Synthesis and Characterization of Polystyrene-Supported Piperazine-Substituted Triazoles by CuAAC and First Evaluation for Metal Ion Extraction
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Organic Syntheses
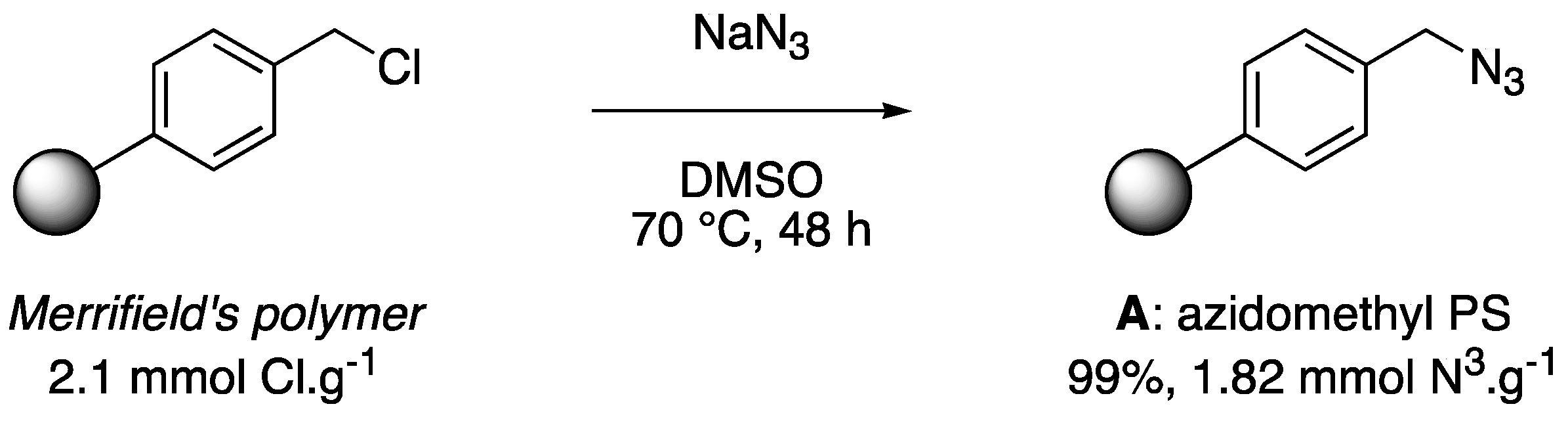
2.2.1. Syntheses of Azidomethyl Polystyrene (A) and Piperazine Propargyl Carbamates (2a–2g)
Synthesis of Azidomethyl Polystyrene (A) (Scheme 1, Section 3.1)
Synthesis of Propargyl Carbamate Derivatives of Piperazine 2a–g (Scheme 2, Section 3.1)
2.2.2. Synthesis of Polymer-Supported Triazolic Piperazines 3a–g (Scheme 3, Section 3.2)
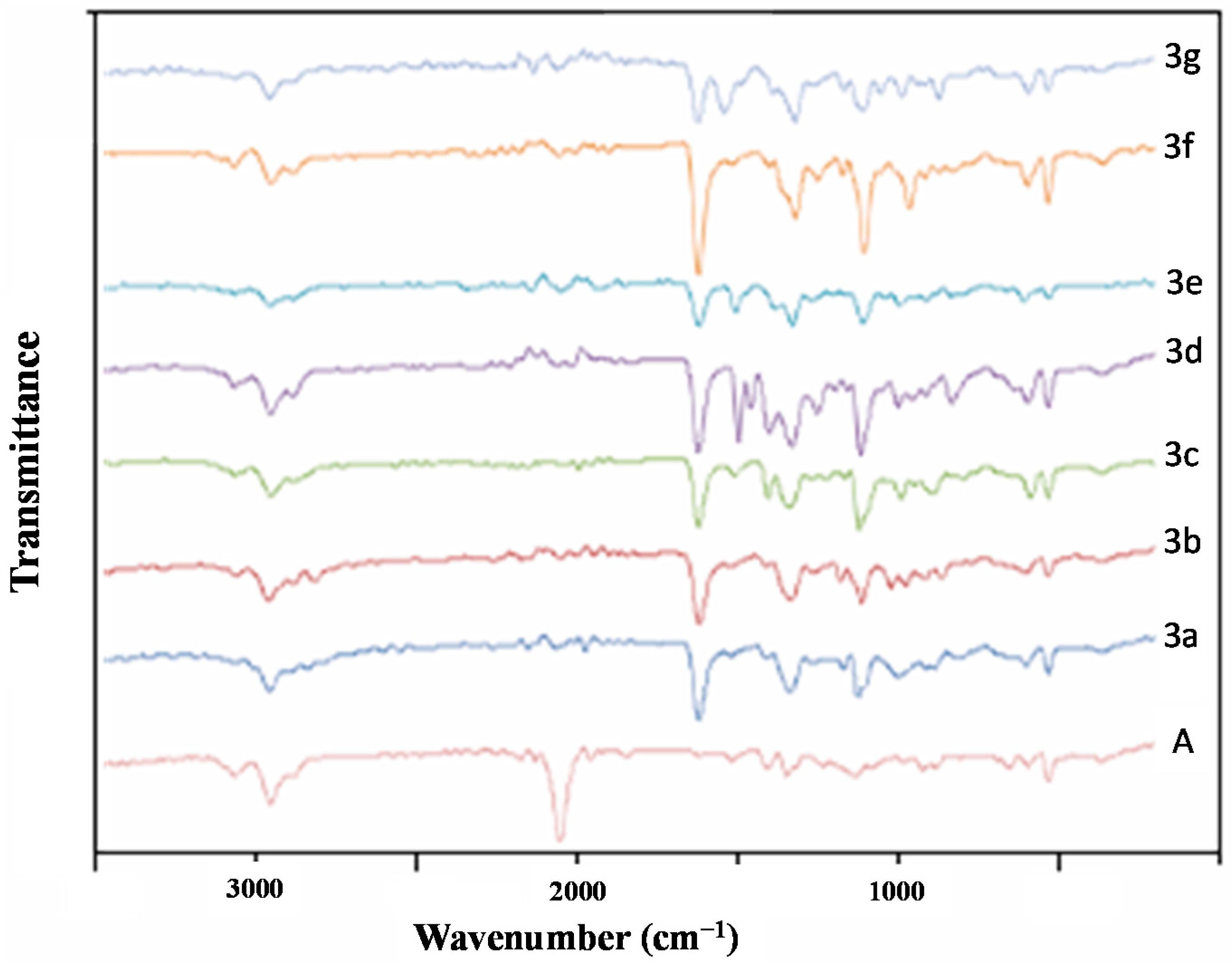
2.3. Characterizations by ATR/FTIR of Polymer-Supported Triazolic Piperazines
2.4. Metal Ion Extraction Method and Quantification
3. Results and Discussion
3.1. Synthesis and Characterization of Azidomethyl Polystyrene (A) and Propargyl Carbamate Derivatives of Piperazine 2a–g
3.2. Synthesis and ATR/FTIR Analysis of Polymer-Supported Triazolic Piperazines 3a–g
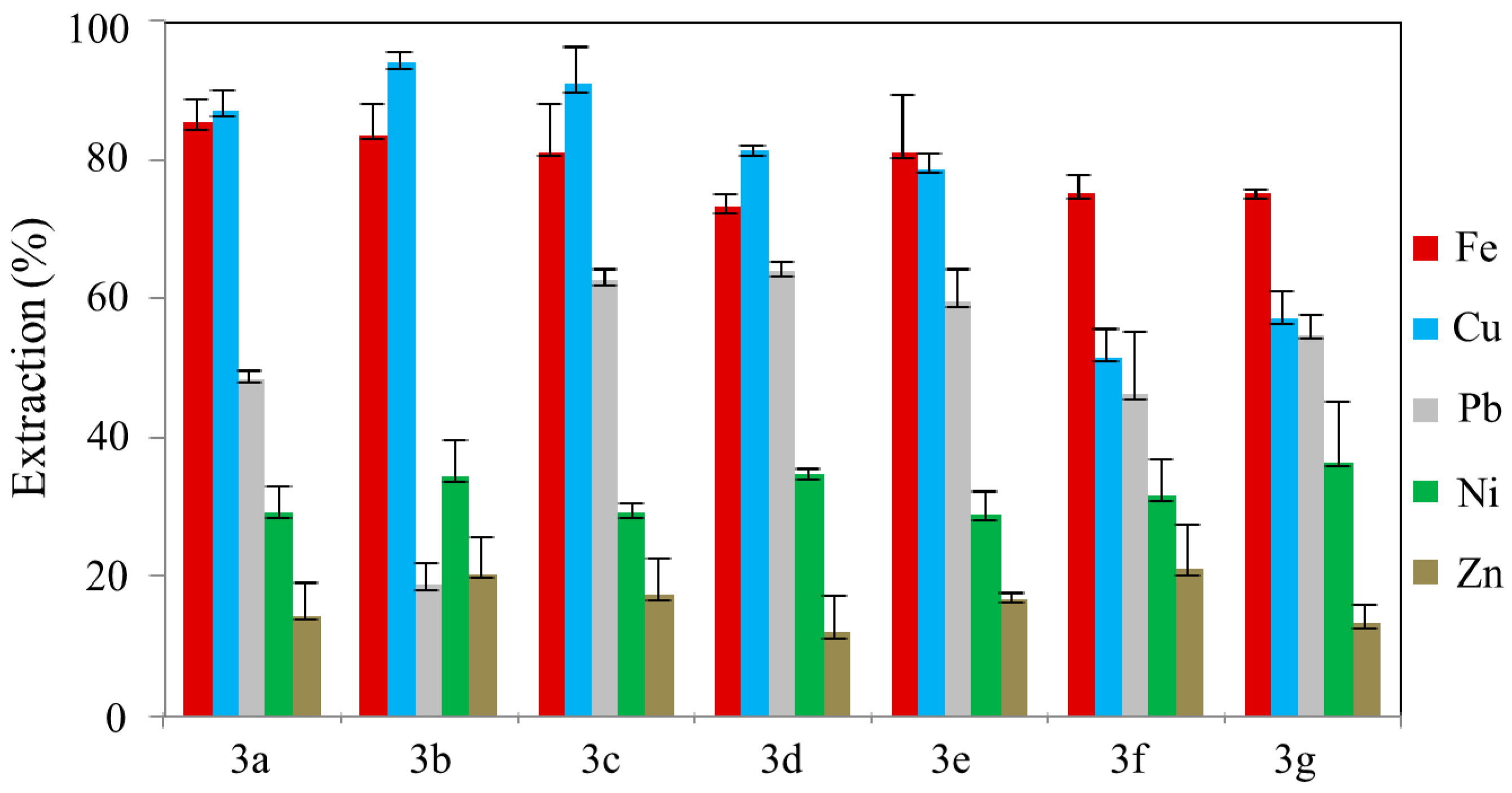
3.3. Metal Ion Extractions by the Polymer-Supported Triazolic Piperazines 3a–g
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rifi, E.H.; Leroy, M.J.F.; Brunette, J.P.; Schloesser-Becker, C. Extraction of copper, cadmium and related metals with poly(sodium acrylate-acrylic acid) hydrogels. Solvent Extr. Ion Exch. 1994, 12, 1103–1119. [Google Scholar] [CrossRef]
- Hodgkin, J.H.; Eibl, R. Gold extraction with poly(diallylamine) resins. React. Polym. 1988, 9, 285–291. [Google Scholar] [CrossRef]
- Rivas, B.L.; Klattenhoff, D.; Perich, I.M. Kinetics of uranium sorption from acidic sulphate solutions onto crosslinked polyethyleneimine based resins. 10. Polym. Bull. 1990, 23, 219–223. [Google Scholar] [CrossRef]
- Anspach, W.M.; Marinsky, J.A. Complexing of nickel(II) and cobalt(II) by a polymethacrylic acid gel and its linear polyelectrolyte analog. J. Phys. Chem. 1975, 79, 433–439. [Google Scholar] [CrossRef]
- Nishide, H.; Oki, N.; Tsuchida, E. Complexation of poly(acrylic acid)s with uranyl ion. Eur. Polym. J. 1982, 18, 799–802. [Google Scholar] [CrossRef]
