Preparation of the Water-Soluble Pyrene-Containing Fluorescent Polymer by One-Pot Method
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents
2.2. Instruments and Measurements
2.3. Synthesis of N-Butoxycarbonyl-Ethylene Diamine (N-Boc-EDA)
2.4. Synthesis of N-Butoxycarbonyl-Acryloyl Ethylene Diamine (N-Boc-AEDA)S
2.5. Synthesis of S-1-Dodecyl-S′-(α,α′-Dimethyl-α″-Acetic Acid)trithiocarbonate and S-1-Dodecyl-S′-[poly(N-Boc-Acryloyl Ethylene Diamine)-2′-Methyl Propionic Acid]trithiocarbonate (P1)

2.6. Synthesis of 6-Bromohexyl Acrylate (BHA)
2.7. Synthesis of 1-(Acryloyloxy Hexyloxy)pyrene (AHP)


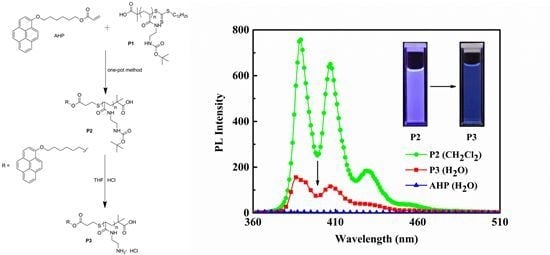
2.8. Synthesis of 1-{3′-S-[poly(N-Butoxycarbonyl-Acryloyl Ethylene Diamine)-2″-Methyl Propionic acid]}propionyloxy Hexyloxy Pyrene (P2) and 1-{3′-S-[poly(Acryloyl Ethylene Diamine Hydrochloride)-2′-Methyl Propionic acid]}propionyloxy Hexyloxy Pyrene (P3)
3. Results and Discussion
3.1. The reaction Mechanism of the One-Pot Reaction
3.2. FTIR Analysis of AHP, P2, and P3

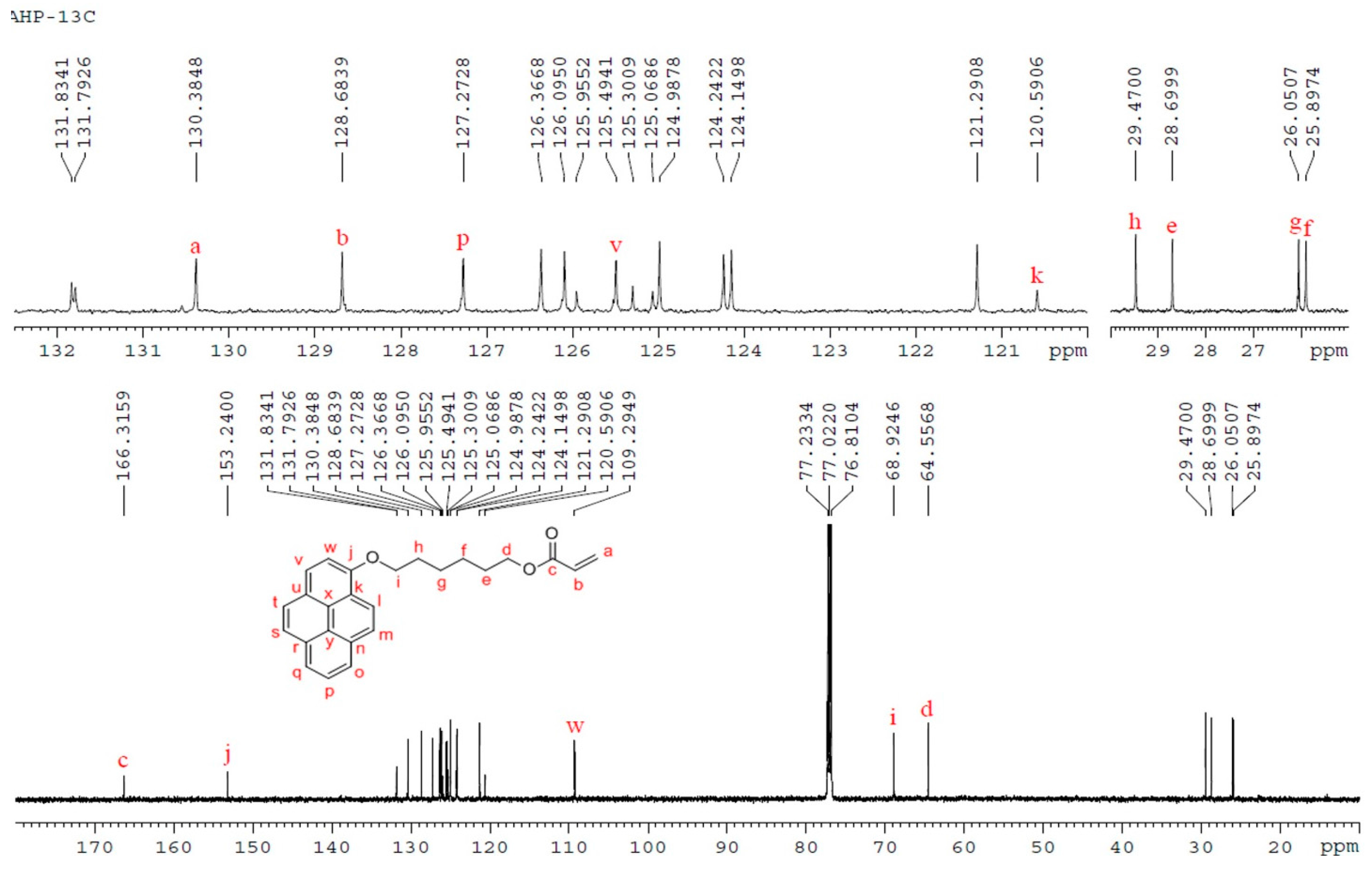
3.3. NMR Analysis of P3

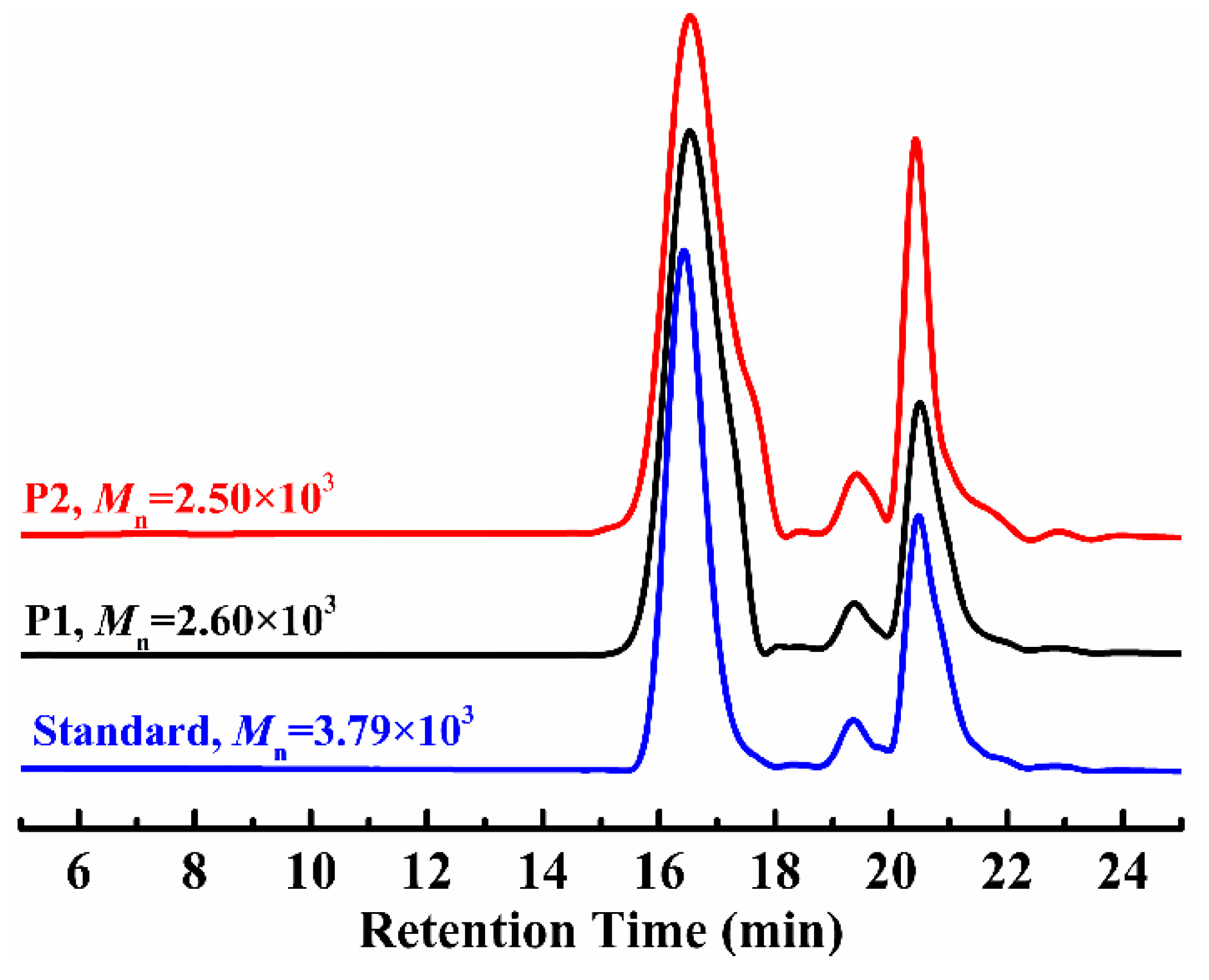
3.4. GPC Analysis
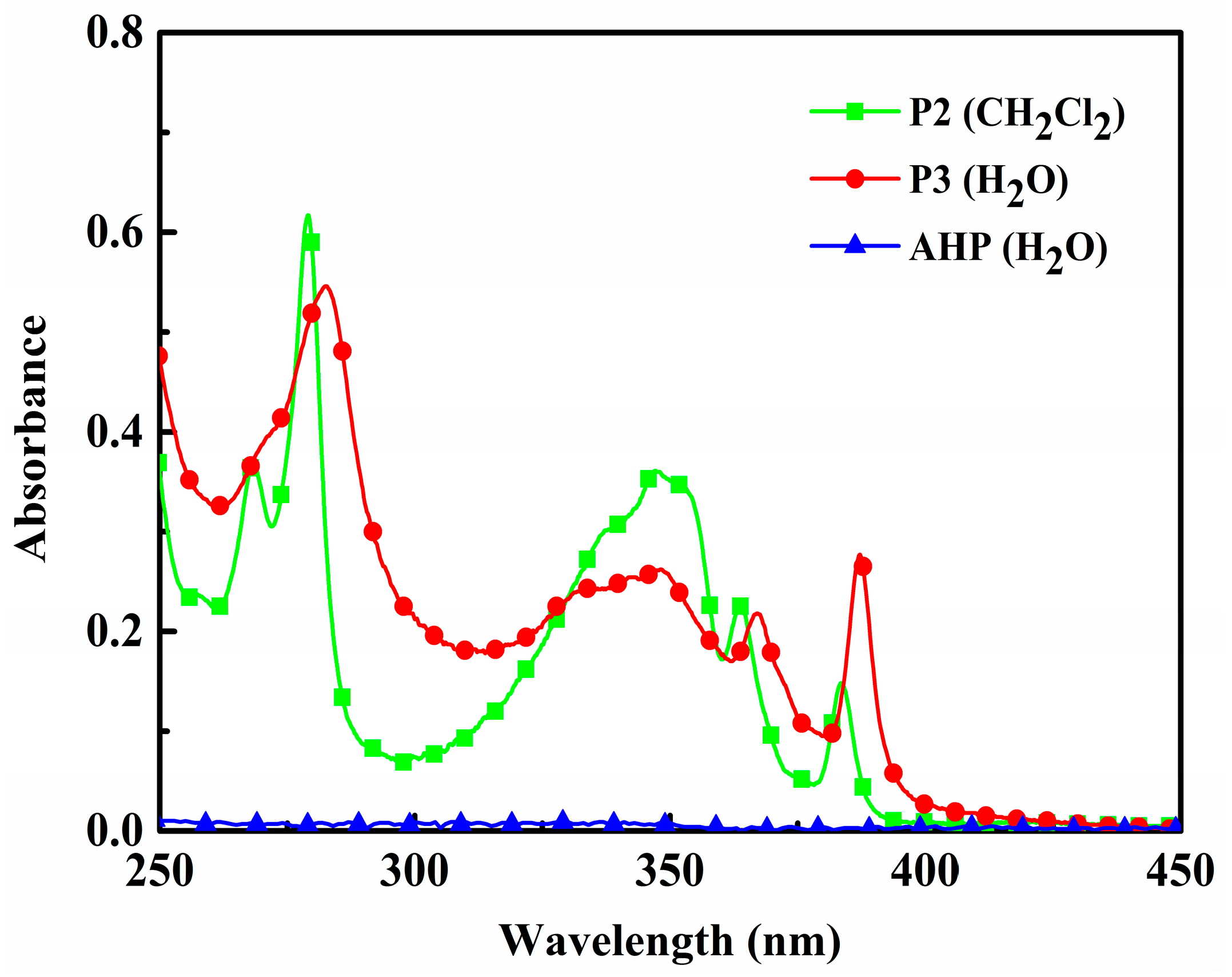
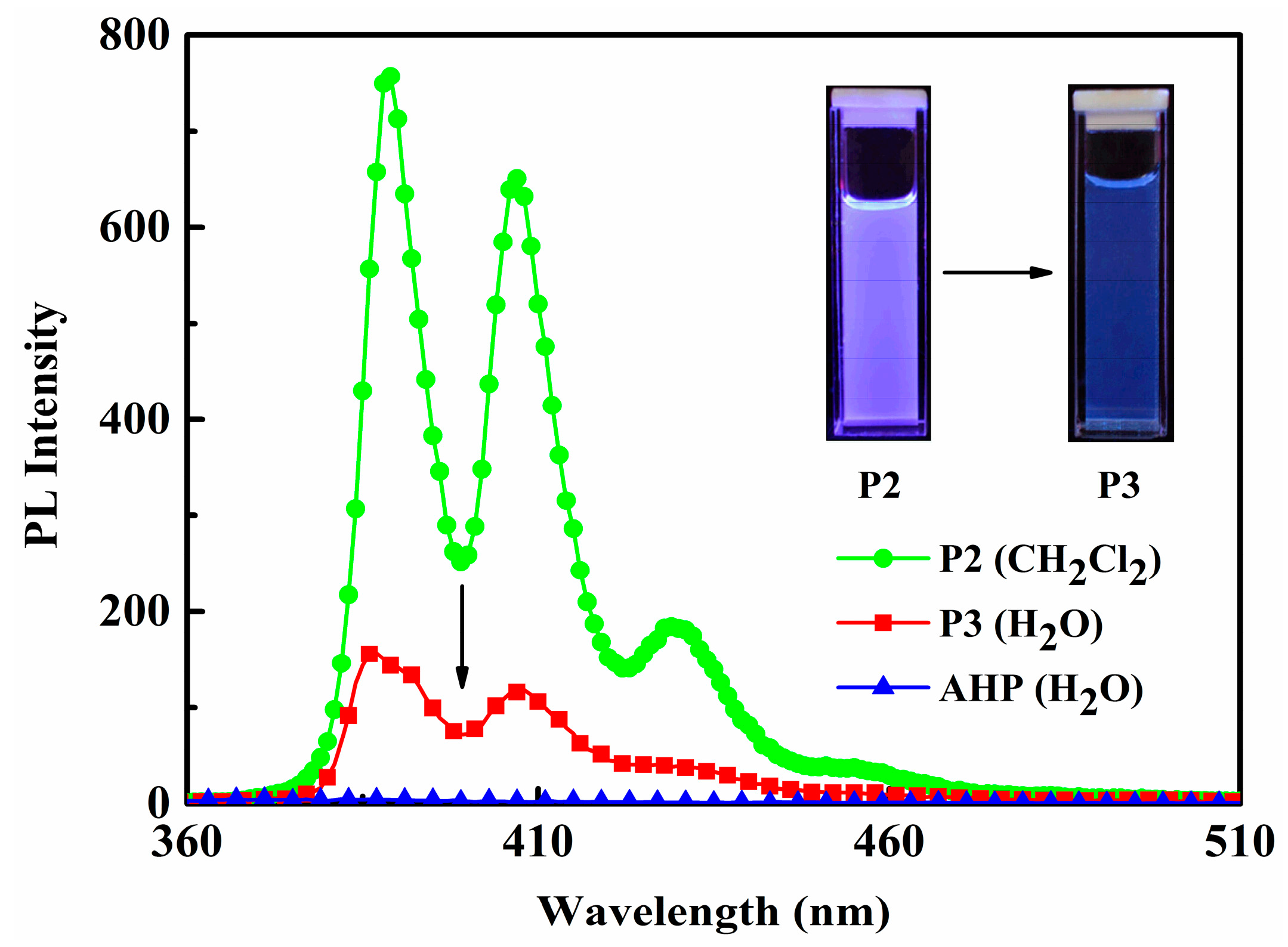
3.5. UV-VIS and PL Analysis


4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miranda, O.R.; Creran, B.; Rotello, V.M. Array-based sensing with nanoparticles: “Chemical noses” for sensing biomolecules and cell surfaces. Curr. Opin. Chem. Biol. 2010, 14, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.; Scott, M.D.; Haldar, M.K.; Ganguly, B.; Srivastava, D.K.; Friesner, D.L.; Mallik, S. Fluorescent water soluble polymers for isozyme-selective interactions with matrix metalloproteinase-9. Bioorg. Med. Chem. Lett. 2011, 21, 2007–2010. