Recent Advances for Flame Retardancy of Textiles Based on Phosphorus Chemistry
Abstract
:1. Introduction
- Lower the developed heat to below that necessary to carry on the combustion process.
- Modify the pyrolysis process to lower the quantity of flammable volatiles developed, favoring, at the same time, the creation the char, i.e., a carbonaceous residue that also limits the heat and mass transfer between the textile material and the flame.
- Isolate the flame from the oxygen/air supply.
- Release flame inhibitors (like chlorinated and brominated compounds) when the textile approaches the ignition temperature.
- Lower the heat flow back to the fabric, hence limiting or preventing further pyrolysis.
- Favor the formation of a char or an intumescent protective layer when the textile interacts with a flame or a heat source.
- 1950–1980: The “golden period” of flame retardant research, involving the appearance of the first patents on FRs based on organophosphorus compounds for cellulosic textiles (i.e., cotton). During this period, inherently FR synthetic fibers bearing aromatic structures were also developed.
- 1980–late 1990s: Very limited advances of the research in FRs were achieved during this period.
- 2000 onward: Several efforts were carried out in the design of char-former flame retardant additives, possibly containing phosphorus-based products. Another goal was the investigation into the possibility of replacing bromine derivatives with other less toxic and efficient products. Furthermore, during this period, nanotechnology was demonstrated to show outstanding potential for conferring flame retardant features to fibers and fabrics, through the use of nanoparticles having different aspect ratios. In particular, the exploitation of both top-down (using preformed nanoparticle suspensions) and bottom-up (exploiting the generation of single nanoparticles or nanoparticle assemblies—even hybrid organic-inorganic structures) was successfully considered [4,5].
- Any new flame retardant should show equivalent or superior ease of application.
- Any new flame retardant should not emit formaldehyde during application or service.
- Any new flame retardant should provide comparable textile service-life features, specifically referring to durability, comfort, tensile properties, outward appearance and aesthetics.
- Any new flame retardant should possess an overall comparable cost-effectiveness to the already existing chemicals and preferably be less costly.