- Pollack, G.H. Water, energy and life: Fresh views from the water’s edge. Int. J. Des. Nat. Ecodyn. 2010, 5, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Rifi, E.H.; Rastegar, F.; Brunette, J.P. Uptake of cesium, strontium and europium by a poly(sodium acrylate-acrylic acid) hydrogel. Talanta 1995, 42, 811–816. [Google Scholar] [CrossRef]
- Wang, X.; Weiss, R.A. A facile method for preparing sticky, hydrophobic polymer surfaces. Langmuir 2012, 28, 3298–3305. [Google Scholar] [CrossRef] [PubMed]
- Loret, J.F.; Brunette, J.P.; Leroy, M.J.F.; Candau, S.J.; Prévost, M. Liquid-lipophilic gel extraction of precious metals. Solvent Extr. Ion Exch. 1988, 6, 585–603. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Mornane, P.; Potter, I.D.; Perera, J.M.; Cattrall, R.W.; Kolev, S.D. Extraction and transport of metal ions and small organic compounds using polymer inclusion membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41. [Google Scholar] [CrossRef]
- Peterson, J.; Nghiem, L.D. Selective extraction of cadmium by polymer inclusion membranes containing PVC and Aliquat 336: Role base polymer and extractant. IJETM 2010, 12, 359–368. [Google Scholar] [CrossRef]
- Kebiche Senhadji, O.; Sahi, S.; Kahloul, N.; Tingry, S.; BenAmor, M.; Seta, P. Extraction du Cr(VI) par membrane polymère à inclusion. Sci. Technol. A 2008, 27, 43–50. [Google Scholar]
- Guibaud, G.; Baudu, M.; Dollet, P.; Condat, M.L.; Dagot, C. Role des polymeres extracellulaires dans l’adsorption du cadmium par les boues activées role of extracellular polymers in cadmium adsorption by activated sludges. Environ. Technol. 1999, 20, 1045–1054. [Google Scholar] [CrossRef]
- Upitis, A.; Peterson, J.; Lukey, C.; Nghiem, L.D. Metallic ion extraction using polymer inclusion membranes (PIMs): Optimising physical strength and extraction rate. Desalin. Water Treat. 2009, 6, 41–47. [Google Scholar] [CrossRef]
- Zotti, G.; Zecchin, S.; Schiavon, G.; Berlin, A.; Penso, M. Ionochromic and potentiometric properties of the novel polyconjugated polymer from anodic coupling of 5,5’-Bis(3,4-(ethylenedioxy)thien-2-yl)-2,2’-bipyridine. Chem. Mater. 1999, 11, 3342–3351. [Google Scholar] [CrossRef]
- Brembilla, A.; Cuny, J.; Roizard, D.; Lochon, P. Un nouveau polymère catalyseur bifonctionnel: Le polyvinyl-5 (6)benzimidazoleméthanethiol Synthèse et catalyse de l’hydrolyse de l’acétate de p-nitrophényle. Eur. Polym. J. 1983, 19, 729–735. [Google Scholar] [CrossRef]
