–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, Y.-L. Sucrose Metabolism: Gateway to Diverse Carbon Use and Sugar Signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Omondi, J.O.; Lazarovitch, N.; Rachmilevitch, S.; Kukew, T.; Yermiyahu, U.; Yasuor, H.; Naftali, L.; Shimon, R.; Uri, Y. Potassium and storage root development: Focusing on photosynthesis, metabolites and soluble carbohydrates in cassava. Physiol. Plant. 2020, 169, 169–178. [Google Scholar] [CrossRef]
- Geiger, D. Plant glucose transporter structure and function. Pflügers Arch. Eur. J. Physiol. 2020, 472, 1111–1128. [Google Scholar] [CrossRef] [PubMed]
- Fetike, J.; Fernie, A.R. Intracellular and cell-to-apoplast compartmentation of carbohydrate metabolism. Trends Plant Sci. 2015, 20, 490–497. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, S.; Liang, Y.; Li, B.; Ma, S.; Wang, Z.; Ma, B.; Li, M. Nitrogen Levels Regulate Sugar Metabolism and Transport in the Shoot Tips of Crabapple Plants. Front. Plant Sci. 2021, 12, 626149. [Google Scholar] [CrossRef]
- Sweetlove, L.J.; Beard, K.F.; Nunes-Nesi, A.; Fernie, A.R.; Ratcliffe, R.G. Not just a circle: Flux modes in the plant TCA cycle. Trends Plant Sci. 2010, 15, 462–470. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Liu, M.; Liu, Y.; Strappe, P.; Sun, H.; Zhou, Z. Comparative non-targeted metabolomic analysis reveals insights into the mechanism of rice yellowing. Food Chem. 2020, 308, 125621. [Google Scholar] [CrossRef]
- Xiao, J.; Gu, C.; He, S.; Zhu, D.; Huang, Y.; Zhou, Q. Widely targeted metabolomics analysis reveals new biomarkers and mechanistic insights on chestnut (Castanea mollissima Bl.) calcification process. Food Res. Int. 2021, 141, 110128. [Google Scholar] [CrossRef] [PubMed]
- Tilsner, J.; Kassner, N.; Struck, C.; Lohaus, G. Amino acid contents and transport in oilseed rape (Brassica napus L.) under different nitrogen conditions. Planta 2005, 221, 328–338. [Google Scholar] [CrossRef]
- Urbanczyk-Wochniak, E.; Fernie, A.R. Metabolic profiling reveals altered nitrogen nutrient regimes have diverse effects on the metabolism of hydroponically-grown tomato (Solanum lycopersicum) plants. J. Exp. Bot. 2005, 56, 309–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goel, P.; Singh, A.K. Abiotic Stresses Downregulate Key Genes Involved in Nitrogen Uptake and Assimilation in Brassica juncea L. PLoS ONE 2015, 10, e0143645. [Google Scholar] [CrossRef]
- Cao, Y.-W.; Qu, R.-J.; Tang, X.-Q.; Sun, L.-Q.; Chen, Q.-Q.; Miao, Y.-J. UPLC-Triple TOF-MS/MS based metabolomics approach to reveal the influence of nitrogen levels on Isatis indigotica seedling leaf. Sci. Hortic. 2020, 266, 109280. [Google Scholar] [CrossRef]
- Zhang, Y.L. The Characteristics of Soil Granularity and its Environmental Significance in Typical Black Soil Area in Northeast China. Master’s Thesis, Harbin Normal University, Harbin, China, 2017. [Google Scholar]
- Guo, J. Study on the Evaluation of Cultivated Land Ecological Security in Lindian Country. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2020. [Google Scholar] [CrossRef]
- Azam, M.; Qadri, R.; Aslam, A.; Khan, I.M.; Khan, A.S.; Anwar, R.; Ghani, A.M.; Ejaz, S.; Hussain, Z.; Iqbal, A.M.; et al. Effects of different combinations of N, P and K at different time interval on vegetative, reproductive, yield and quality traits of mango (Mangifera Indica. L) cv. Dusehri. Braz. J. Biol. 2021, 82, e235612. [Google Scholar] [CrossRef]