- Any new flame retardant should possess equivalent or even lower toxicity and environmental impact.
- Air permeability of the treated textiles should be maintained, irrespective of the possible high amounts of chemicals needed to provide flame retardant features.
- Any new flame retardant should not cause any alteration in the hue of the dye and/or dyeability of the fibers/fabrics.
2. Assessment of the Fire Behavior of Textiles
3. Phosphorus Chemistry in FRs: An Overview
4. Chemical Phosphorus-Based FRs for Textiles
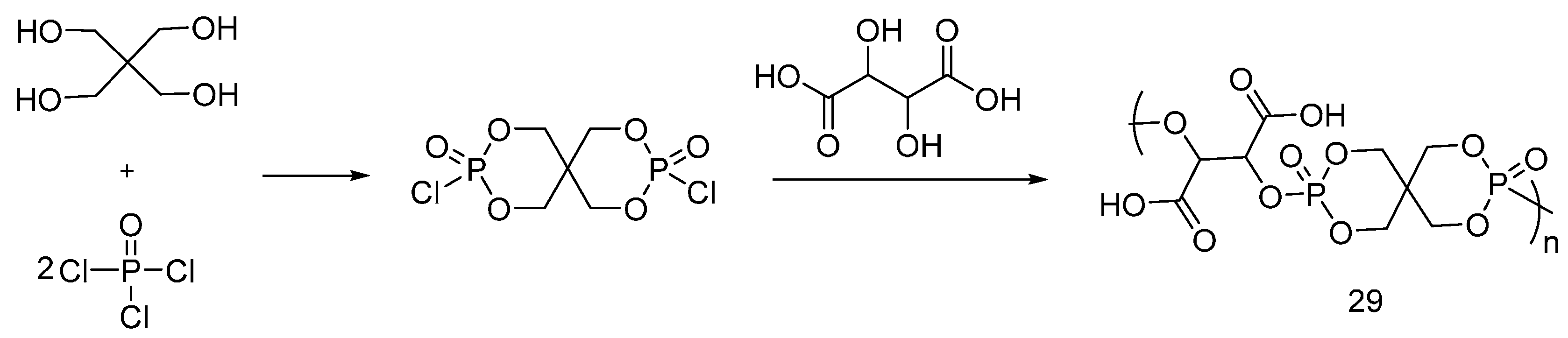
4.1. Dioxaphosphorinane Derivatives for PET Fibers
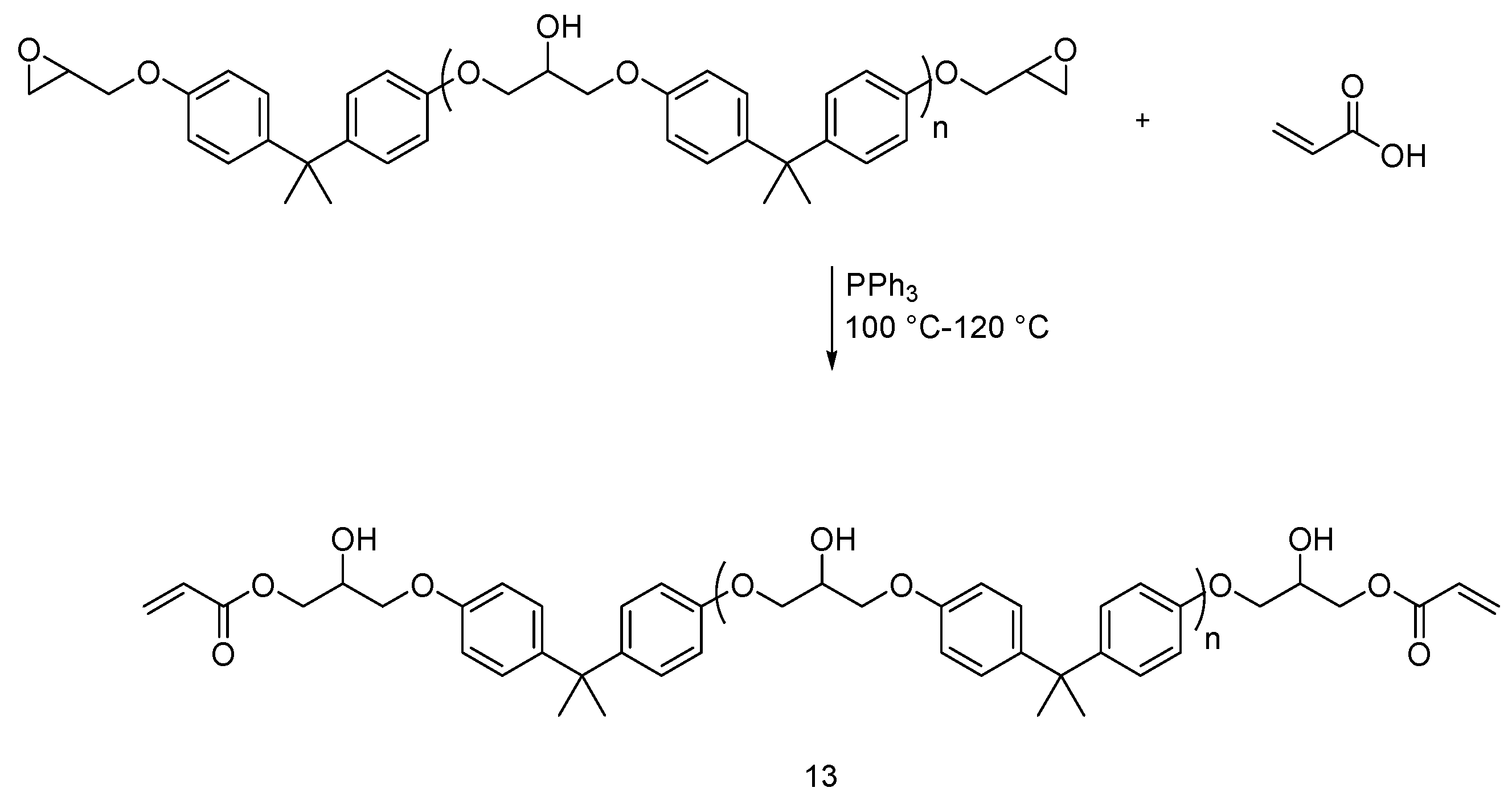
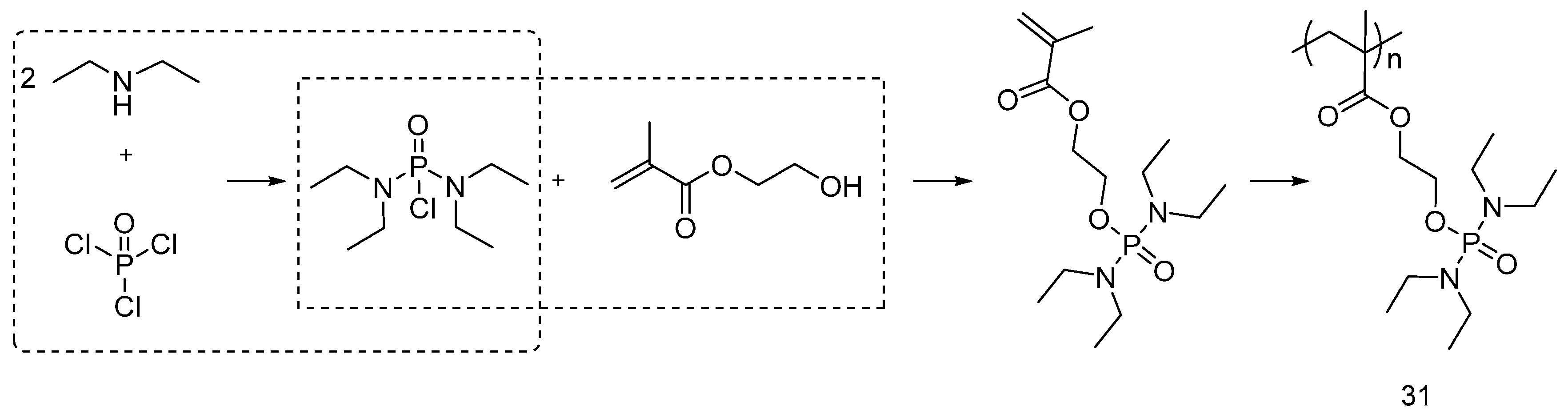
4.2. UV-Curable Flame Retardants Coatings
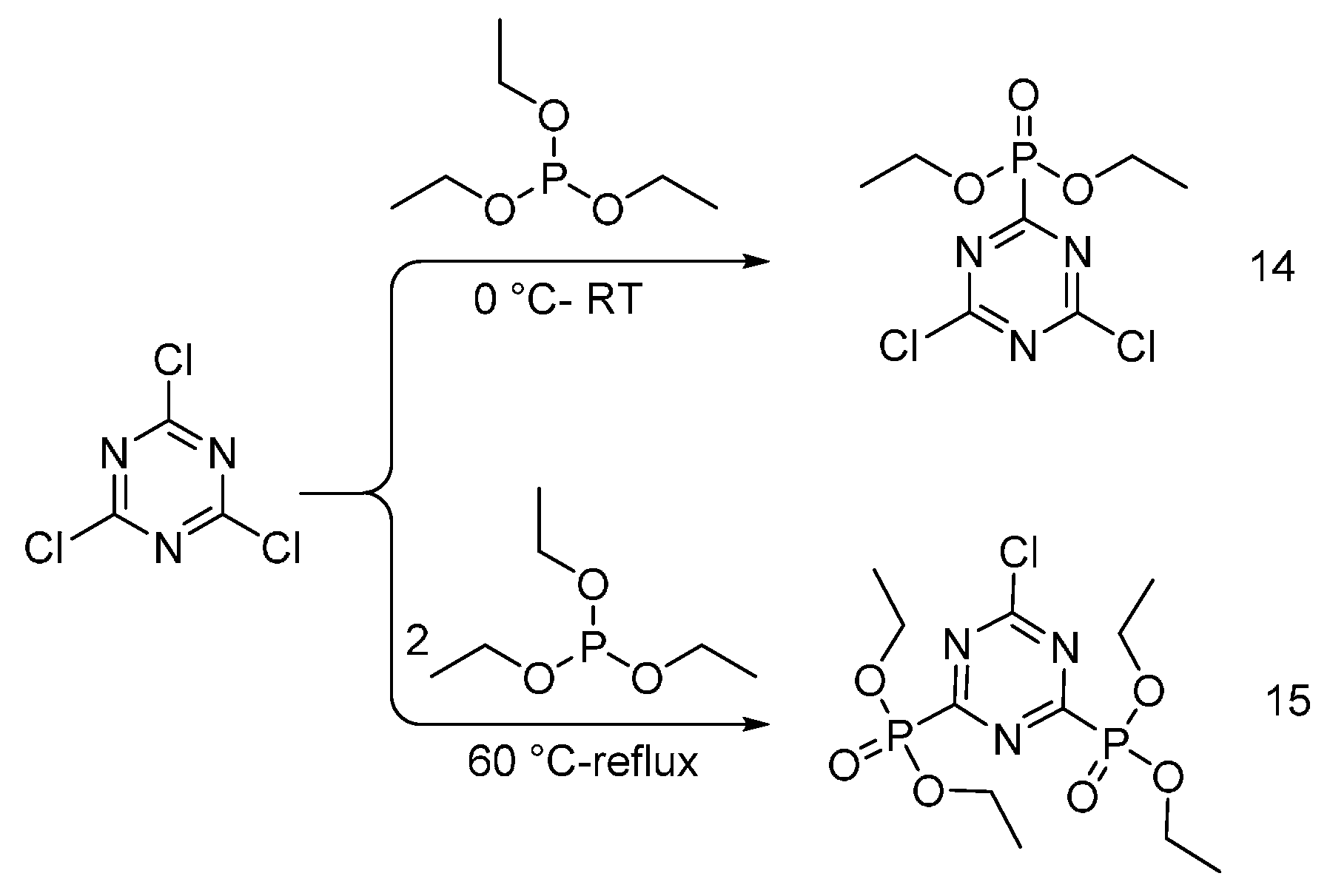
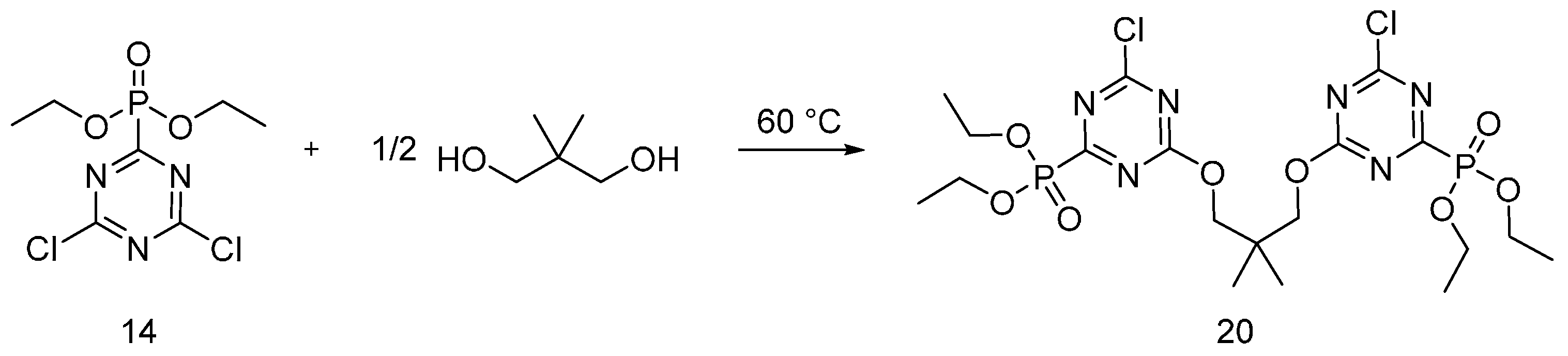
4.3. Triazine-Based Flame Retardants
4.4. Hybrid Organic-Inorganic Flame Retardants
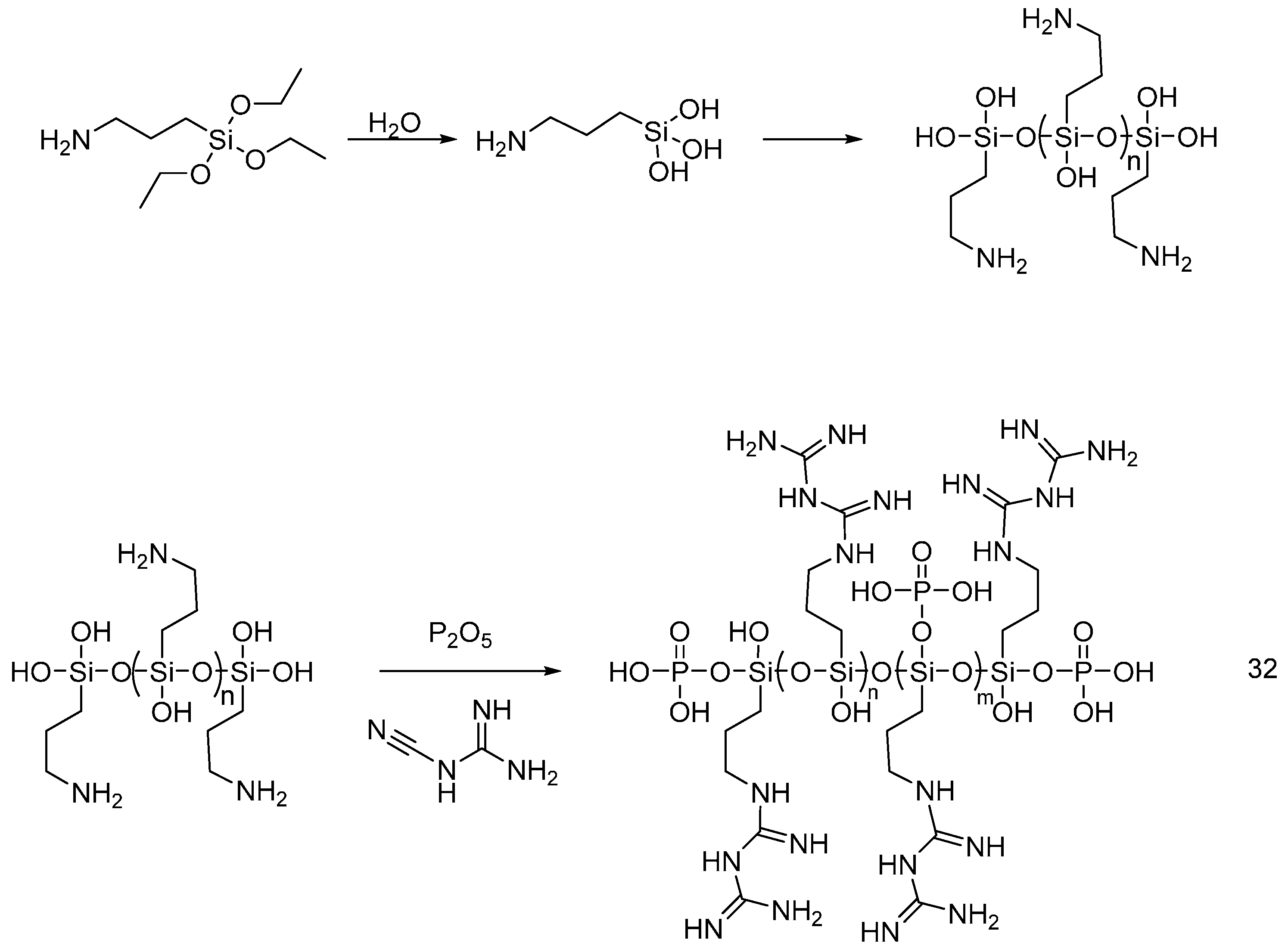
4.5. Flame Retardants as Polymer-Additives
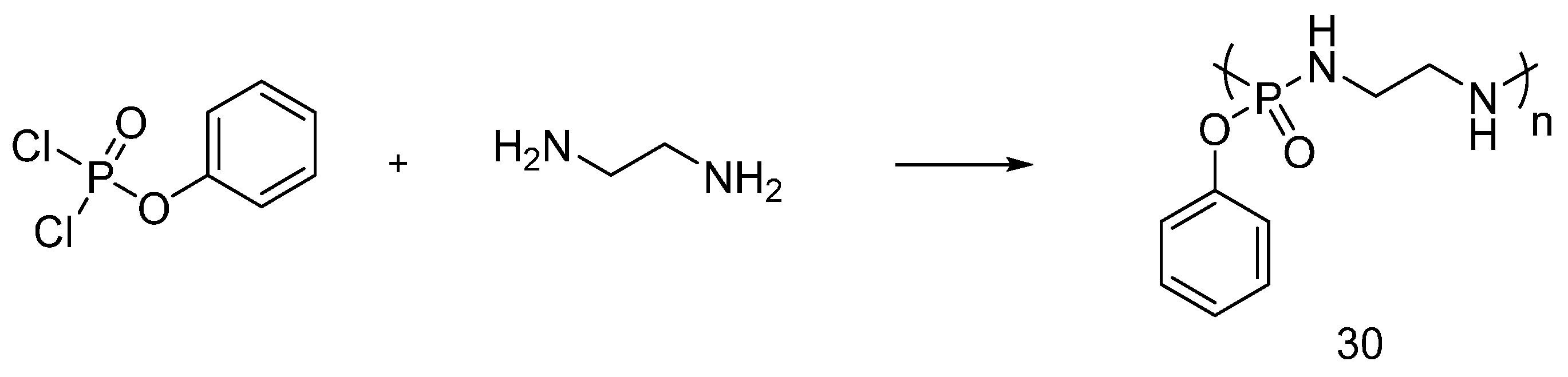
4.6. Phosphoramidate Flame Retardants
4.7. Miscellaneous and Potential Phosphorus-Based Flame Retardants for Textile Applications
5. Phosphorus-Containing Biomacromolecules as Flame Retardants
5.1. Caseins
- αS1-caseins: They contain eight or nine bound phosphate groups/mol and represent the major protein fraction of bovine milk;
- αS2-caseins: They show a structure similar to αS1-casein, as they contains, according to its four differentially phosphorylated isoforms, around 10–13 phosphate groups/mol;
- β-caseins: They are less phosphorylated with respect to αS1- and αS2- counterparts. They include glutamines bearing amino groups and they display a single major phosphorylation site near the N-terminus. Bovine β-caseins can be sourced in a single, fully phosphorylated form containing five phosphate groups/mol;
- κ-caseins: As regard to any other casein, these constituents have the smallest phosphate component. In particular, the phosphorylated regions, present as single sites, are positioned in the C-terminal region of the biomacromolecule.
5.2. Deoxyribonucleic Acid
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Department for Communities and Local Government. Fire statistics: Great Britain April 2013 to March 2014. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/456652/Fire_Statistics_Great_Britain_2013-14___PDF_Version_.pdf (accessed on 15 June 2016).
- Fire safety–Vocabulary. Available online: https://www.iso.org/obp/ui/#iso:std:iso:13943:ed-2:v1:en (accessed on 15 June 2016).