- Rhazi, M.; Desbrières, J.; Tolaimate, A.; Rinaudo, M.; Vottero, P.; Alagui, A.; El Meray, M. Influence of the nature of the metal ions on the complexation with chitosan: Application to the treatment of liquid waste. Eur. Polym. J. 2002, 38, 1523–1530. [Google Scholar] [CrossRef]
- Kozlowski, C.A.; Walkowiak, W. Applicability of liquid membranes in chromium(VI) transport with amines as ion carriers. J. Membr. Sci. 2005, 266, 143–150. [Google Scholar] [CrossRef]
- Kozlowski, C.; Apostoluk, W.; Walkowiak, W.; Kita, A. Removal of Cr(VI), Zn(II) and Cd(II) ions by transport across polymer inclusion membranes with basic ion carriers. Physicochem. Probl. Miner. Process. 2002, 36, 115–122. [Google Scholar]
- Toy, P.H.; Janda, K.D. Soluble polymer-supported organic synthesis. Acc. Chem. Res. 2000, 33, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Girard, C.; Önen, E.; Aufort, M.; Beauvière, S.; Samson, E.; Herscovici, J. Reusable polymer-supported catalyst for the [3+2] huisgen cycloaddition in automation protocols. Org. Lett. 2006, 8, 1689–1692. [Google Scholar] [CrossRef] [PubMed]
- Jlalia, I.; Beauvineau, C.; Beauvière, S.; Onen, E.; Aufort, M.; Beauvineau, A.; Khaba, E.; Herscovici, J.; Meganem, F.; Girard, C. Automated synthesis of a 96 product-sized library of triazole derivatives using a solid phase supported copper catalyst. Molecules 2010, 15, 3087–3120. [Google Scholar] [CrossRef] [PubMed]
- Ouerghui, A.; Elamari, H.; Ghammouri, S.; Slimi, R.; Meganem, F.; Girard, C. Polystyrene-supported triazoles for metal ions extraction: Synthesis and evaluation. Reac. Funct. Polym. 2014, 74, 37–45. [Google Scholar] [CrossRef]
- Li, C.; Finn, M.G. Click chemistry in materials synthesis. II. Acid-swellable crosslinked polymers made by copper-catalyzed azide–alkyne cycloaddition. J. Polym. Sci. A Polym. Chem. 2006, 44, 5513–5518. [Google Scholar] [CrossRef]
- Tasdelen, M.A.; Yilmaz, G.; Iskin, B.; Yagci, Y. Photoinduced free radical promoted copper(I)-catalyzed click chemistry for macromolecular syntheses. Macromolecules 2012, 45, 56–61. [Google Scholar] [CrossRef]