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, A.; Miranda, O.R.; Phillips, R.; Kim, I.B.; Jerry, D.J.; Bunz, U.H.; Rotello, V.M. Array-based sensing of normal, cancerous, and metastatic cells using conjugated fluorescent polymers. J. Am. Chem. Soc. 2010, 132, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.D.; Dutta, R.; Haldar, M.K.; Guo, B.; Friesner, D.L.; Mallik, S. Differentiation of prostate cancer cells using flexible fluorescent polymers. Anal. Chem. 2012, 84, 17–20. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Park, T.; Kim, J.; Heo, J.S.; Kim, H.S.; Kim, H.O.; Kim, E. Highly fluorescent conjugated polyelectrolyte for protein sensing and cell-compatible chemosensing applications. ACS Appl. Mater. Interfaces 2014, 5, 3305–3311. [Google Scholar] [CrossRef] [PubMed]
- Traina, C.A.; Bakus II, R.C.; Bazan, G.C. Design and synthesis of monofunctionalized, water-soluble conjugated polymers for biosensing and imaging applications. J. Am. Chem. Soc. 2011, 133, 12600–12607. [Google Scholar] [CrossRef] [PubMed]
- Schillén, K.; Anghel, D.F.; Miguel, M.G.; Lindman, B. Association of naphthalene-labeled poly(acrylic acid) and interaction with cationic surfactants fluorescence studies. Langmuir 2000, 16, 10528–10539. [Google Scholar] [CrossRef]