- Horrocks, A.R. Flame retardant challenges for textiles and fibers: New chemistry versus innovatory solutions. Polym. Degrad. Stab. 2011, 96, 377–392. [Google Scholar] [CrossRef]
- Yu, H.D.; Regulacio, M.D.; Ye, E.; Han, M.Y. Chemical routes to top-down nanofabrication. Chem. Soc. Rev. 2013, 42, 6006–6018. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, M.; Sawadaishi, T. Bottom-up strategy of materials fabrication: A new trend in nanotechnology of soft materials. Curr. Opin. Colloid Interface Sci. 2001, 6, 11–16. [Google Scholar] [CrossRef]
- Van der Veen, I.; De Boer, J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 2012, 88, 1119–1153. [Google Scholar] [CrossRef] [PubMed]
- Gaan, S.; Mauclaire, L.; Rupper, P.; Salimova, V.; Tran, T.-T.; Heuberger, M. Thermal degradation of cellulose acetate in presence of bis-phosphoramidates. J. Anal. Appl. Pyrolysis 2011, 90, 33–41. [Google Scholar] [CrossRef]
- Hirsch, C.; Striegl, B.; Mathes, S.; Adlhart, C.; Edelmann, M.; Bono, E.; Gaan, S.; Salmeia, K.A.; Hoelting, L.; Krebs, A.; et al. Multiparameter toxicity assessment of novel DOPO-derived organophosphorus flame retardants. Arch. Toxicol. 2016, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.M., III; Coston, B.B.; Duckett, C.W. Treated Inherently Flame Resistant Polyester Fabrics. U.S. Patent 6,759,127, 6 July 2004. [Google Scholar]
- Sato, M.; Endo, S.; Araki, Y.; Matsuoka, G.; Gyobu, S.; Takeuchi, H. The Flame-retardant Polyester Fiber: Improvement of Hydrolysis Resistance. J. Appl. Polym. Sci. 2000, 78, 1134–1138. [Google Scholar] [CrossRef]
- Horrocks, A.R.; Anand, S.C. Handbook of Technical Textiles. Available online: https://textlnfo.files.wordpress.com/2012/10/handbook_of_technical_textile_.pdf (accessed on 15 June 2016).
- Muthu, S.S. Handbook of Sustainable Apparel Production; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Horrocks, A.R.; Tunc, M.; Price, D. The burning behaviour of textiles and its assessment by oxygen-index methods. Text. Prog. 1989, 18, 1–186. [Google Scholar] [CrossRef]
- Tata, J.; Alongi, J.; Carosio, F.; Frache, A. Optimization of the procedure to burn textile fabrics by cone calorimeter: Part I. Combustion behavior of polyester. Fire Mater. 2011, 35, 397–409. [Google Scholar] [CrossRef]
- Tata, J.; Alongi, J.; Frache, A. Optimization of the procedure to burn textile fabrics by cone calorimeter: Part II. Results and discussion on nanoparticle finished polyester. Fire Mater. 2012, 36, 527–536. [Google Scholar] [CrossRef]
- Lyon, R.E.; Walters, R.N.; Stoliarov, S.I.; Safronava, N. Principles and practices of microscale combustion calorimetry. Available online: http://www.fire.tc.faa.gov/pdf/TC-12-53.pdf (accessed on 15 June 2016).
- Walters, R.N.; Safronava, N.; Lyon, R.E. A microscale combustion calorimeter study of gas phase combustion of polymers. Combust. Flame 2015, 162, 855–863. [Google Scholar] [CrossRef]
- Granzow, A. Flame retardation by phosphorus compounds. Acc. Chem. Res. 1978, 11, 177–183. [Google Scholar] [CrossRef]
- Gaan, S.; Sun, G. Effect of nitrogen additives on thermal decomposition of cotton. J. Anal. Appl. Pyrolysis 2009, 84, 108–115. [Google Scholar] [CrossRef]
- Negrell-Guirao, C.; Boutevin, B.; David, G.; Fruchier, A.; Sonnier, R.; Lopez-Cuesta, J.-M. Synthesis of polyphosphorinanes Part II. Preparation, characterization and thermal properties of novel flame retardants. Polym. Chem. 2011, 2, 236–243. [Google Scholar] [CrossRef]
- Xing, W.; Jie, G.; Song, L.; Hu, S.; Lv, X.; Wang, X.; Hu, Y. Flame retardancy and thermal degradation of cotton textiles based on UV-curable flame retardant coatings. Thermochim. Acta. 2011, 513, 75–82. [Google Scholar] [CrossRef]
- Edwards, B.; Hauser, P.; EI-Shafei, A. Nonflammable cellulosic substrates by application of novel radiation-curable flame retardant monomers derived from cyclotriphosphazene. Cellulose 2015, 22, 275–287. [Google Scholar] [CrossRef]
- Edwards, B.; Rudolf, S.; Hauser, P.; EI-Shafei, A. Preparation, Polymerization, and Performance Evaluation of Halogen-Free Radiation Curable Flame Retardant Monomers for Cotton Substrates. Ind. Eng. Chem. Res. 2015, 54, 577–584. [Google Scholar] [CrossRef]
- Mayer-Gall, T.; Knittel, D.; Gutmann, J.S.; Opwis, K. Permanent Flame Retardant Finishing of Textiles by Allyl-Functionalized Polyphosphazenes. ACS Appl. Mater. Interfaces 2015, 7, 9349–9363. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, Z.; Onen, A.; Gungor, A. Preparation of flame retardant epoxyacrylate-based adhesive formulations for textile applications. J. Adhes. Sci. Technol. 2016, 30, 1765–1778. [Google Scholar] [CrossRef]
- Chang, S.; Condon, B.; Nguyen, T.-M.; Graves, E.; Smith, J. Antiflammable Properties of Capable Phosphorus-Nitrogen-Containing Triazine Derivatives on Cotton. In Fire and Polymers VI: New Advances in Flame Retardant Chemistry and Science; American Chemical Society: Washington, DC, USA, 2012; Volume 1118, pp. 123–137. [Google Scholar]
- Chang, S.C.; Condon, B.; Graves, E.; Uchimiya, M.; Fortier, C.; Easson, M.; Wakelyn, P. Flame retardant properties of triazine phosphonates derivative with cotton fabric. Fibers Polym. 2011, 12, 334–339. [Google Scholar] [CrossRef]
- Easson, M.; Condon, B.; Yoshioka-Tarver, M.; Childress, S.; Slopek, R.; Bland, J.; Thach-Mien, N.; SeChin, C.; Graves, E. Cyanuric Chloride Derivatives for Cotton Textile Treatment-Synthesis, Analysis, and Flammability Testing. AATCC Rev. 2011, 11, 60–66. [Google Scholar]
- Nguyen, T.-M.D.; Chang, S.; Condon, B.; Slopek, R. Synthesis of a novel flame retardant containing phosphorus-nitrogen and its comparison for cotton fabric. Fiber Polym. 2012, 13, 963–970. [Google Scholar] [CrossRef]
- Li, X.; Chen, H.; Wang, W.; Liu, Y.; Zhao, P. Synthesis of a formaldehyde-free phosphorus–nitrogen flame retardant with multiple reactive groups and its application in cotton fabrics. Polym. Degrad. Stab. 2015, 120, 193–202. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Zhao, G.-Z.; Liu, Y.-Q. Preparation and thermal behavior of novel sulfur-nitrogen flame retardant containing triazine ring for cotton fabrics. Asian J. Chem. 2014, 26, 4419–4422. [Google Scholar]
- Zhao, P.; Zhang, M.; Wu, D.; Liu, Y. Synthesis of a novel triazine flame retardant containing sulfur and its application to cotton fabrics. Korean J. Chem. Eng. 2013, 30, 1687–1690. [Google Scholar] [CrossRef]
- Zhao, P.; Li, X.; Zhang, M.; Liu, S.; Liang, W.; Liu, Y. Highly flame-retarding cotton fabrics with a novel phosphorus/nitrogen intumescent flame retardant. Korean J. Chem. Eng. 2014, 31, 1592–1597. [Google Scholar] [CrossRef]
- Alongi, J.; Carosio, F.; Malucelli, G. Current emerging techniques to impart flame retardancy to fabrics: An overview. Polym. Degrad. Stab. 2014, 106, 138–149. [Google Scholar] [CrossRef]
- Alongi, J.; Malucelli, G. State of the art and perspectives on sol-gel derived hybrid architectures for flame retardancy of textiles. J. Mater. Chem. 2012, 22, 21805–21809. [Google Scholar] [CrossRef]
- Hu, S.; Hu, Y.; Song, L.; Lu, H. Effect of modified organic-inorganic hybrid materials on thermal properties of cotton fabrics. J. Therm. Anal. Calorim. 2011, 103, 423–427. [Google Scholar] [CrossRef]
- Vasiljević, J.; Jerman, I.; Jakša, G.; Alongi, J.; Malucelli, G.; Zorko, M.; Tomšič, B.; Simončič, B. Functionalization of cellulose fibres with DOPO-polysilsesquioxane flame retardant nanocoating. Cellulose 2015, 22, 1893–1910. [Google Scholar] [CrossRef]
- Šehić, A.; Tomšič, B.; Jerman, I.; Vasiljević, J.; Medved, J.; Simončič, B. Synergistic inhibitory action of P- and Si-containing precursors in sol–gel coatings on the thermal degradation of polyamide 6. Polym. Degrad. Stab. 2016, 128, 245–252. [Google Scholar] [CrossRef]
- Yang, Z.; Fei, B.; Wang, X.; Xin, J.H. A novel halogen-free and formaldehyde-free flame retardant for cotton fabrics. Fire Mater. 2012, 36, 31–39. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Y.-T.; Wang, X.; Acuña, P.; Zhu, P.; Wagenknecht, U.; Heinrich, G.; Zhang, X.-Q.; Wang, R.; Wang, D.-Y. Effect of phosphorus-containing inorganic–organic hybrid coating on the flammability of cotton fabrics: Synthesis, characterization and flammability. Chem. Eng. J. 2016, 294, 167–175. [Google Scholar] [CrossRef]
- Wang, S.; Sui, X.; Li, Y.; Li, J.; Xu, H.; Zhong, Y.; Zhang, L.; Mao, Z. Durable flame retardant finishing of cotton fabrics with organosilicon functionalized cyclotriphosphazene. Polym. Degrad. Stab. 2016, 128, 22–28. [Google Scholar] [CrossRef]
- Liu, W.; Chen, L.; Wang, Y.-Z. A novel phosphorus-containing flame retardant for the formaldehyde-free treatment of cotton fabrics. Polym. Degrad. Stab. 2012, 97, 2487–2491. [Google Scholar] [CrossRef]
- Dong, Z.; Yu, D.; Wang, W. Preparation of a New Flame Retardant Based on Phosphorus-Nitrogen (P-N) Synergism and Its Application on Cotton Fabrics. Adv. Mater. Res. 2012, 381–384. [Google Scholar] [CrossRef]
- Abou-Okeil, A.; El-Sawy, S.M.; Abdel-Mohdy, F.A. Flame retardant cotton fabrics treated with organophosphorus polymer. Carbohydr. Polym. 2013, 92, 2293–2298. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Lu, Z.; Zhu, P.; Zhang, F.; Zhang, X. Combustion behaviors of cotton fabrics treated by a novel guanidyl- and phosphorus-containing polysiloxane flame retardant. J. Therm. Anal. Calorim. 2015, 119, 349–357. [Google Scholar] [CrossRef]
- Dong, C.; Lu, Z.; Zhang, F. Preparation and properties of cotton fabrics treated with a novel guanidyl- and phosphorus-containing polysiloxane antimicrobial and flame retardant. Mater. Lett. 2015, 142, 35–37. [Google Scholar] [CrossRef]
- Dong, C.; Lu, Z.; Zhang, F.; Zhu, P.; Wang, P.; Che, Y.; Sui, S. Combustion behaviors of cotton fabrics treated by a novel nitrogen- and phosphorus-containing polysiloxane flame retardant. J. Therm. Anal. Calorim. 2016, 123, 535–544. [Google Scholar] [CrossRef]
- Dong, C.; Lu, Z.; Zhang, F.; Zhu, P.; Zhang, L.; Sui, S. Preparation and properties of cotton fabrics treated with a novel polysiloxane water repellent and flame retardant. Mater. Lett. 2015, 152, 276–279. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, B.; Zhang, H.; Zhou, X. Synthesis and application of a sulfur-containing phosphoric amide flame retardant for nylon fabric. Fire Mater. 2016. [Google Scholar] [CrossRef]
- Gaan, S.; Viktoriya, S.; Ottinger, S.; Heuberger, M.; Ritter, A. Phosphoramidate Flame Retardants. PCT Patent WO 2009,1530,34 A1, 23 December 2009. [Google Scholar]
- Nguyen, T.-M.; Chang, S.; Condon, B.; Slopek, R.; Graves, E.; Yoshioka-Tarver, M. Structural Effect of Phosphoramidate Derivatives on the Thermal and Flame Retardant Behaviors of Treated Cotton Cellulose. Ind. Eng. Chem. Res. 2013, 52, 4715–4724. [Google Scholar] [CrossRef]
- Nguyen, T.-M.; Chang, S.; Condon, B.; Smith, J. Fire Self-Extinguishing Cotton Fabric: Development of Piperazine Derivatives Containing Phosphorous-Sulfur-Nitrogen and Their Flame Retardant and Thermal Behaviors. Mater. Sci. Appl. 2014, 5, 789–802. [Google Scholar] [CrossRef]
- Nguyen, T.-M.; Chang, S.; Condon, B. The comparison of differences in flammability and thermal degradation between cotton fabrics treated with phosphoramidate derivatives. Polym. Adv. Technol. 2014, 25, 665–672. [Google Scholar] [CrossRef]
- Engel, R. (Ed.) Handbook of Organophosphorus Chemistry; CRC Press: Boca Raton, FL, USA, 1992.