- Liu, Y.; Díaz, D.D.; Accurso, A.A.; Sharpless, K.B.; Fokin, V.V.; Finn, M.G. Click chemistry in materials synthesis. III. Metal-adhesive polymers from Cu(I)-catalyzed azide–alkyne cycloaddition. J. Polym. Sci. A Polym. Chem. 2007, 45, 5182–5189. [Google Scholar] [CrossRef]
- Dag, A.; Durmaz, H.; Demir, E.; Hizal, G.; Tunca, U. Heterograft copolymers via double click reactions using one-pot technique. J. Polym. Sci. A Polym. Chem. 2008, 46, 6969–6977. [Google Scholar] [CrossRef]
- Opsteen, J.A.; Brinkhuis, R.P.; Teeuwen, R.L.M.; Löwik, D.W.P.M.; van Hest, J.C.M. “Clickable” polymersomes. Chem. Commun. 2007, 3136–3138. [Google Scholar] [CrossRef] [PubMed]
- Riva, R.; Schmeits, S.; Jérôme, C.; Jérôme, R.; Lecomte, P. Combination of ring-opening polymerization and “click chemistry”: Toward functionalization and grafting of poly(ε-caprolactone). Macromolecules 2007, 40, 796–803. [Google Scholar] [CrossRef]
- Löber, S.; Rodriguez-Loaiza, P.; Gmeiner, P. Click linker: Efficient and high-yielding synthesis of a new family of SPOS resins by 1,3-dipolar cycloaddition. Org. Lett. 2003, 5, 1753–1755. [Google Scholar] [CrossRef] [PubMed]
- Iskin, B.; Yilmaz, G.; Yagci, Y. ABC type miktoarm star copolymers through combination of controlled polymerization techniques with thiol-ene and azide-alkyne click reactions. J. Polym. Sci. A Polym. Chem. 2011, 49, 2417–2422. [Google Scholar] [CrossRef]
- Xue, X.; Zhu, J.; Zhang, Z.; Cheng, Z.; Tu, Y.; Zhu, X. Synthesis and characterization of azobenzene-functionalized poly(styrene)-b-poly(vinyl acetate) via the combination of RAFT and “click” chemistry. Polymer 2010, 51, 3083–3090. [Google Scholar] [CrossRef]
- Shin, J.A.; Lim, Y.G.; Lee, K.H. Synthesis of polymers including both triazole and tetrazole by click reaction. Bull. Korean Chem. Soc. 2011, 32, 547–552. [Google Scholar] [CrossRef]
- Struthers, H.; Mindt, T.L.; Schibli, R. Metal chelating systems synthesized using the copper(I) catalyzed azide-alkyne cycloaddition. Dalton Trans. 2009, 39, 675–696. [Google Scholar] [CrossRef] [PubMed]
- Urankar, D.; Pinter, B.; Pevec, A.; de Proft, F.; Turel, I.; Košmrlj, J. Click-triazole N2 coordination to transition-metal ions is assisted by a pendant pyridine substituent. Inorg. Chem. 2010, 49, 4820–4829. [Google Scholar] [CrossRef] [PubMed]