- Agbede, T.M. Effect of tillage, biochar, poultry manure and NPK 15-15-15 fertilizer, and their mixture on soil properties, growth and carrot (Daucus carota L.) yield under tropical conditions. Heliyon 2021, 24, e07391. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumari, R.; Agrawal, M.; Agrawal, S. Modification in growth, biomass and yield of radish under supplemental UV-B at different NPK levels. Ecotoxicol. Environ. Saf. 2011, 74, 897–903. [Google Scholar] [CrossRef]




| Classification | Quantity | Metabolites |
|---|---|---|
| Organic acids and derivatives | 28 | 1-Aminocyclopentanecarboxylic acid |
| 5-O-Feruloylquinic acid | ||
| Benzoic acid | ||
| Butanedioic acid | ||
| Caffeic acid | ||
| Chlorogenic acid | ||
| Citric acid | ||
| D-Gluconic acid | ||
| Galactaric acid | ||
| Gallic acid | ||
| Glycolic acid | ||
| Hydroxybenzoic acid | ||
| Isophthalic acid | ||
| Lactic Acid | ||
| Malic acid | ||
| Oxalic acid | ||
| Palmitic Acid | ||
| Phenyllactic acid | ||
| Phosphonic acid | ||
| Phthalic acid | ||
| Pipecolic acid | ||
| Propanedioic acid | ||
| Propenoic acid | ||
| Protocatechoic acid | ||
| Quininic acid | ||
| Succinic acid | ||
| Sulfurous acid | ||
| Trifluorobenzoic acid | ||
| Sugars | 16 | Sucrose |
| Arabinofuranose | ||
| Arabinose | ||
| D-Cellobiose | ||
| D-Fructose | ||
| D-Glucose | ||
| D-Mannose | ||
| D-Xylose | ||
| Erythritol | ||
| Galactoseoxime | ||
| Lactose | ||
| Levoglucosan | ||
| L-Rhamnose | ||
| Maltose | ||
| Sedoheptulose | ||
| α-Mannobiose | ||
| Polyols | 6 | Benzylaminooctanol |
| Benzenediol | ||
| Cuminyl alcohol | ||
| Inositol | ||
| Muco-Inositol | ||
| Ribitol | ||
| Glycosides | 3 | α-Lyxofuranoside |
| α-D-glucopyranoside | ||
| L-Galactopyranoside | ||
| Alkyl | 9 | Cyclohexene |
| Decane | ||
| Disiloxane | ||
| Ether | ||
| Heptane | ||
| Nonane | ||
| Silane | ||
| Trisiloxane | ||
| Undecane | ||
| Phenylpropanoid | 1 | Dihydroxybenzoate |
| Alkaloids | 6 | Isoquinolinium |
| Ethanolamine | ||
| Copper phthalocyanine | ||
| Heptabarbital | ||
| Benzopyran-4-one | ||
| Carbamate | ||
| Amino acid and derivatives | 8 | Alanine |
| Aminobutanoic acid | ||
| Glycine | ||
| L-5-Oxoproline | ||
| L-Proline | ||
| Serine | ||
| Urea | ||
| L-Valine | ||
| Lipids and derivatives | 7 | Glyceric acid |
| Glycerol monostearate | ||
| Stearic acid | ||
| Glycerol | ||
| Ribonic acid | ||
| Adenosine | ||
| 1-Monopalmitin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Li, W.; Zhang, Y.; Guo, Y.; Duan, Z.; Tang, Z.; Abozeid, A. Metabolomics Analysis Reveals Potential Mechanisms in Bupleurum L. (Apiaceae) Induced by Three Levels of Nitrogen Fertilization. Agronomy 2021, 11, 2291. https://doi.org/10.3390/agronomy11112291
Sun J, Li W, Zhang Y, Guo Y, Duan Z, Tang Z, Abozeid A. Metabolomics Analysis Reveals Potential Mechanisms in Bupleurum L. (Apiaceae) Induced by Three Levels of Nitrogen Fertilization. Agronomy. 2021; 11(11):2291. https://doi.org/10.3390/agronomy11112291
Chicago/Turabian StyleSun, Jialin, Weinan Li, Ye Zhang, Yun Guo, Zejia Duan, Zhonghua Tang, and Ann Abozeid. 2021. "Metabolomics Analysis Reveals Potential Mechanisms in Bupleurum L. (Apiaceae) Induced by Three Levels of Nitrogen Fertilization" Agronomy 11, no. 11: 2291. https://doi.org/10.3390/agronomy11112291