- Nguyen, T.-M.D.; Chang, S.; Condon, B.; Uchimiya, M.; Fortier, C. Development of an environmentally friendly halogen-free phosphorus–nitrogen bond flame retardant for cotton fabrics. Polym. Adv. Technol. 2012, 23, 1555–1563. [Google Scholar] [CrossRef]
- Jiang, W.; Jin, F.-L.; Park, S.-J. Synthesis of a novel phosphorus-nitrogen-containing intumescent flame retardant and its application to fabrics. J. Ind. Eng. Chem. 2015, 27, 40–43. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Javeri, N.; Shekarriz, S. Flame retardant cotton fibers produced using novel synthesized halogen-free phosphoramide nanoparticles. Carbohydr. Polym. 2015, 118, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Kaur, I. Gamma-induced polymerization and grafting of a novel phosphorus-, nitrogen-, and sulfur-containing monomer on cotton fabric to impart flame retardancy. J. Appl. Polym. Sci. 2012, 125, 1506–1512. [Google Scholar] [CrossRef]
- Wang, L.-H.; Ren, Y.-L.; Wang, X.-L.; Zhao, J.-Y.; Zhang, Y.; Zeng, Q.; Gu, Y.-T. Fire retardant viscose fiber fabric produced by graft polymerization of phosphorus and nitrogen-containing monomer. Cellulose 2016, 23, 2689–2700. [Google Scholar] [CrossRef]
- Alongi, J.; Colleoni, C.; Rosace, G.; Malucelli, G. Thermal and fire stability of cotton fabrics coated with hybrid phosphorus-doped silica films. J. Therm. Anal. Calorim. 2012, 110, 1207–1216. [Google Scholar] [CrossRef]
- Zhou, T.; He, X.; Guo, C.; Yu, J.; Lu, D.; Yang, Q. Synthesis of a novel flame retardant phosphorus/nitrogen/siloxane and its application on cotton fabrics. Text. Res. J. 2015, 85, 701–708. [Google Scholar] [CrossRef]
- Zheng, D.; Zhou, J.; Zhong, L.; Zhang, F.; Zhang, G. A novel durable and high-phosphorous-containing flame retardant for cotton fabrics. Cellulose 2016, 23, 2211–2220. [Google Scholar] [CrossRef]
- Gao, W.-W.; Zhang, G.-X.; Zhang, F.-X. Enhancement of flame retardancy of cotton fabrics by grafting a novel organic phosphorus-based flame retardant. Cellulose 2015, 22, 2787–2796. [Google Scholar] [CrossRef]
- Butnaru, I.; Fernandez-Ronco, M.P.; Czech-Polak, J.; Heneczkowski, M.; Bruma, M.; Gaan, S. Effect of meltable triazine-dopo additive on rheological, mechanical, and flammability properties of PA6. Polymers 2015, 7, 1541–1563. [Google Scholar] [CrossRef]
- Stelzig, T.; Bommer, L.; Gaan, S.; Buczko, A. Dopo-Based Hybrid Flame Retardants. PCT Patent WO 2015,1401,05 A1, 24 September 2015. [Google Scholar]
- Alongi, J.; Bosco, F.; Carosio, F.; Di Blasio, A.; Malucelli, G. A new era for flame retardant materials? Mater. Today 2014, 17, 152–153. [Google Scholar] [CrossRef]
- Malucelli, G.; Bosco, F.; Alongi, J.; Carosio, F.; Di Blasio, A.; Mollea, C.; Cuttica, F.; Casale, A. Biomacromolecules as novel green flame retardant systems for textiles: An overview. RSC Adv. 2014, 4, 46024–46039. [Google Scholar] [CrossRef]
- Malucelli, G.; Carosio, F.; Alongi, J.; Fina, A.; Frache, A.; Camino, G. Materials Engineering for Surface-Confined Flame Retardancy. Mater. Sci. Eng. R Rep. 2014, 84, 1–20. [Google Scholar] [CrossRef]
- Malucelli, G. Layer-by-Layer nanostructured assemblies for the fire protection of fabrics. Mater. Lett. 2016, 166, 339–342. [Google Scholar] [CrossRef]
- Wang, L.; Yoshida, J.; Ogata, N. Self-Assembled Supramolecular Films Derived from Marine Deoxyribonucleic Acid (DNA)-Cationic Surfactant Complexes: Large-Scale Preparation and Optical and Thermal Properties. Chem. Mater. 2001, 13, 1273–1281. [Google Scholar] [CrossRef]
- Alongi, J.; Carletto, R.A.; Bosco, F.; Carosio, F.; Di Blasio, A.; Cuttica, F.; Antonucci, V.; Giordano, M.; Malucelli, G. Caseins and hydrophobins as novel green flame retardants for cotton fabrics. Polym. Degrad. Stab. 2014, 99, 111–117. [Google Scholar] [CrossRef]