- Slimi, R.; Girard, C. “High-throughput” evaluation of polymer-supported triazolic appendages for metallic cations extraction. Metals 2015, 5, 418–427. [Google Scholar] [CrossRef]
- Arseniyadis, S.; Wagner, A.; Mioskowski, C. A straightforward preparation of amino–polystyrene resin from Merrifield resin. Tetrahedron Lett. 2002, 43, 9717–9719. [Google Scholar] [CrossRef]
- Ouerghui, A.; Elamari, H.; Dardouri, M.; Ncib, S.; Meganem, F.; Girard, C. Chemical modifications of poly(vinyl chloride) to poly(vinyl azide) and “clicked” triazole bearing groups for application in metal cation extraction. React. Funct. Polym. 2016, 100, 191–197. [Google Scholar] [CrossRef]
- Eldridge, D.S.; Crawford, R.J.; Harding, I.H. The role of metal ion-ligand interactions during divalent metal ion adsorption. J. Colloid Interface Sci. 2015, 454, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Gilson, R.; Durrant, M.C. Estimation of the pKa values of water ligands in transition metal complexes using density functional theory with polarized continuum model solvent corrections. Dalton Trans. 2009, 10223–10230. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.R.; Fokin, V.V. Polymer-supported copper(I) catalysts for the experimentally simplified azide–alkyne cycloaddition. QSAR Comb. Sci. 2007, 26, 1274–1279. [Google Scholar] [CrossRef]
- Bergbreiter, D.E.; Hamilton, P.N.; Koshti, N.M. Self-separating homogeneous Copper (I) catalysts. J. Am. Chem. Soc. 2007, 129, 10666–10667. [Google Scholar] [CrossRef] [PubMed]
- Meudtner, R.M.; Hecht, S. Responsive backbones based on alternating triazole-pyridine/benzene copolymers: From helically folding polymers to metallosupramolecularly crosslinked gels. Macromol. Rapid Commun. 2008, 29, 347–351. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Y.; Wen, X.; Ding, C.; Li, Y. Dual-functional click-triazole: A metal chelator and immobilization linker for the construction of a heterogeneous palladium catalyst and its application for the aerobic oxidation of alcohols. Chem. Commun. 2012, 48, 2979–2981. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D. Palladium catalysis using dendrimers: Molecular catalysts versus nanoparticles. Tetrahedron 2010, 21, 1041–1054. [Google Scholar] [CrossRef]