- Costa, T.; Miguel, M.G.; Lindman, B.; Schillén, K.; Lima, J.C.; Seixas de Melo, J. Self-assembly of a hydrophobically modified naphthalene-labeled poly(acrylic acid) polyelectrolyte in water: Organic solvent mixtures followed by steady-state and time-resolved fluorescence. J. Phys. Chem. B 2005, 109, 3243–3251. [Google Scholar] [CrossRef] [PubMed]
- Oyama, H.T.; Tang, W.T.; Frank, C.W. Complex formation between poly(acry1ic acid) and pyrene-labeled poly(ethylene glycol) in aqueous solution. Macromolecules 1987, 20, 474–480. [Google Scholar] [CrossRef]
- Hemker, D.J.; Garza, V.; Frank, C.W. Complexation of poly(acry1ic acid) and poly(methacry1ic acid) with pyrene-end-labeled poly(ethy1ene glycol). pH and fluorescence measurements. Macromolecules 1990, 23, 4411–4418. [Google Scholar] [CrossRef]
- Zhang, W.J.; Peng, B.; Tian, F.; Qin, W.J.; Qian, X.H. Facile preparation of well-defined hydrophilic core-shell upconversion nanoparticles for selective cell membrane glycan labeling and cancer cell imaging. Anal. Chem. 2014, 86, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, K.; Hong, S.; Kim, H.; Kwon, Y.J.; Song, R. Intracellular protein target detection by quantum dots optimized for live cell imaging. Bioconjug. Chem. 2011, 22, 1576–1586. [Google Scholar] [CrossRef] [PubMed]
- Roth, P.J.; Boyer, C.; Lowe, A.B.; Davis, T.P. RAFT polymerization and thiol chemistry: Acomplementary pairing for implementing modern macromolecular design. Macromol. Rapid Commun. 2011, 32, 1123–1143. [Google Scholar] [CrossRef] [PubMed]