- Carosio, F.; Di Blasio, A.; Cuttica, F.; Alongi, J.; Malucelli, G. Flame retardancy of polyester and polyester-cotton blends treated with caseins. Ind. Eng. Chem. Res. 2014, 53, 3917–3923. [Google Scholar] [CrossRef]
- Alongi, J.; Carletto, R.A.; Di Blasio, A.; Carosio, F.; Bosco, F.; Malucelli, G. DNA: A novel, green, natural flame retardant and suppressant for cotton. J. Mater. Chem. A 2013, 1, 4779–4785. [Google Scholar] [CrossRef]
- Alongi, J.; Carletto, R.A.; Di Blasio, A.; Cuttica, F.; Carosio, F.; Bosco, F.; Malucelli, G. Intrinsic intumescent-like flame retardant properties of DNA-treated cotton fabrics. Carbohydr. Polym. 2013, 96, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Bosco, F.; Casale, A.; Mollea, C.; Terlizzi, M.E.; Gribaudo, G.; Alongi, J.; Malucelli, G. DNA coatings on cotton fabrics: Effect of molecular size and pH on flame retardancy. Surf. Coat. Technol. 2015, 272, 86–95. [Google Scholar] [CrossRef]
- Carosio, F.; Di Blasio, A.; Alongi, J.; Malucelli, G. Green DNA-based flame retardant coatings assembled through Layer by Layer. Polymer 2013, 54, 5148–5153. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Yuan, M.; Guo, R. Preparation and characterization of casein-stabilized gold nanoparticles for catalytic applications. Colloids Surf. A 2013, 417, 18–25. [Google Scholar] [CrossRef]
- Alongi, J.; Malucelli, G. Thermal degradation of cellulose and cellulosic substrates. In Reactions and Mechanisms in Thermal Analysis of Advanced Materials; Tiwari, A., Raj, B., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2015; Volume 14, pp. 301–332. [Google Scholar]
- Sönmezoğlu, S.; Sönmezoğlu, Ö.A. Optical and Dielectric Properties of Double Helix DNA Thin Films. Mater. Sci. Eng. C 2011, 31, 1619–1624. [Google Scholar] [CrossRef]
- Sönmezoğlu, S.; Sönmezoğlu, Ö.A.; Çankaya, G.; Yıldırım, A.; Serin, N. Electrical characteristics of DNA-based metal-insulator-semiconductor structures. J. Appl. Phys. 2010, 107, 124518. [Google Scholar] [CrossRef]
- Alongi, J.; Cuttica, F.; Di Blasio, A.; Carosio, F.; Malucelli, G. Intumescent features of nucleic acids and proteins. Thermochim. Acta 2014, 591, 31–39. [Google Scholar] [CrossRef]
- Alongi, J.; Milnes, J.; Malucelli, G.; Bourbigot, S.; Kandola, B. Thermal degradation of DNA-treated cotton fabrics under different heating conditions. J. Anal. Appl. Pyrol. 2014, 18, 212–221. [Google Scholar] [CrossRef]
- Alongi, J.; Di Blasio, A.; Milnes, J.; Malucelli, G.; Bourbigot, S.; Camino, G. Thermal degradation of DNA, an all-in-one natural intumescent flame retardant. Polym. Degrad. Stab. 2015, 113, 110–118. [Google Scholar] [CrossRef]


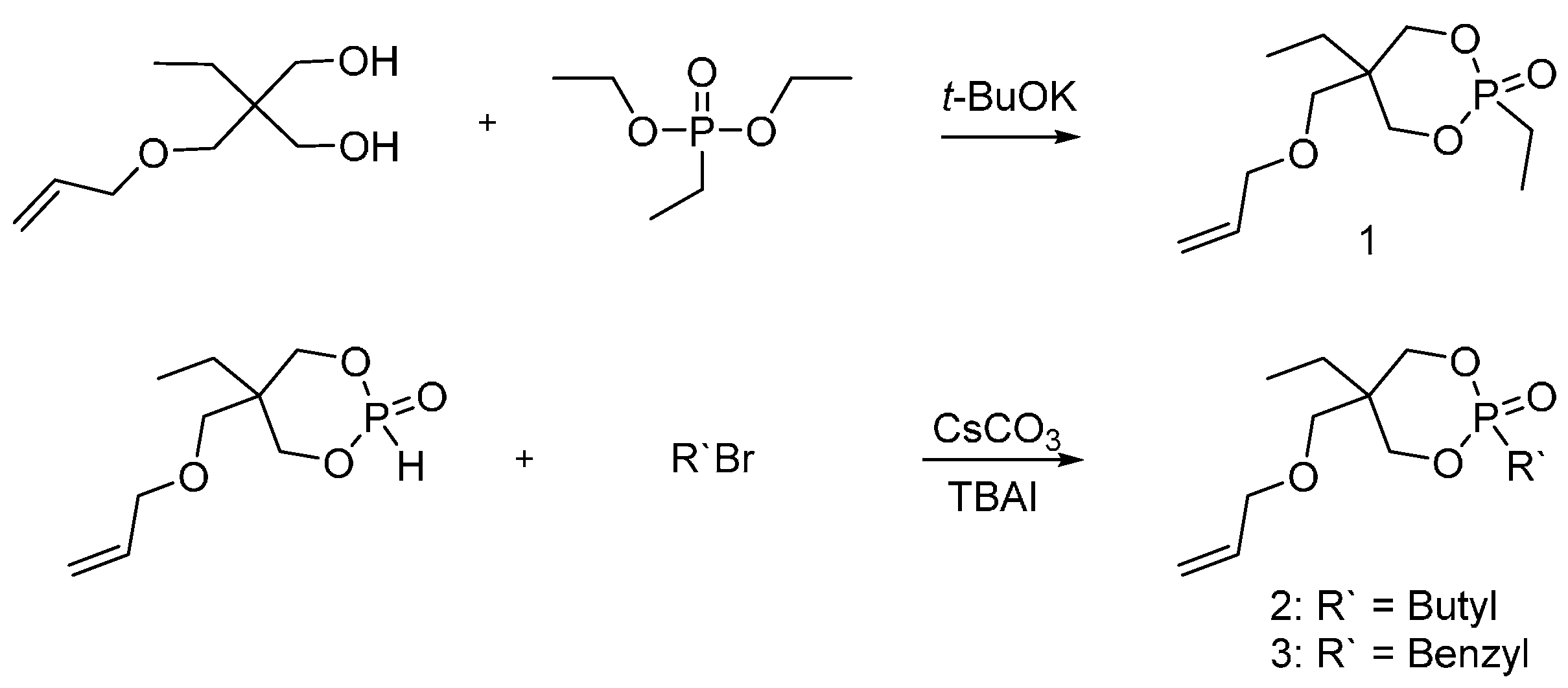

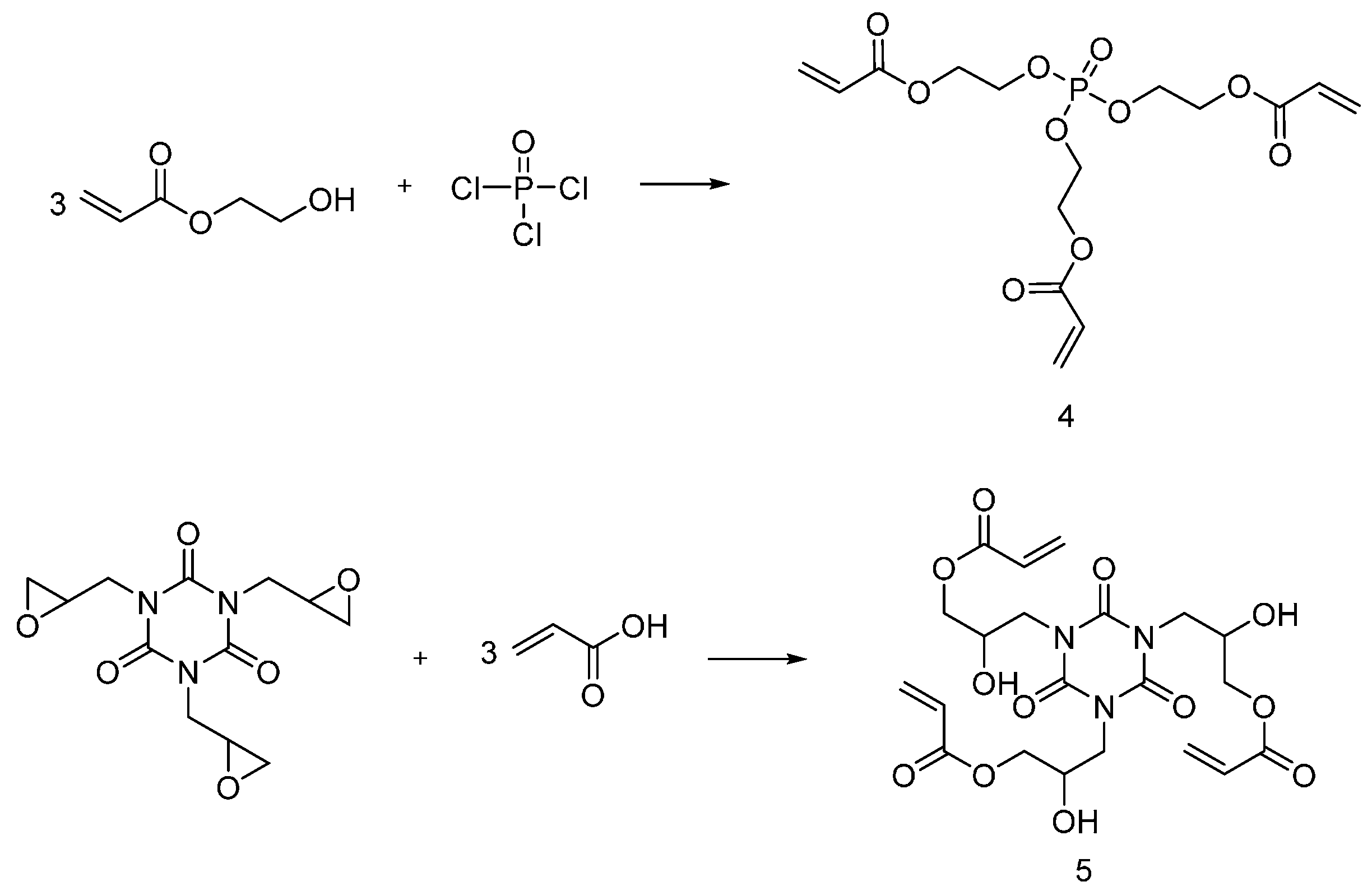
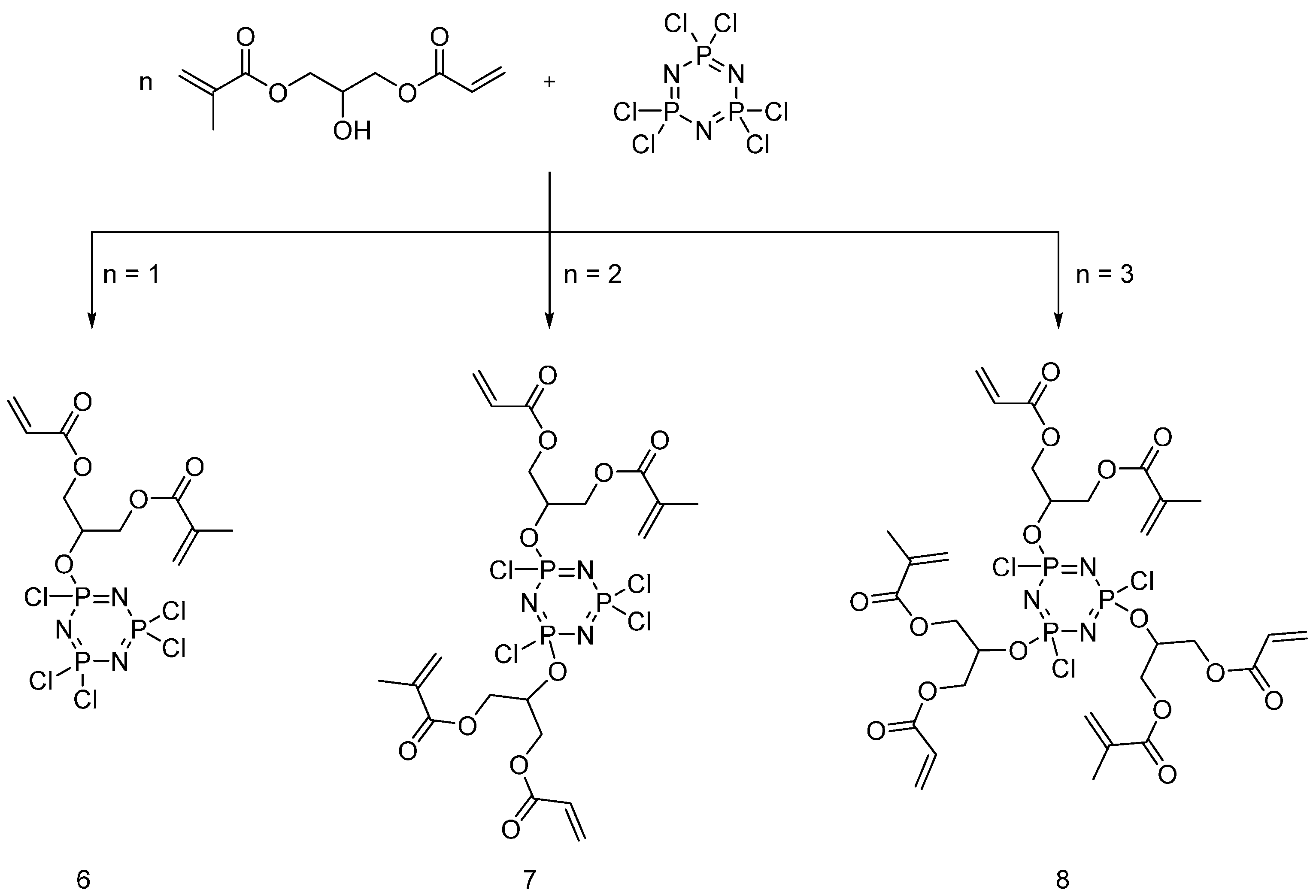
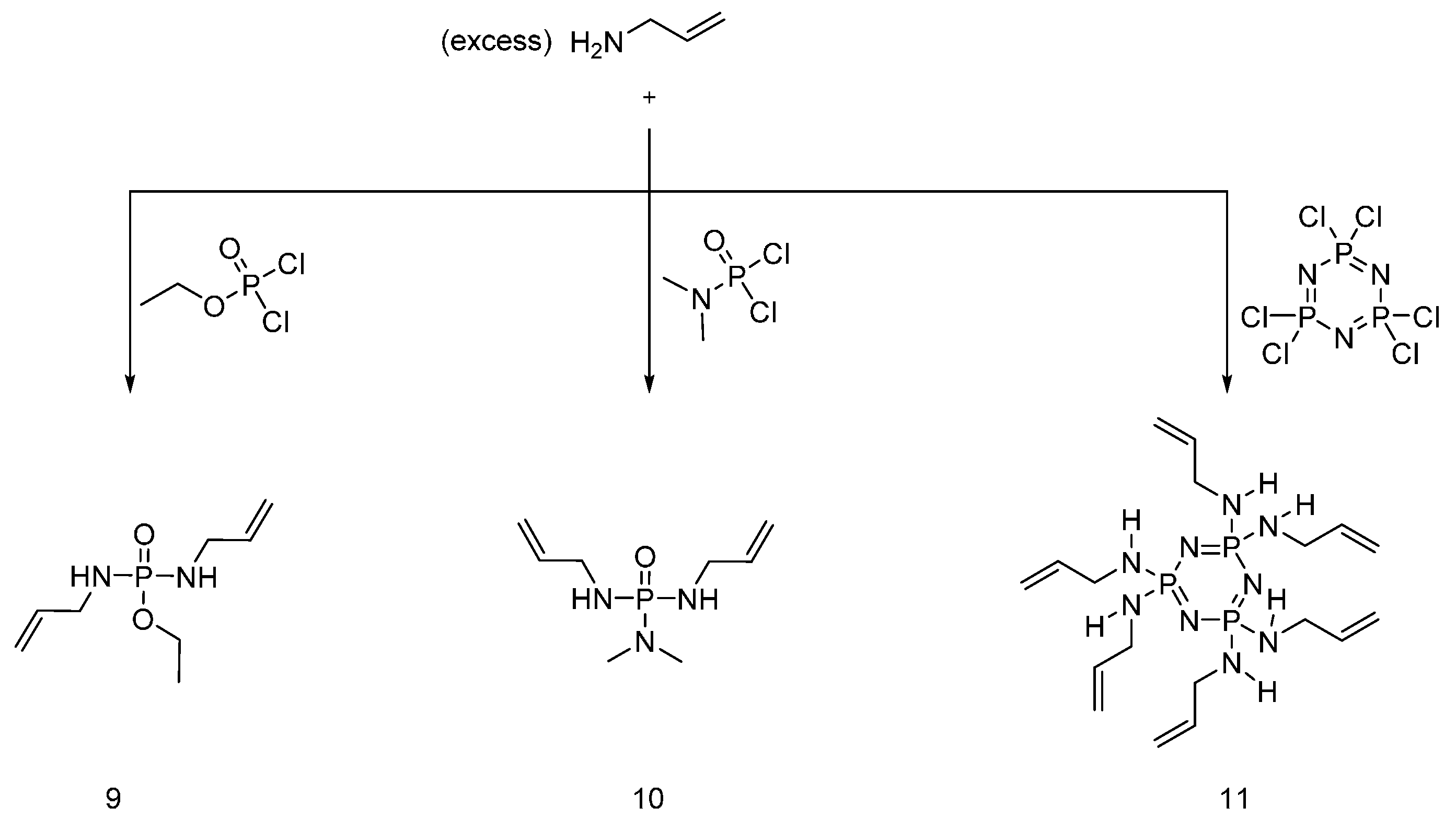
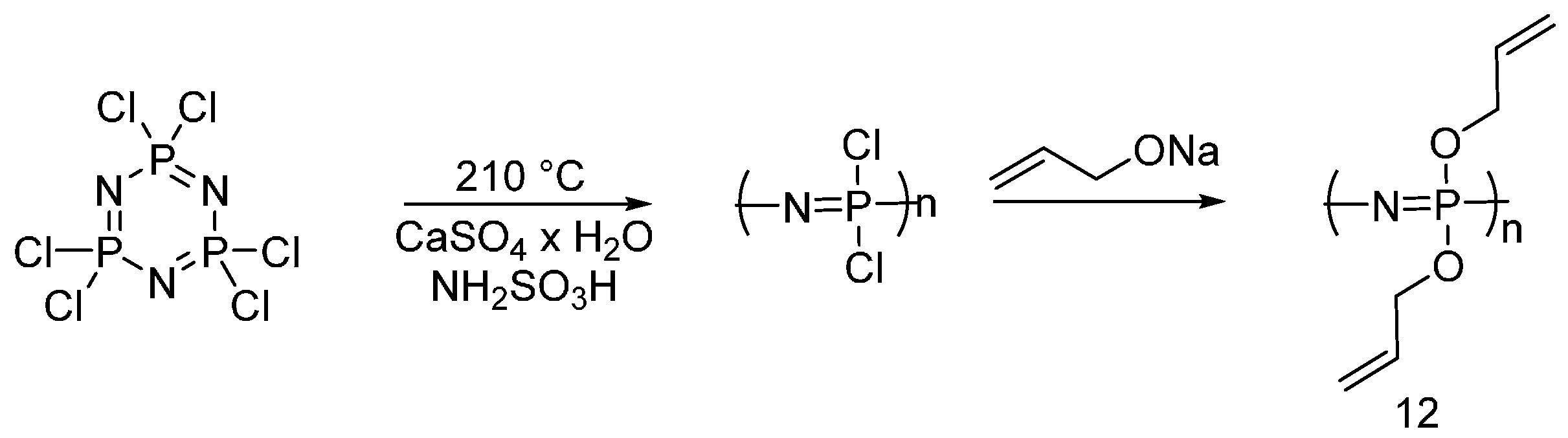

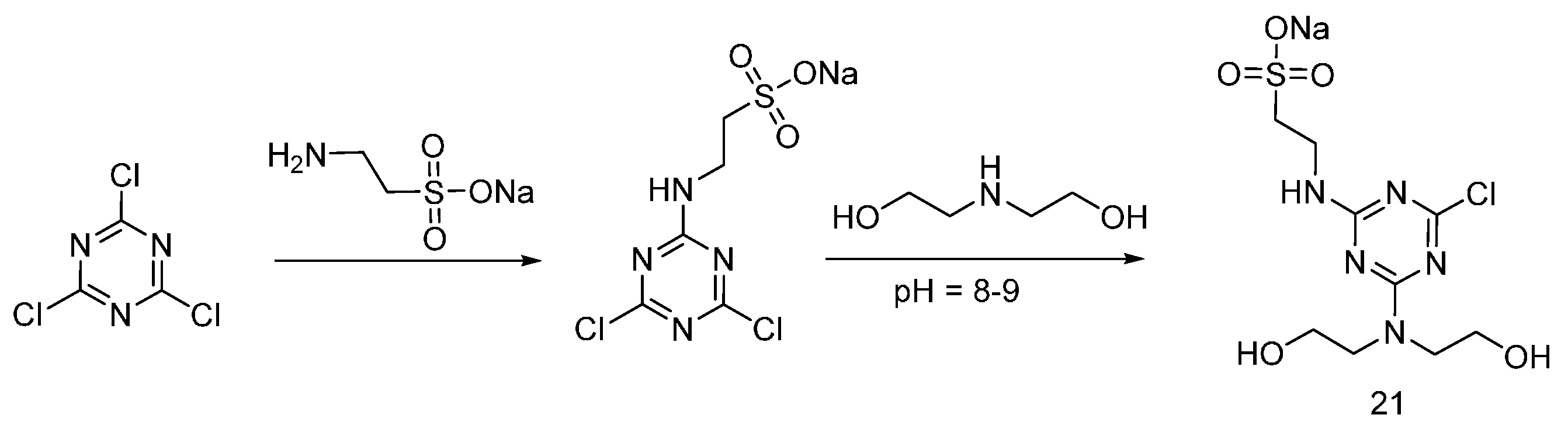

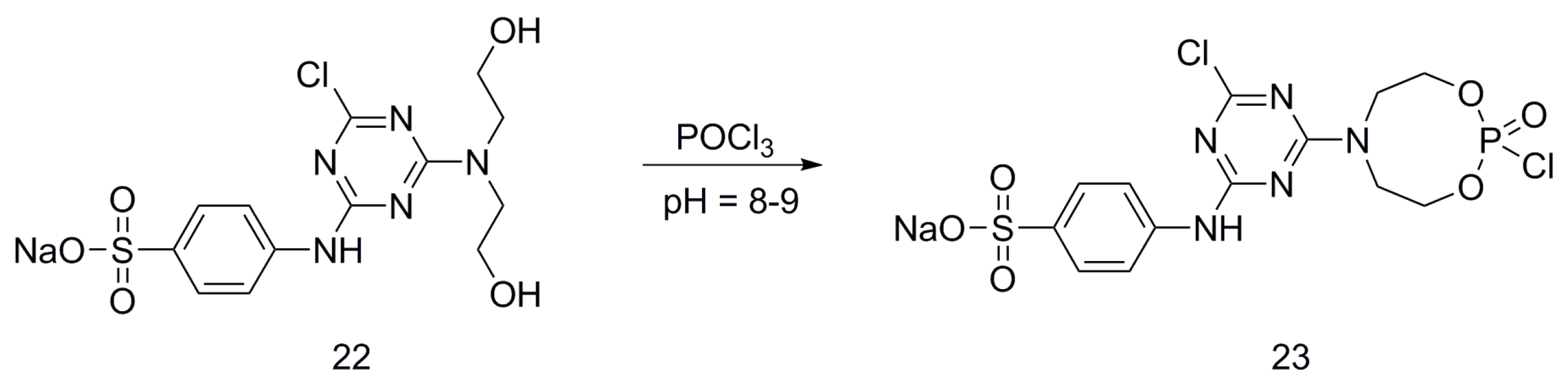

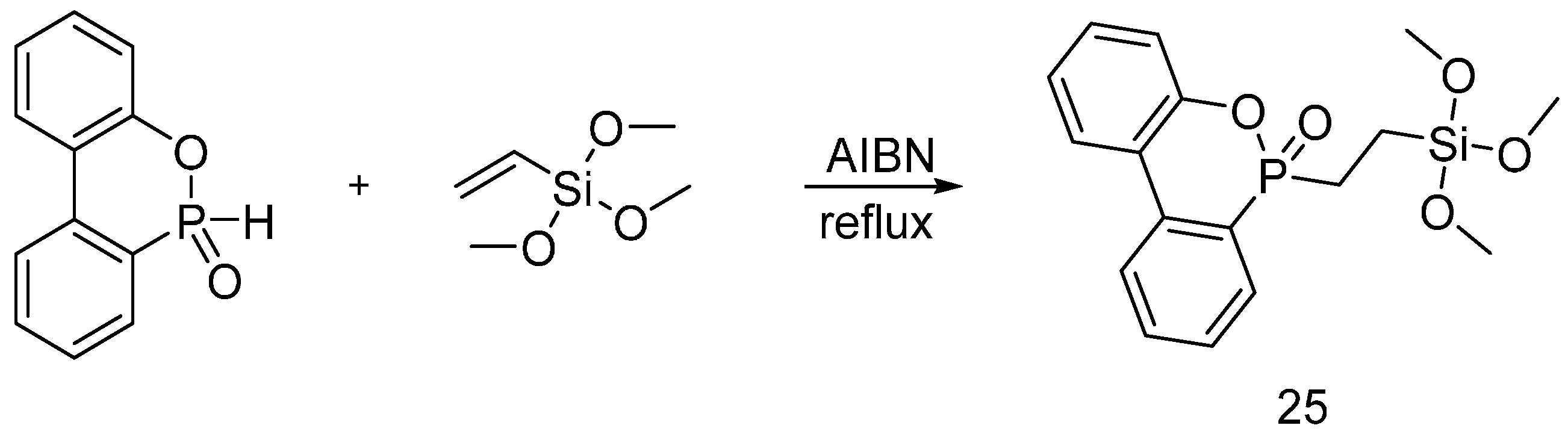
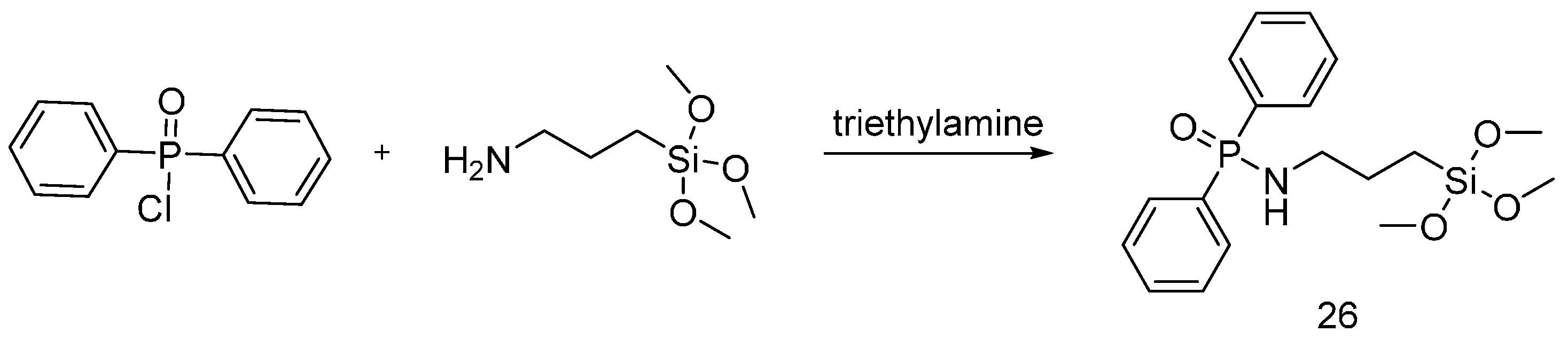

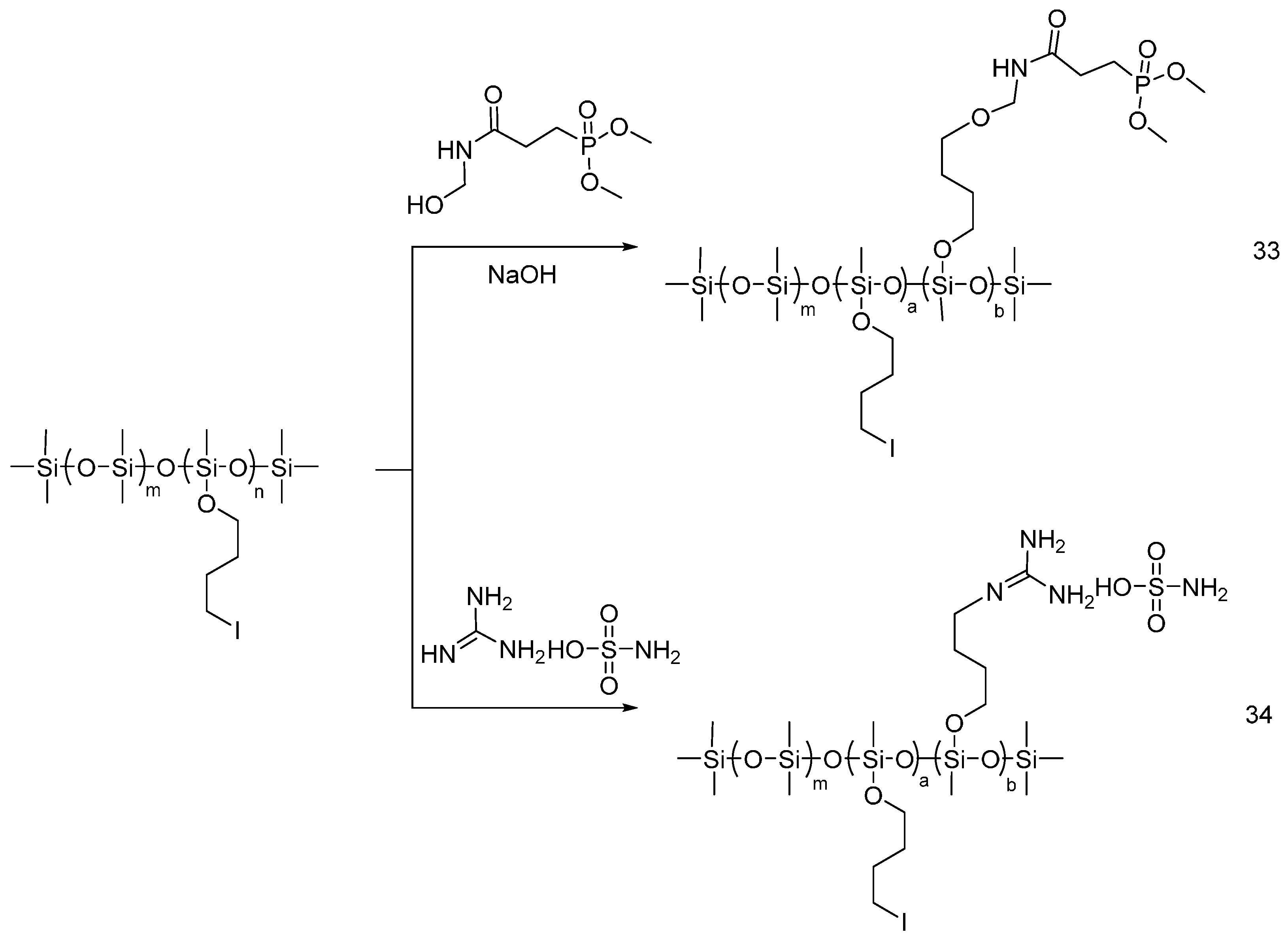
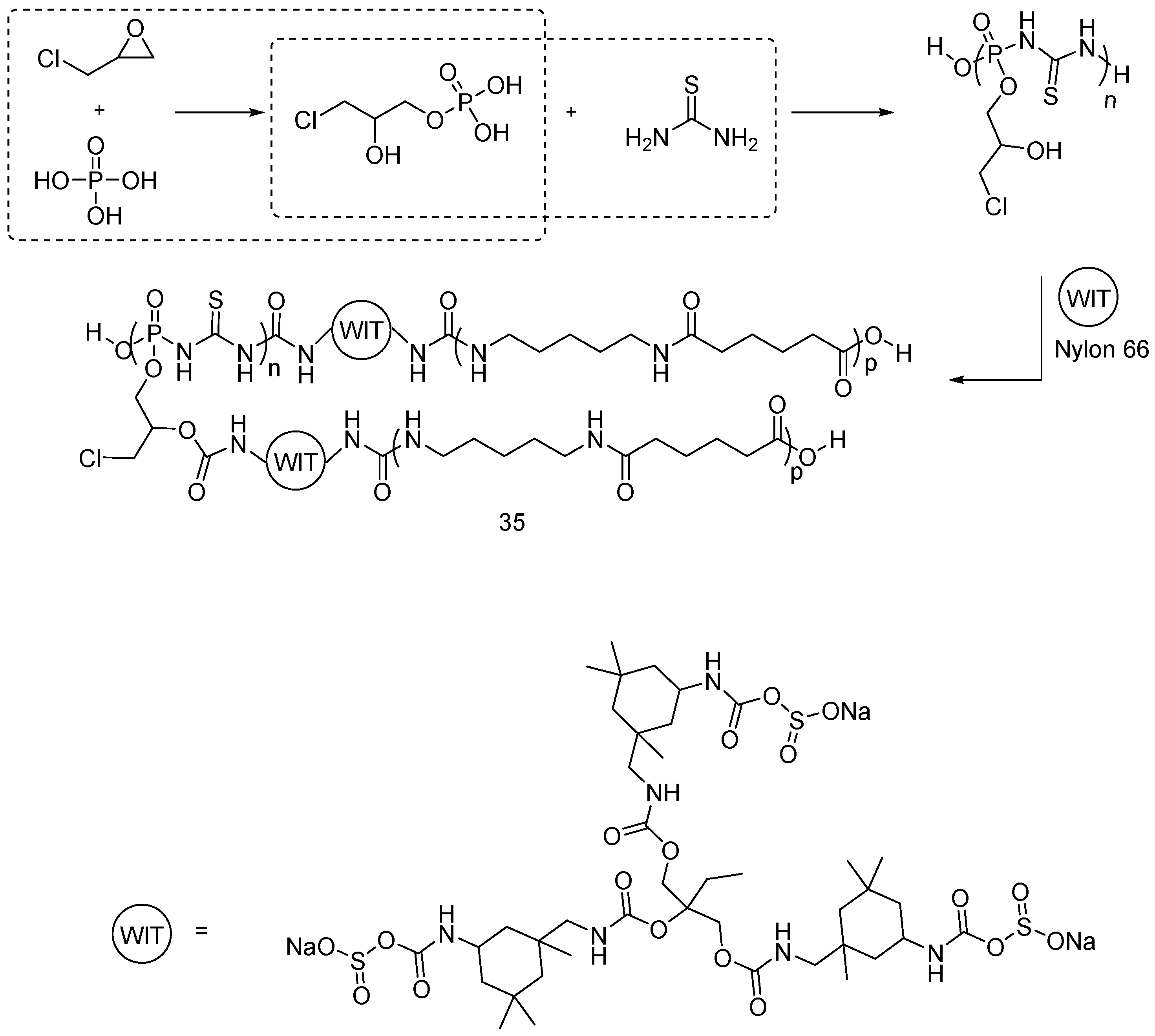

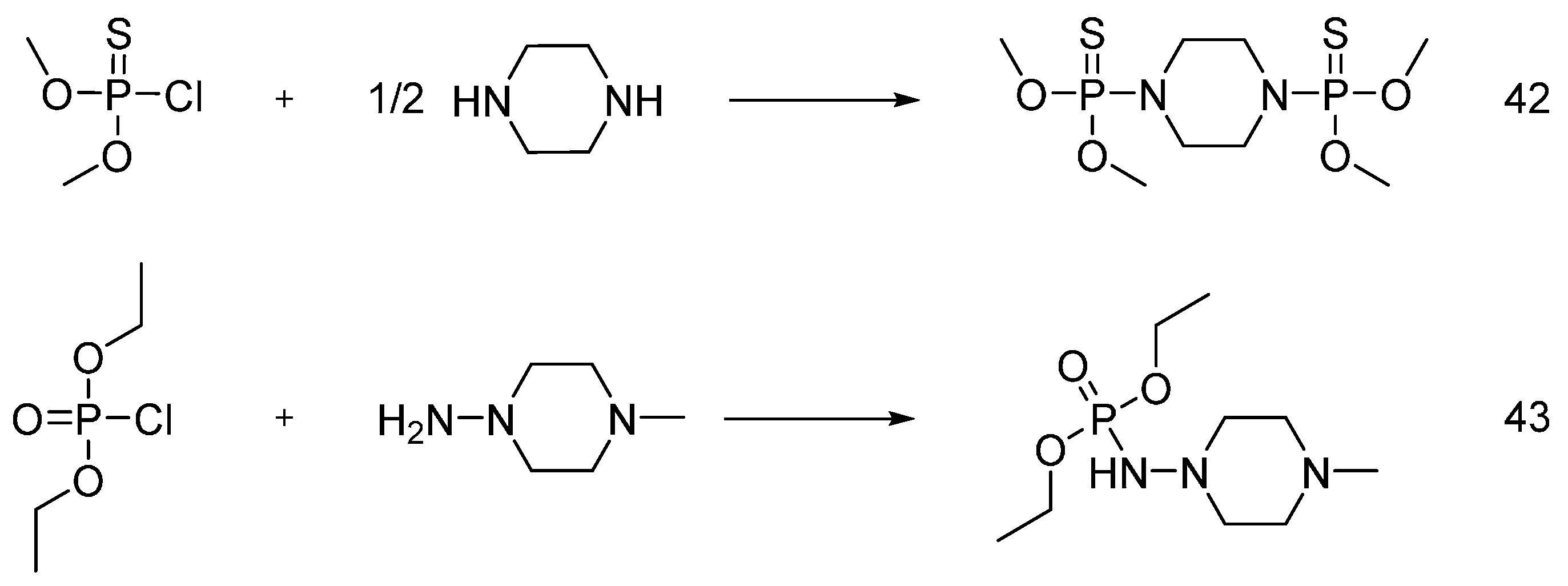


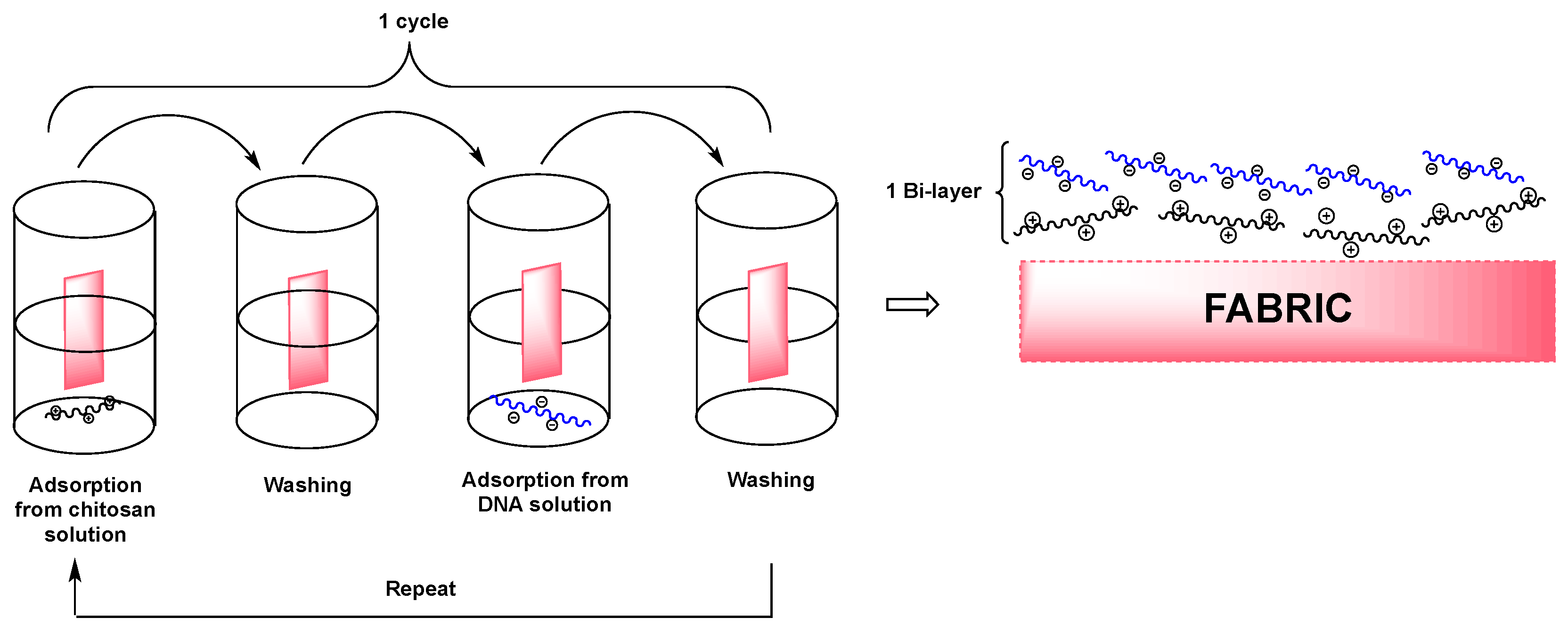
| Type of P-based FR | Textile material | Highlights | Durability (washing fastness) | Ref. |
|---|---|---|---|---|
| Dioxaphosphorinane derivatives | PET | New oligomers were synthesized and their burning behavior was compared to Antiblaze 19®. The new oligomers showed higher thermal stability and more char residue comparing to Antiblaze 19®. The thermal stability and flame retardant properties were studied only on the oligomeric derivatives. Treated PETs with these oligomers were not investigated. | ND * | [20] |
| UV-curable flame retardants | Cotton | Cotton fabric was treated with UV-curable flame retardants and cured under UV-lamp in presence of photoinitiator. LOI values increased with increasing the FR content. MCC showed a decrease in HRC and HRR and THC with increasing the FR content. As increasing the monomer concentration and UV exposure time, the coating yield increased. Relatively small monomers were not suitable for UV-curing as they might evaporate during curing process. | Yes | [21,22,23] |