- Watanabe, W.; Maekawa, T.; Miyazaki, Y.; Kida, T.; Takeshita, K.; Mori, A. Quadruple click: A facile pathway leading to tetrakis(4-(1,2,3-triazolyl)methyl)-ethylenediamine derivatives as a new class of extracting agents for soft metal ions. Chem. Asian J. 2012, 7, 1679–1683. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, A.; Moth-Poulsen, K.; Eklöf, J.; Nydén, M.; Kann, N. One-pot synthesis of TBTA-functionalized coordinating polymers. React. Funct. Polym. 2014, 82, 1–8. [Google Scholar] [CrossRef]



| Polymer a | Mass increase (g) b | Total mass (g) | Substitution (mmol·g−1) b | 250 mg (mmol) |
|---|---|---|---|---|
| 3a | 0.99 | 3.99 | 1.37 | 0.342 |
| 3b | 1.07 | 4.07 | 1.34 | 0.335 |
| 3c | 1.50 | 4.50 | 1.21 | 0.302 |
| 3d | 1.34 | 4.34 | 1.26 | 0.315 |
| 3e | 1.34 | 4.34 | 1.26 | 0.315 |
| 3f | 1.65 | 4.65 | 1.17 | 0.292 |
| 3g | 1.43 | 4.43 | 1.23 | 0.307 |
| Cation | Anion | pHi a | pHf b | pK1 c |
|---|---|---|---|---|
| Fe3+ | Cl− | 3.71 ± 0.01 | 3.19 ± 0.02 | 2.2 |
| Cu2+ | SO42− | 6.90 ± 0.01 | 7.06 ± 0.61 | 7.6 |
| Pb2+ | NO3− | 5.07 ± 0.01 | 5.26 ± 0.59 | 7.7 |
| Ni2+ | Cl− | 6.13 ± 0.01 | 7.36 ± 0.62 | 9.9 |
| Zn2+ | SO42− | 6.64 ± 0.01 | 5.71 ± 0.29 | 9.5 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slimi, R.; Ben Othman, R.; Sleimi, N.; Ouerghui, A.; Girard, C. Synthesis and Characterization of Polystyrene-Supported Piperazine-Substituted Triazoles by CuAAC and First Evaluation for Metal Ion Extraction. Polymers 2016, 8, 187. https://doi.org/10.3390/polym8050187
Slimi R, Ben Othman R, Sleimi N, Ouerghui A, Girard C. Synthesis and Characterization of Polystyrene-Supported Piperazine-Substituted Triazoles by CuAAC and First Evaluation for Metal Ion Extraction. Polymers. 2016; 8(5):187. https://doi.org/10.3390/polym8050187
Chicago/Turabian StyleSlimi, Riadh, Raja Ben Othman, Noomene Sleimi, Abid Ouerghui, and Christian Girard. 2016. "Synthesis and Characterization of Polystyrene-Supported Piperazine-Substituted Triazoles by CuAAC and First Evaluation for Metal Ion Extraction" Polymers 8, no. 5: 187. https://doi.org/10.3390/polym8050187