Function and Autonomous Behavior of Self-Oscillating Polymer Systems
Abstract
:1. Introduction
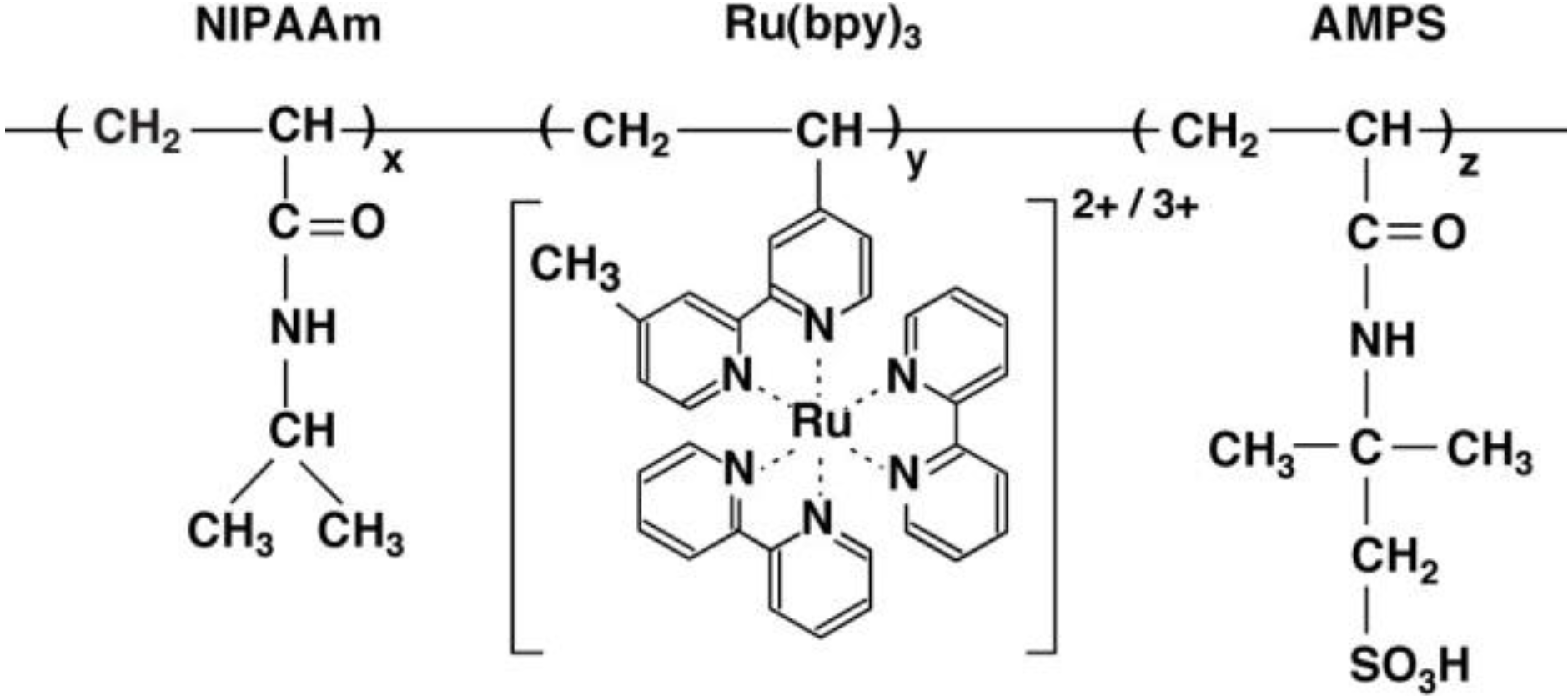
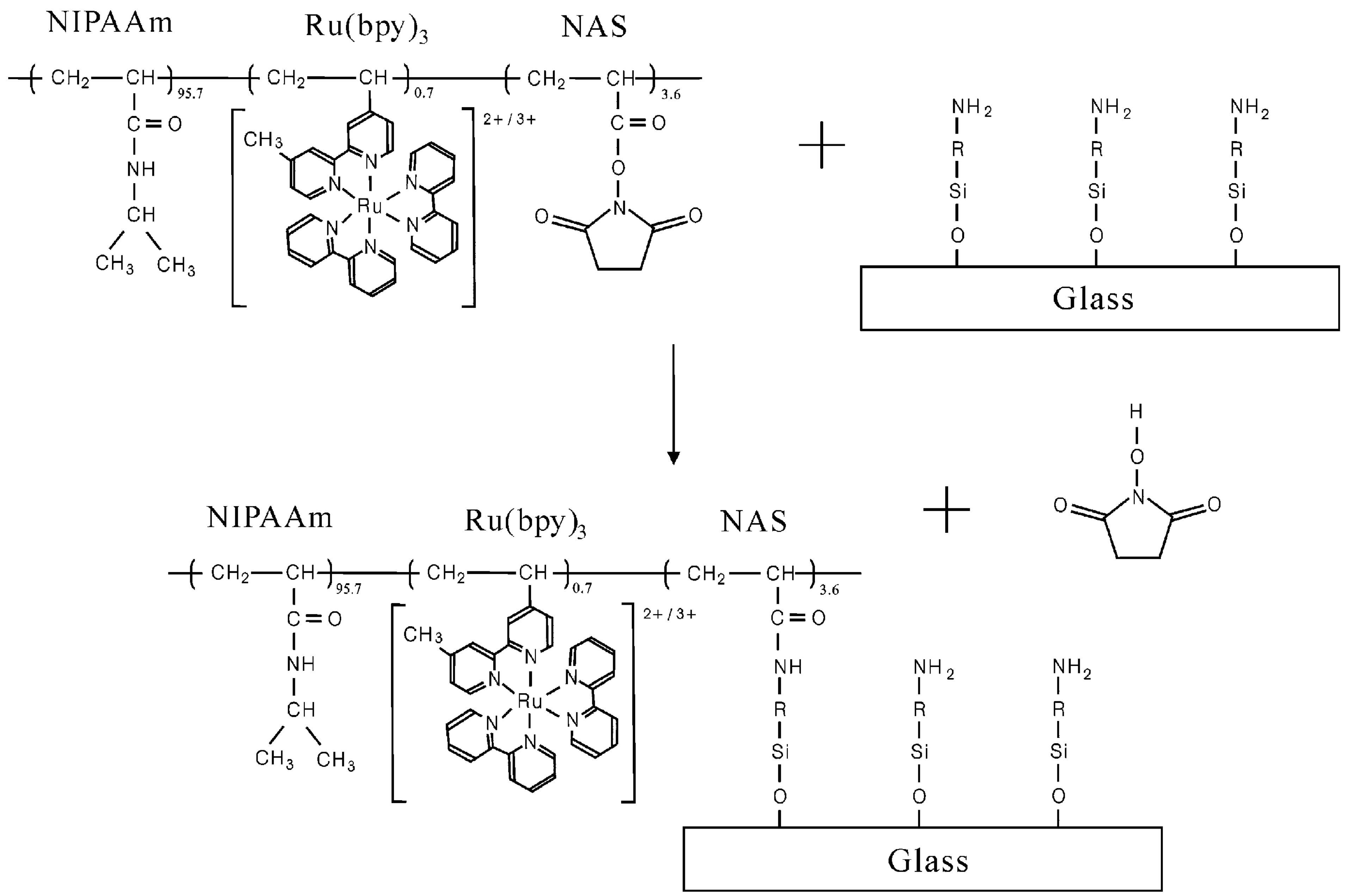
2. Strong-Acid-Free Gel
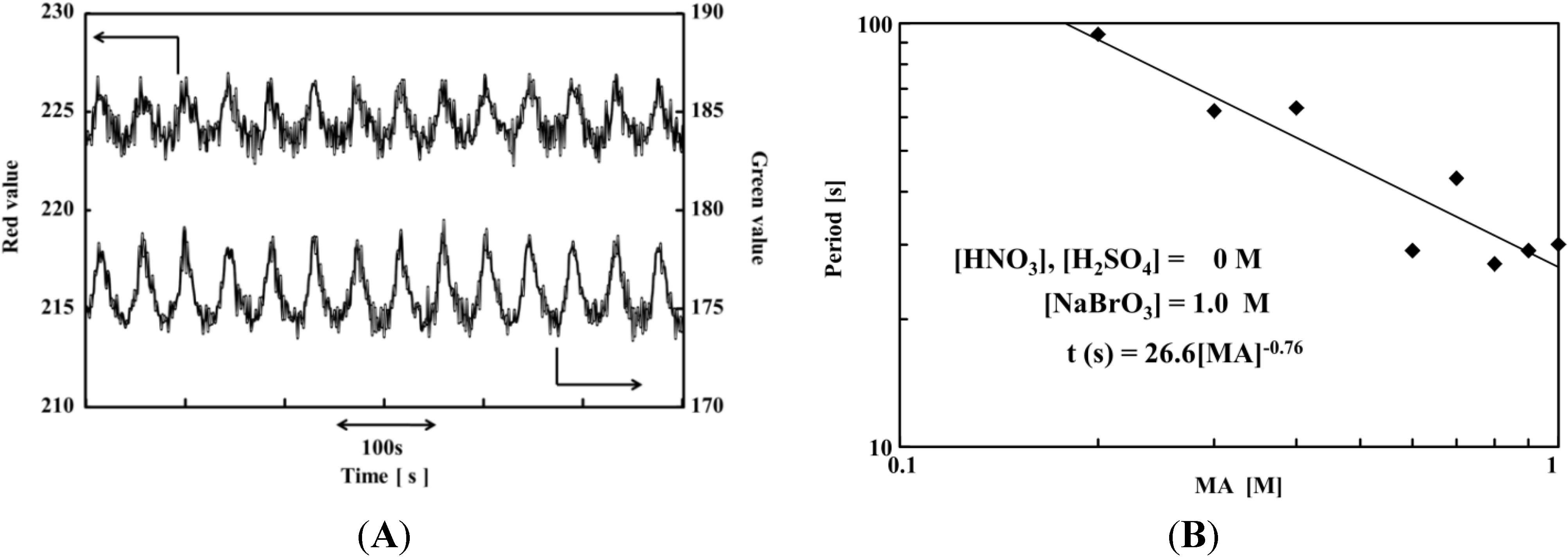
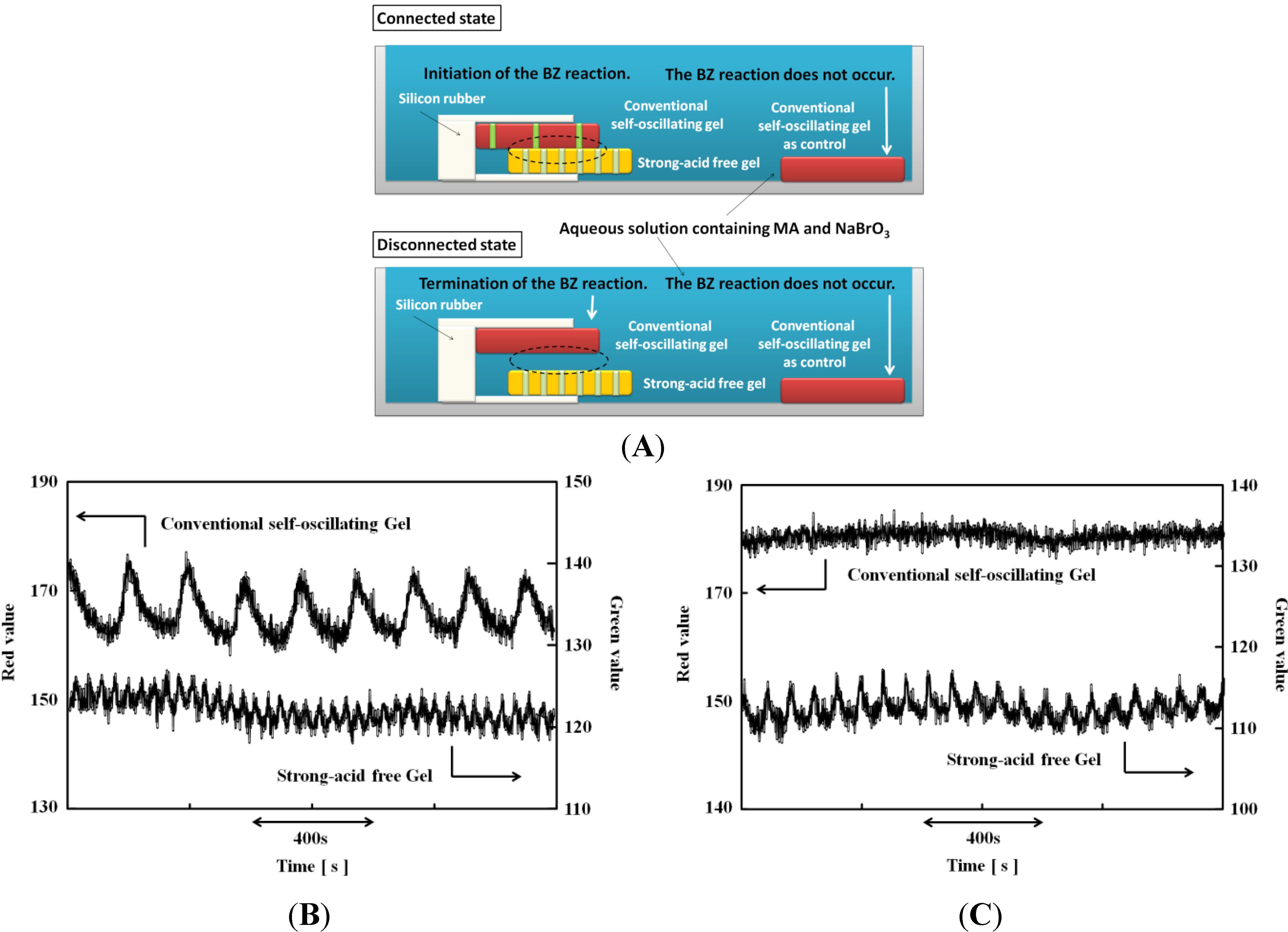
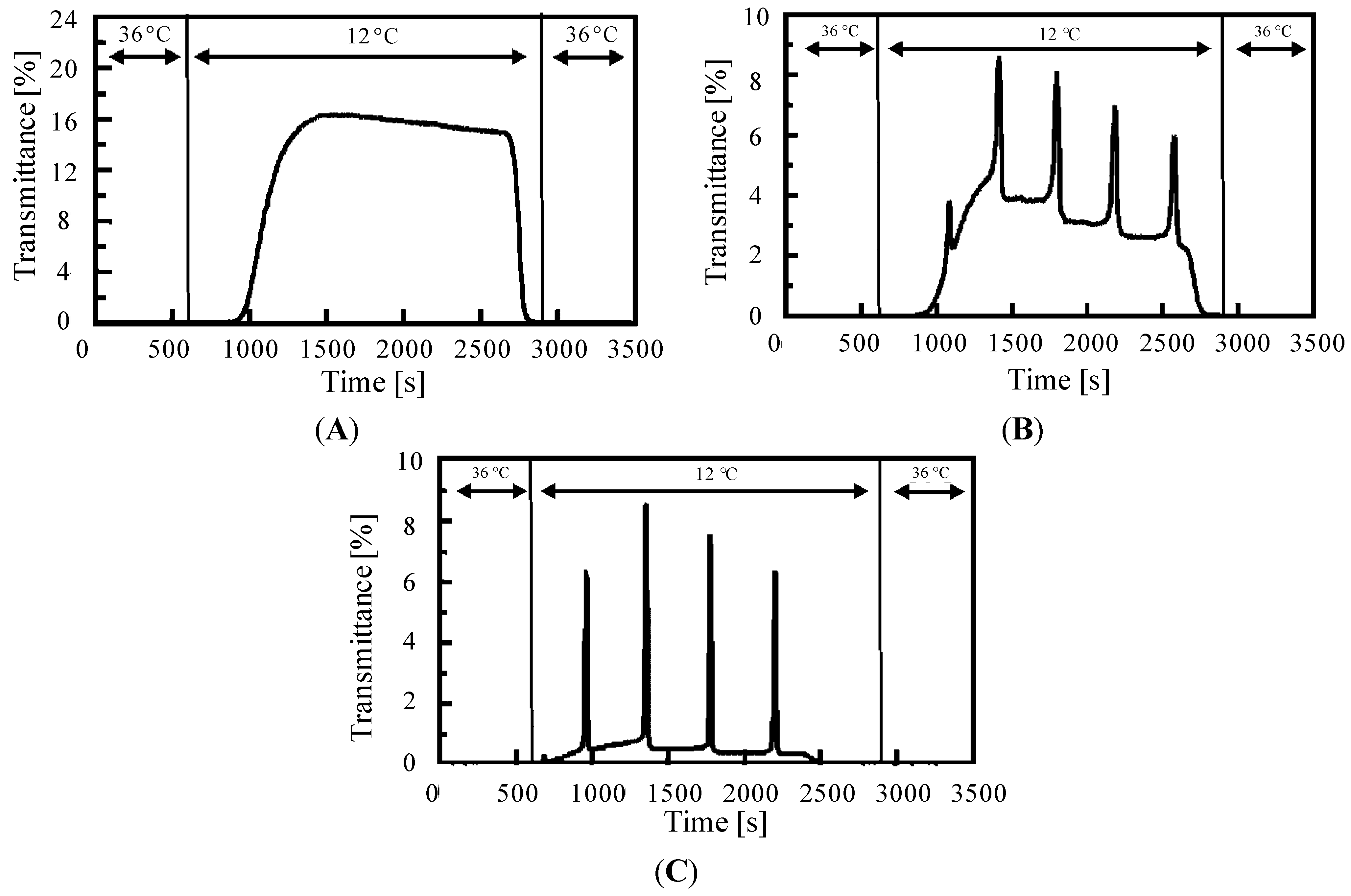
3. On/Off Switching of the AMPS-Containing Self-Oscillating Polymer Chain
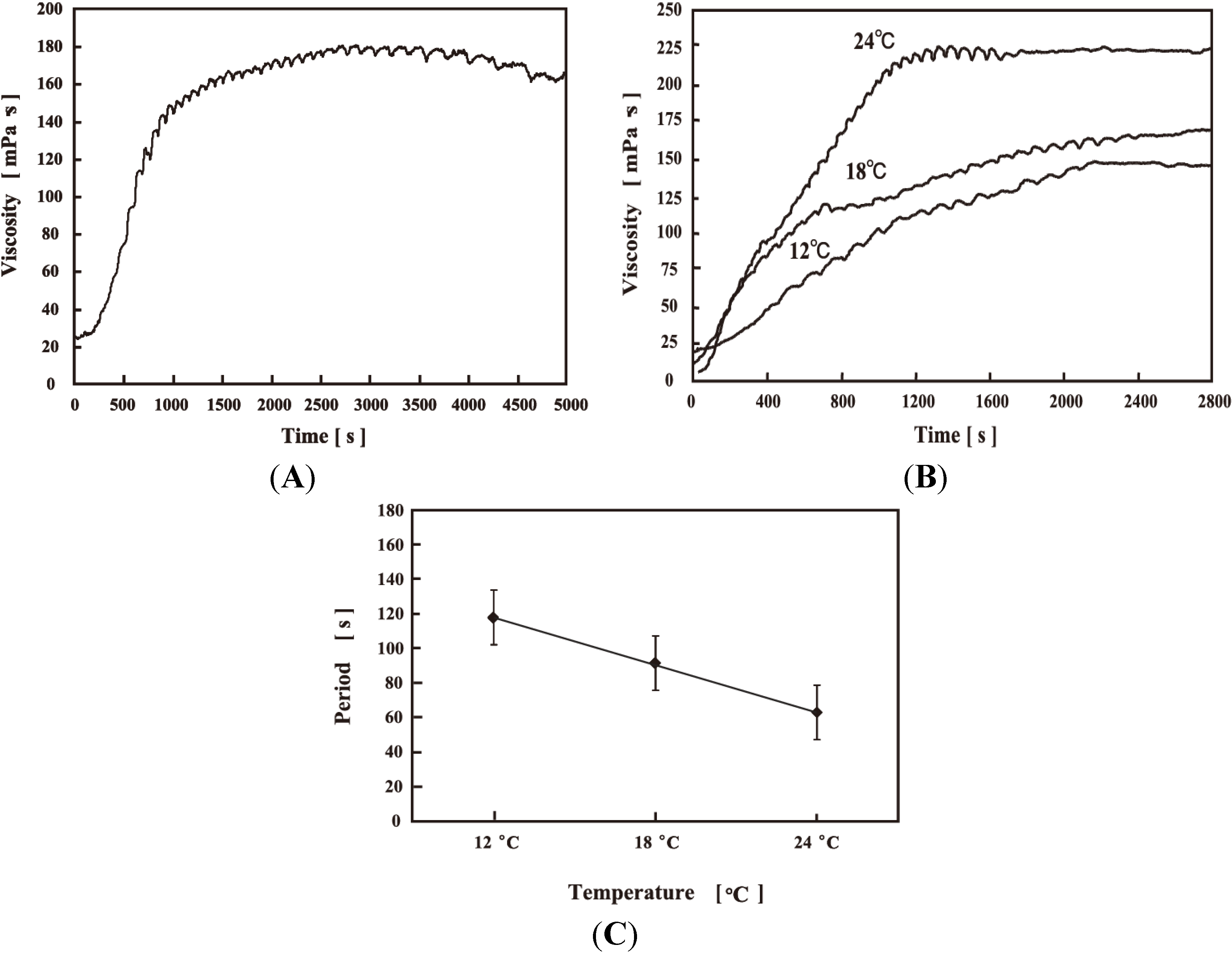
4. Viscosity Self-Oscillation of the AMPS-Containing Polymer Solution