- Harvison, M.A.; Davis, T.P.; Lowe, A.B. Macromolecular thiolysis of oxiranes: End-group modification of RAFT prepared homopolymers. Polym. Chem. 2011, 2, 1347–1354. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Barry Sharpless, K. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Kappe, C.O.; Van der Eycken, E. Click chemistry under non-classical reaction conditions. Chem. Soc. Rev. 2010, 39, 1280–1290. [Google Scholar] [CrossRef] [PubMed]
- Clavé, G.; Campidelli, S. Efficient covalent functionalisation of carbon nanotubes: The use of “click chemistry”. Chem. Sci. 2011, 2, 1887–1896. [Google Scholar] [CrossRef]
- Negishi, K.; Mashiko, Y.; Yamashita, E.; Otsuka, A.; Hasegawa, T. Synthesis of well-defined, water-soluble hyperbranched polyamides by chain-growth condensation polymerization of AB2 monomer. Polymers 2012, 4, 1170–1182. [Google Scholar]
- Moses, J.E.; Moorhouse, A.D. The growing applications of click chemistry. Chem. Soc. Rev. 2007, 8, 1249–1262. [Google Scholar] [CrossRef] [PubMed]
- Hoyle, C.E.; Bowman, C.N. Thiol-ene click chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef] [PubMed]
- Korthals, B.; Morant-Miñana, M.C.; Schmid, M.; Mecking, S. Functionalization of polymer nanoparticles by thiol-ene addition. Macromolecules 2010, 43, 8071–8078. [Google Scholar] [CrossRef]
- Prasath, R.A.; Gokmen, M.T.; Espeel, P.; du Prez, F.E. Thiol-ene and thiol-yne chemistry in microfluidics: A straight forward method towards macroporous and nonporous functional polymer beads. Polym. Chem. 2010, 1, 685–692. [Google Scholar] [CrossRef]
- Iskin, B.; Yilmaz, G.; Yagci, Y. ABC type miktoarm star copolymers through combination of controlled polymerization techniques with thiol-ene and azide-alkyne click reactions. J. Polym. Sci. Polym. Chem. 2011, 49, 2417–2422. [Google Scholar] [CrossRef]