| Cotton and Cotton/polyester blend | Allyl-functionalized polyphosphazene additive was investigated, avoiding the disadvantage of small molecules. The treated fabrics showed good flame retardancy with char formation. The additive acts in the condensed phase mode of action | Yes | [24] | |
| Polyester/Polyamide blend | UV-curable epoxy based oligomer formulation. Vinyl phosphonic acid (VPA) was incorporated in the formula. The thermal stability, char formation and LOI values of the treated fabrics increased with increasing the concentration of the VPA acid The peel strength values increased gradually up to 50.8 N/cm with increasing VPA. | ND | [25] | |
| Triazine-based flame retardants | Cotton | The triazine-based flame retardants are derivatives of cyanuric chloride. These additives are considered as active flame retardants, forming ether bond by replacement the chlorine atoms with the hydroxyl groups pf cellulose. The fabrics were treated with flame retardants by impregnation. Thermal analysis showed a char formation which proves the condensed phase mode of action. LOI values increased with increasing the add-on of flame retardants. With increasing the flame retardant concentration, the treated fabrics did not show any afterglow, which can be considered as self-extinction. | Yes | [26,27,28,29,30,31,32,33] |
| Hybrid organic-inorganic flame retardants | Cotton | The fabrics were treated with flame retardants using the sol-gel technique. Increasing the sol-gel precursors on treated fabrics, showed a decrease of the HRC, PHRR and TGA onset and an increase of the char residue. Increasing the solid dry add-on increased relatively the after flame with no afterglow. Increasing the solid dry add-on increased the LOI value and decrease of the THR and HRC. | Yes | [34,35,36,37,38,39,40,41] |
| PA6 | The PA6 samples were treated with different concentrations of equimolar mixtures of the flame retardant and TEOS. Combination of P- and Si-based additives improved the thermos-oxidative stability and combustion behavior by increasing the char residue and reducing the HRC and PHRR of treated samples, respectively. | ND | [38] | |
| Polymeric flame retardant additives | Cotton | The cotton fabrics were treated by dipping/soaking in a solution of the polymeric flame retardants. Binders or crosslinkers were used when needed to bind the polymer permanently onto fabrics. LOI values of the treated fabrics showed an increase with increasing the add-on. The vertical burning test of the treated fabrics showed a reduction of the afterglow time and char length. Cone calorimetry showed a decrease of HRR THR and CO2/CO ratio with increasing the add-on. | Partially studied | [42,43,44,45,46,47,48,49] |
| Nylon fabrics | The nylon fabrics were dip-treated in a solution containing FR and crosslinker. MCC analyses showed a decrease of HRR, THR and HRC. | Yes | [49] | |
| Phosphoramidate derivatives | Cotton | LOI values of the treated fabrics increased with increasing the phosphorus content. The treated fabrics with add-on beyond 5 wt % were found self-extinction. The thermal stability and the flame retardancy performance of phosphoramidates are influenced by the nature of chemical substituents and type of cotton fabrics. | Partially studied | [50,51,52,53,54,55,56,57] |
| Nylon and polyester | The vertical flame test showed better flame retardancy of treated nylon fibers. | ND | [56] |
| Chemical structure | Textile material | Highlights | Ref. |
|---|---|---|---|