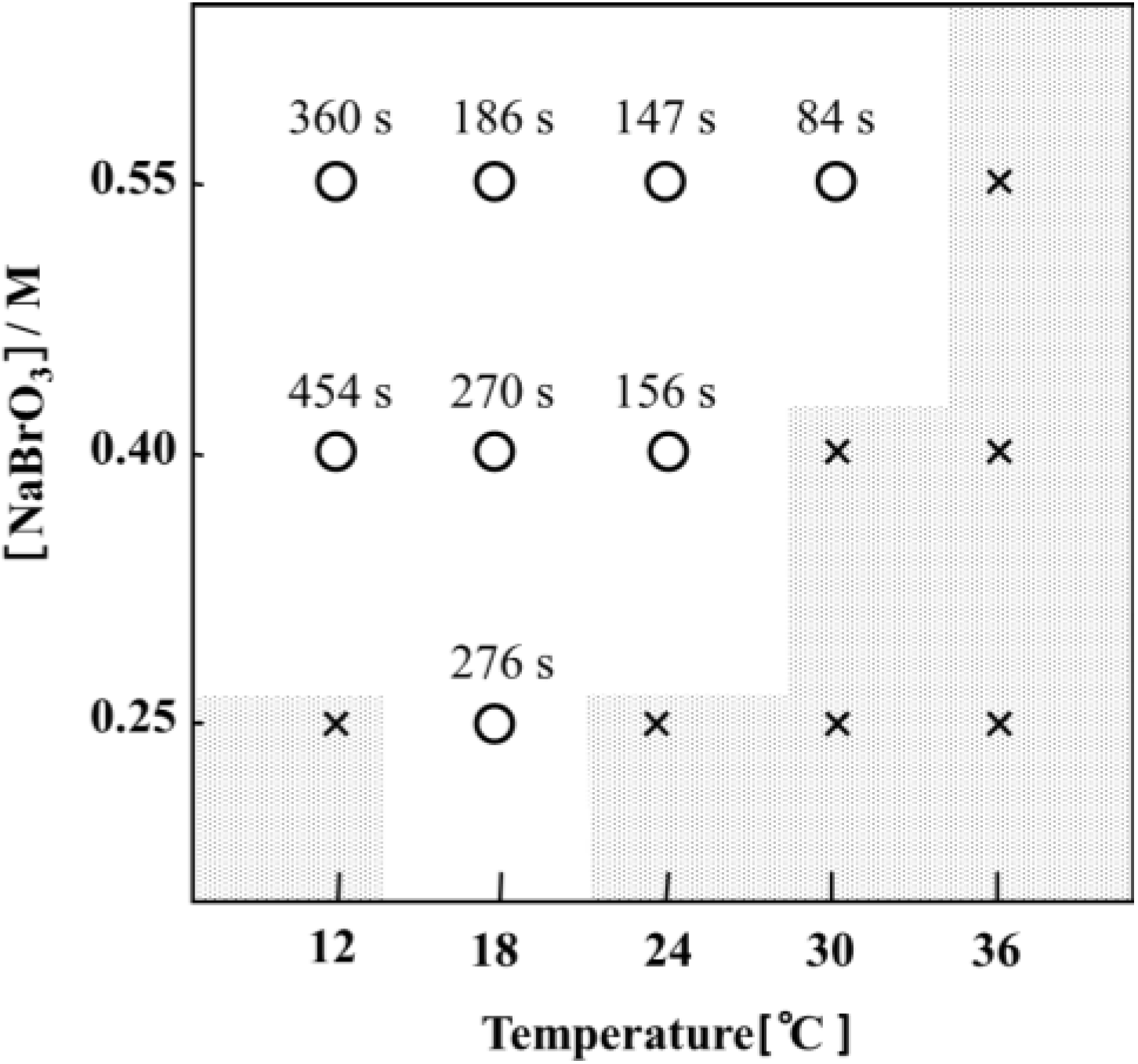
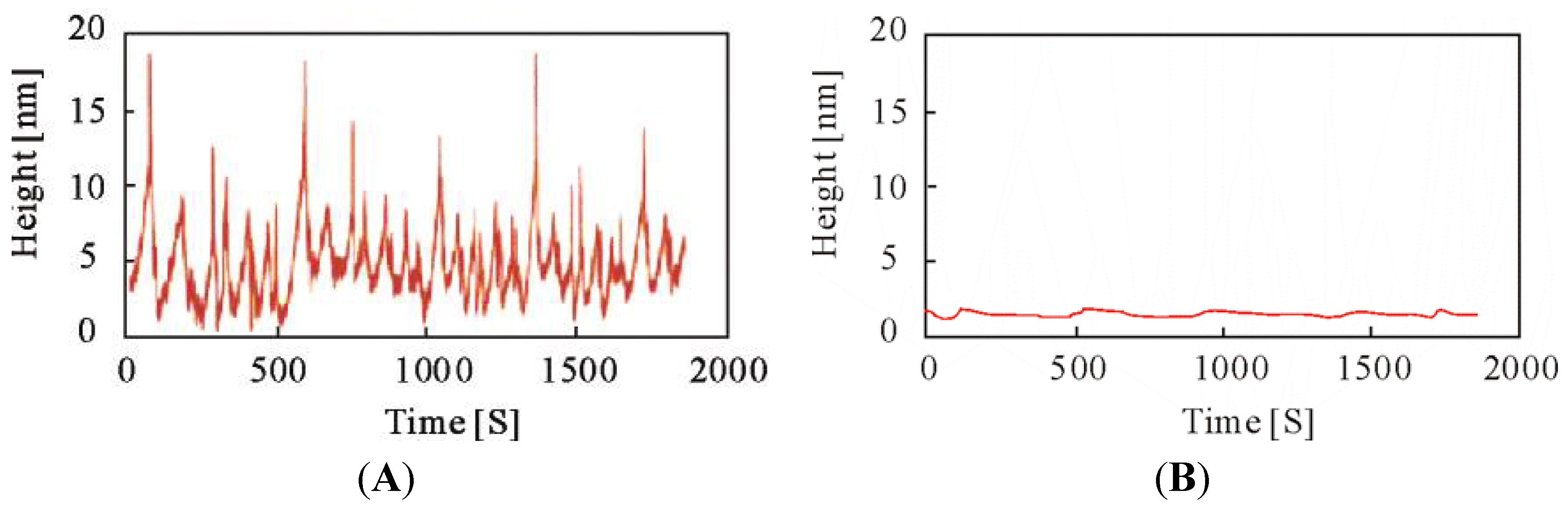
5. Direct Observation of the Autonomous Oscillation of the Single Polymer Chain
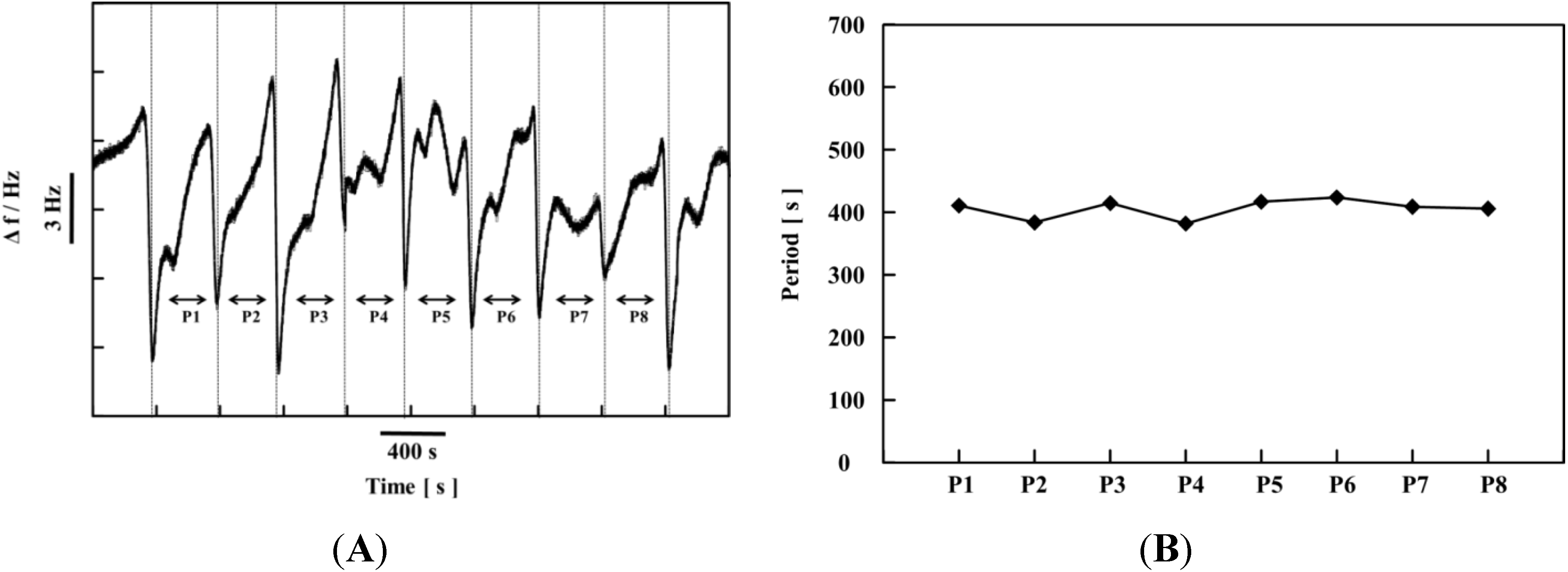
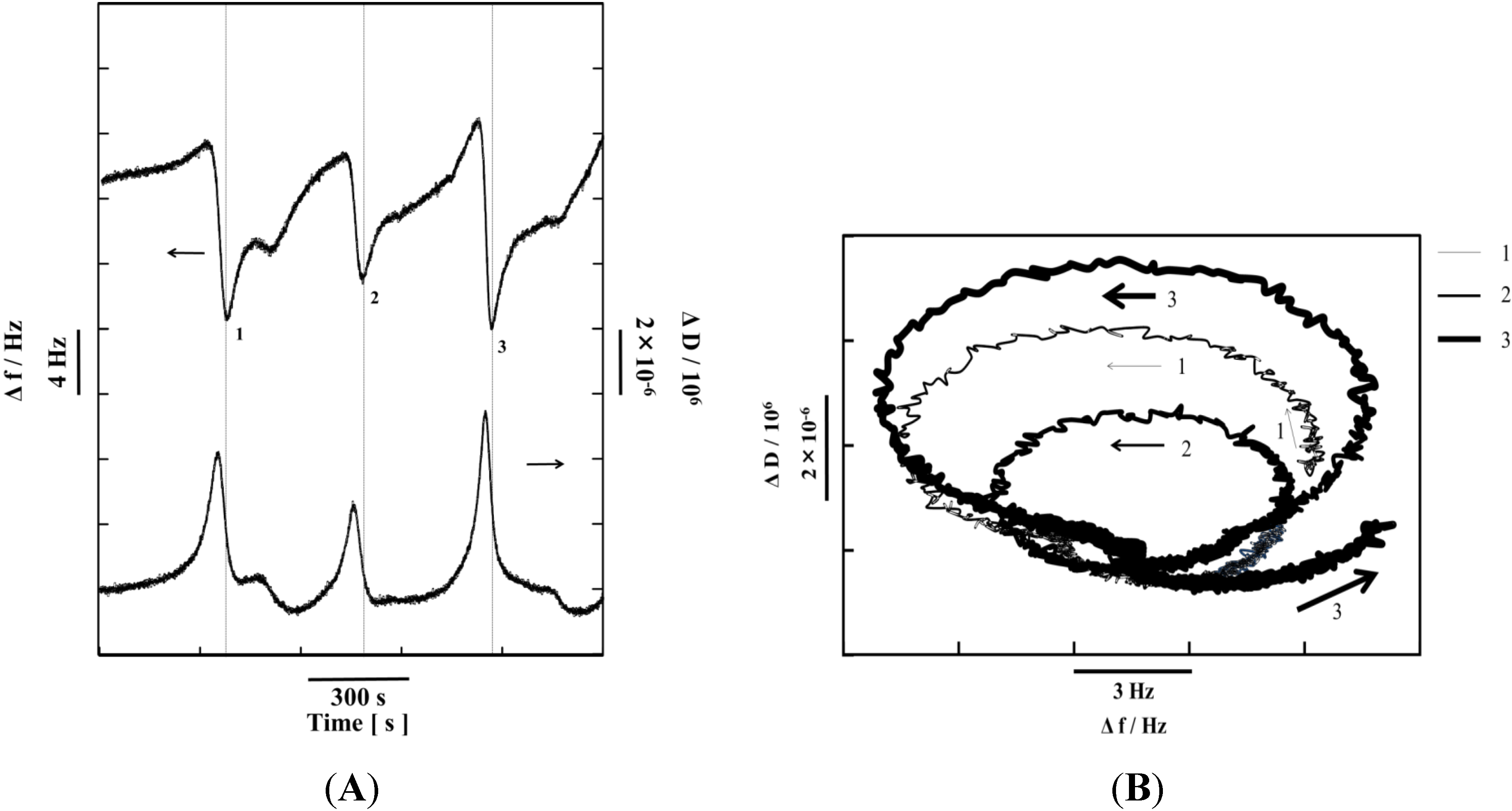
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Oguro, K.; Kawami, Y.; Takenaka, H. An Actuator Element of Polyelectrolyte Gel Membrane-Electrode Composite. Bull. Gov. Ind. Res. Inst. Osaka 1992, 43, 21–24. [Google Scholar]