- Montañnez, M.I.; Campos, L.M.; Antoni, P.; Hed, Y.; Walter, M.V.; Krull, B.T.; Khan, A.; Hult, A.; Hawker, C.; Malkoch, M. Accelerated growth of dendrimers via thiol-ene and esterification reactions. Macromolecules 2010, 43, 6004–6013. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Kalaitzis, Z.M.; Cao, W. Solvent dependent morphologies in thiol-ene photopolymerization: A facile route to the synthesis of resorcinarene nanocapsules. J. Mater. Chem. 2010, 20, 6539–6543. [Google Scholar] [CrossRef]
- Campos, L.M.; Killops, K.L.; Sakai, R.; Paulusse, J.M.J.; Damiron, D.; Drockenmuller, E.; Messmore, B.W.; Hawker, C.J. Development of thermal and photochemical strategies for thiol-ene click polymer functionalization. Macromolecules 2008, 41, 7063–7070. [Google Scholar] [CrossRef]
- Zimerman, O.E.; Cosa, J.J.; Previtallj, C.M. Binding of ionic pyrene derivatives to polyelectrolytes. A UV absorption and fluorescence study. J. Macromol. Sci. A 1994, 31, 859–872. [Google Scholar] [CrossRef]
- Nakagawa, K.; Numata, Y.; Ishino, H.; Tanaka, D.; Kobayashi, T.; Tokunaga, E. Excimer luminescence from nonresonantly excited pyrene and perylene molecules in solution. J. Phys. Chem. A 2013, 117, 11449–11455. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, E.; Delgado-Pinar, E.; Pitarch-Jarque, J.; Alarcón, J.; García-España, E. Boehmite supported pyrene polyamine systems as probes for iodide recognition. J. Phys. Chem. C 2013, 117, 14325–14331. [Google Scholar] [CrossRef]