 | cotton | The FR monomer was grafted onto cotton fabrics using gamma chamber. TG analysis of the treated fabrics showed a decrease of the onset decomposition temperature and increase of char formation. The burning test showed that treated fabrics burnt much slower than untreated fabrics. | [58] |
 | Viscose fiber fabric | The FR was applied to viscose fiber fabric through grafting polymerization. LOI values increased by increasing the grafting percentage. LOI values were almost unchanged after many washing cycles which showed the durability of the covalent bond. Cone calorimetry data of treated fabric showed a significant decrease of PHRR and THR compared to untreated fabric. | [59] |
 | cotton | The FR was used as sol-gel precursor. It was hydrolyzed with 3-aminopropyltriethoxysilane (APTES) in deionized water in presence of HCl followed by the addition of a crosslinker. The fabrics were treated by impregnation in solutions and cured at 150 °C after processing. The burning test showed that all treated cotton fabrics burned completely with significant reduction of burning time and burning rate compared to untreated fabrics. The FR exhibited condensed phase mode of action as TG analysis showed an increase of char formation. | [60] |
 | cotton | The cotton fabrics were treated by soaking in finishing baths of FR, each at different concentrations but all at pH = 5. The FR can react with cellulose without using other crosslinkers. LOI values of treated fabrics increased by increasing the FR concentration. The treated fabrics were found durable up to 50 washing cycles as no change had been noticed on the LOI values or the physical performance of the treated fabrics. | [61] |
 | cotton | The cotton fabrics were treated by impregnation in an aqueous solution of FR, binder, crosslinker and pyrovatex. LOI values of the treated fabrics with different binders and crosslinkers were below 21 which means they can ignite easily and burn rapidly. | [44] |
 | cotton | The fabrics were treated by immersion in an aqueous solution of the FR in the presence of a buffer and shrinking agent. LOI measurements showed an increase in LOI values with increases in the FR concentration. The treated fabrics showed durability up to 30 laundry cycles which was considered as semi-durable flame-resistant. The vertical burning test showed no afterflame or afterglow and a decrease in the char length with an increase in the FR concentration. | [62] |
 | cotton | The fabrics were treated by immersion in an aqueous solution of the FR in the presence of a shrinking agent The treated fabrics showed durability up to 30 laundry cycles which was considered as semi-durable flame-resistant The vertical burning test showed no afterflame or afterglow and a decrease in the char length with an increase in the FR concentration. | [63] |
 | PA6 fibers | Meltable flame retardant which facilitates the compounding process. Rheological measurements showed that this FR behaves as a plasticizer for PA6. TG analysis showed higher char formation of formulated PA6 with the FR compared to neat PA6, while PCFC showed a predominant action in the gas-phase mode. UL-94 test showed a V0 rating with 15 wt % of FR in PA6. | [64] |
 | PET and PBT fibers | The UL-94 test for the formulated PET and PBT fibers with FR showing a V0 rating. | [65] |
| Type of P-based FR | Textile material | Highlights | Durability (washing fastness) | Ref. |
|---|---|---|---|---|
| Casein | Cotton | Anticipation of cotton degradation as assessed by TG analyses (T10%onset values: 285 °C vs. 329 °C for treated and untreated fabrics, respectively). High residue (18%) at 600 °C in nitrogen (TG analyses). Significant increase of the total burning time (+40%), and strong reduction of the total burning rate (−35%) with respect to untreated cotton in flammability tests. Remarkable increase of the final residue after flammability tests (+34%). Strong reduction of TTI (−52%) and of PHRR (−27%) with respect to untreated cotton (Cone calorimetry tests). | No | [71] |