- Baughman, R.H.; Cui, C.; Zakhidov, A.A.; Iqbal, Z.; Barisci, J.N.; Spinks, G.M.; Wallace, G.G.; Mazzoldi, A.; de Rossi, D.; Rinzler, A.G.; et al. Carbon Nanotube Actuators. Science 1999, 284, 1340–1344. [Google Scholar] [CrossRef]
- Pelrine, R.; Kornbluh, R.; Pei, Q.; Joseph, J. High-Speed Electrically Actuated Elastomers with Strain Greater Than 100%. Science 2000, 287, 836–839. [Google Scholar] [CrossRef]
- Bar-Cohen, Y. EAP Actuators as Artificial Muscles—Reality, Potential, and Challenges; International Society for Optical Engineering (SPIE): Bellingham, WA, USA, 2001; pp. 4–44. [Google Scholar]
- Fukushima, T.; Asaka, K.; Kosaka, A.; Aida, T. Fully Plastic Actuator through Layer-by-Layer Casting with Ionic-Liquid-Based Bucky Gel. Angew. Chem. 2005, 117, 2462–2465. [Google Scholar] [CrossRef]
- Mukai, K.; Asaka, K.; Sugino, T.; Kiyohara, K.; Takeuchi, I.; Terasawa, N.; Futaba, D.N.; Hata, K.; Fukushima, T.; Aida, T. Highly Conductive Sheets from Millimeter-Long Single-Walled Carbon Nanotubes and Ionic Liquids: Application to Fast-Moving, Low-Voltage Electromechanical Actuators Operable in Air. Adv. Mater. 2009, 21, 1582–1585. [Google Scholar] [CrossRef]