- Duhamel, J. Internal dynamics of dendritic molecules probed by pyrene excimer formation. Polymers 2012, 4, 211–239. [Google Scholar] [CrossRef]
- Mahapatra, A.K.; Mondal, S.; Maiti, K.; Manna, S.K.; Maji, R.; Mandal, D.; Mandal, S.; Goswami, S.; Quah, C.K.; Funde, H.K. A pyrene thiazole conjugate as a ratiometric chemosensor with high selectivity and sensitivity for tin (Sn4+) and its application in imaging livecells. RSC Adv. 2014, 4, 56605–56614. [Google Scholar] [CrossRef]
- Zou, L.; Wang, X.; Shi, Y.K.; Wang, J.Y.; Pei, J. Fusion at the non-K-region of pyrene: An alternative strategy to extend the π‑conjugated plane of pyrene. Org. Lett. 2013, 15, 4378–4381. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Sahana, A.; Lohar, S.; Sarkar, B.; Mukhopadhya, S.K.; Banerjee, A.; Das, D. A visible light excitable pyrene-naphthalene conjugate for ON fluorescence sensing of histidine in living cells. RSC Adv. 2014, 4, 7495–7499. [Google Scholar] [CrossRef]
- Edkins, R.M.; Fucke, K.; Peach, M.J.G.; Crawford, A.G.; Marder, T.B.; Beeby, A. Syntheses, structures, and comparison of the photophysical properties of cyclometalated iridium complexes containing the isomeric 1-and 2‑(2′-pyridyl)pyrene ligands. Inorg. Chem. 2013, 52, 9842–9860. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Wu, Y.G.; Bai, L.B.; Wang, Y.; Huang, H.C.; Zhao, H.C.; Ba, X.W. Preparation of pH-sensitive fluorescent poly(acryl-2-aminoethy-lammonium hydrochloride) with porphyrin or fluorene by one-pot method. Chem. J. Chin. Univ. 2014, 35, 1111–1118. [Google Scholar]