| Casein | Polyester | Anticipation of polyester degradation as assessed by TG analyses (T10%onset values: 315 °C vs. 400 °C for treated and untreated fabrics, respectively). Increase of LOI after the biomacromolecule treatment (from 21% to 26%). High residue (22%) at 600 °C in nitrogen (TG analyses). Significant reduction of the total burning rate (−67%) with respect to untreated polyester in flammability tests. Remarkable increase of the final residue after flammability tests (+77%). In cone calorimetry tests, strong reduction of TTI (from 112 to 62 s, for untreated and treated polyester) and increase of the residue (from 2, neat polyester, to 11%, treated fabric). Melt dripping phenomena also observed for the treated fabrics. | No | [72] |
| Casein | Cotton-polyester blend (35%–65%) | Anticipation of fabric blend degradation as assessed by TG analyses (T10%onset values: 332 °C vs. 304 °C for treated and untreated fabrics, respectively). High residue (22%) at 600 °C in nitrogen (TG analyses). Remarkable increase (+64%) of the total burning time and decrease of the total burning rate (−36%) in flammability tests. Cone calorimetry tests: strong reduction of TTI (from 30 to 12 s, for untreated and treated fabric blend) and of PHRR (−15%) and increase of the residue (from 3, neat blend, to 5%, treated fabric). Slight increase of LOI after the biomacromolecule treatment (from 19% to 21%). | No | [72] |
| DNA | Cotton | Anticipation of cotton degradation as assessed by TG analyses (T10%onset values: 244 °C vs. 335 °C for treated and untreated fabrics, respectively). High residue (34%) at 600 °C in nitrogen (TG analyses). Self-extinction; flame out reached within 2 s. Cone calorimetry tests: no ignition under a heat flux of 35 kW/m2. Significant increase of LOI (from 18, untreated cotton, to 28%, DNA-treated cotton). | No | [73] |
| DNA | Cotton | 10 wt % is the minimum biomacromolecule add-on necessary to reach the flame out of cotton. Cone calorimetry tests performed at 50 kW/m2: remarkable decrease of PHRR (−60%). | No | [74] |
| DNA | Cotton | Being equal the final dry add-on on the fabrics, low MW DNA (100–200 bp) is more effective in enhancing the fire behavior of cotton, with respect to high MW DNA (3000–10,000 bp), providing the highest residues after horizontal flame spread tests and self-extinction for about the 86% of the tested samples impregnated at pH = 4. Cone calorimetry tests performed at 35 kW/m2 on fabrics treated with low MW DNA: reduction of THR (−36%) and of PHRR (−40%). | No | [75] |
| DNA | Cotton | LbL treatments with chitosan (20 bi-layers, BL) provide self-extinction to the fabric. Cone calorimetry tests performed at 35 kW/m2 show a significant decrease of TTI (from 39 s, for untreated cotton, to 23 s, for 20 BL-treated fabric), of PHRR (−41%) and of THR (−32%); the LbL treatment provide an increase of the residue (from 2% for untreated cotton, to 13%, for 20 BL-treated fabric). | No | [76] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmeia, K.A.; Gaan, S.; Malucelli, G. Recent Advances for Flame Retardancy of Textiles Based on Phosphorus Chemistry. Polymers 2016, 8, 319. https://doi.org/10.3390/polym8090319
Salmeia KA, Gaan S, Malucelli G. Recent Advances for Flame Retardancy of Textiles Based on Phosphorus Chemistry. Polymers. 2016; 8(9):319. https://doi.org/10.3390/polym8090319
Chicago/Turabian StyleSalmeia, Khalifah A., Sabyasachi Gaan, and Giulio Malucelli. 2016. "Recent Advances for Flame Retardancy of Textiles Based on Phosphorus Chemistry" Polymers 8, no. 9: 319. https://doi.org/10.3390/polym8090319
APA StyleSalmeia, K. A., Gaan, S., & Malucelli, G. (2016). Recent Advances for Flame Retardancy of Textiles Based on Phosphorus Chemistry. Polymers, 8(9), 319. https://doi.org/10.3390/polym8090319