- Lai, J.T.; Filla, D.; Shea, R. Functional polymers from novel carboxyl-terminated trithiocarbonates as highly efficient RAFT agents. Macromolecules 2002, 35, 6754–6756. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, H.J.; Li, X.M.; Qian, Y.X.; Bai, L.B.; Wu, Y.G.; Lv, S.F.; Zhao, H.C. Preparation of the water-soluble fluorene-containing fluorescent polymer by one-pot method. Macromol. Res. 2015, 23, 891–897. [Google Scholar] [CrossRef]
- Hayashida, O.; Kaku, Y. Synthesis of dabsyl-appended cyclophanes and their heterodimer formation with pyrene-appended cyclophanes. J. Org. Chem. 2013, 78, 10437–10442. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Han, Y.; Sun, M.H.; Bo, Z.S. Water-soluble dendronized polyfluorenes with an extremely high quantum yield in water. Macromolecules 2007, 13, 4494–4500. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, M.; Tan, H.; Yang, Q.; Wang, A.; Bai, L.; Zhao, H.; Wu, Y. Preparation of the Water-Soluble Pyrene-Containing Fluorescent Polymer by One-Pot Method. Polymers 2015, 7, 2625-2637. https://doi.org/10.3390/polym7121538
Li X, Wang M, Tan H, Yang Q, Wang A, Bai L, Zhao H, Wu Y. Preparation of the Water-Soluble Pyrene-Containing Fluorescent Polymer by One-Pot Method. Polymers. 2015; 7(12):2625-2637. https://doi.org/10.3390/polym7121538
Chicago/Turabian StyleLi, Xiaomeng, Miaomiao Wang, Haijian Tan, Qingmin Yang, Aiqing Wang, Libin Bai, Hongchi Zhao, and Yonggang Wu. 2015. "Preparation of the Water-Soluble Pyrene-Containing Fluorescent Polymer by One-Pot Method" Polymers 7, no. 12: 2625-2637. https://doi.org/10.3390/polym7121538