- Zhang, Q.M.; Li, H.; Poh, M.; Xia, F.; Cheng, Z.-Y.; Xu, H.; Huang, C. An All-Organic Composite Actuator Material with a High Dielectric Constant. Nature 2002, 419, 284–287. [Google Scholar] [CrossRef]
- Wissler, M.; Mazza, E. Modeling of a Pre-strained Circular Actuator Made of Dielectric Elastomer Actuators. Sens. Actuators A 2005, 120, 184–192. [Google Scholar] [CrossRef]
- Shankar, R.; Ghosh, T.K.; Spontak, R.J. Dielectric Elastomers as Next-Generation Polymeric Actuators. Soft Matter 2007, 3, 1116–1129. [Google Scholar] [CrossRef]
- Pons, J.L. Emerging Actuator Technologies: A Micromechatronic Approach; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Smela, E.; Inganäs, O.; Lundström, I. Controlled Folding of Micrometer-Size Structures. Science 1995, 268, 1735–1738. [Google Scholar]
- Baughman, R.H. Conducting Polymer Artificial Muscles. Synth. Met. 1996, 78, 339–353. [Google Scholar] [CrossRef]
- Doi, M.; Matsumoto, M.; Hirose, Y. Deformation of Ionic Polymer Gels by Electric Fields. Macromolecules 1992, 25, 5504–5511. [Google Scholar] [CrossRef]
- Ishiwatari, T.; Kawaguchi, M.; Mitsuishi, M. Oscillatory Reactions in Polymer Systems. J. Polym. Sci. Part A Polym. Chem. 1984, 22, 2699–2704. [Google Scholar] [CrossRef]
- Yoshida, R.; Sakai, T.; Ito, S.; Yamaguchi, T. Self-Oscillation of Polymer Chains with Rhythmical Soluble-Insoluble Changes. J. Am. Chem. Soc. 2002, 124, 8095–8098. [Google Scholar] [CrossRef]
- Yoshida, R.; Takahashi, T.; Yamaguchi, T.; Ichijo, H. Self-Oscillating Gel. J. Am. Chem. Soc. 1996, 118, 5134–5135. [Google Scholar] [CrossRef]
- Zaikin, A.N.; Zhabotinsky, A.M. Concentration Wave Propagation in Two-Dimensional Liquid-Phase Self-Oscillating System. Nature 1970, 225, 535–537. [Google Scholar] [CrossRef]
- Reusser, E.J.; Field, R.J. The Transition from Phase Waves to Trigger Waves in a Model of the Zhabotinsky Reaction. J. Am. Chem. Soc. 1979, 101, 1063–1094. [Google Scholar] [CrossRef]
- Gyorgyi, L.; Turanyi, T.; Field, R.J. Mechanistic Details of the Oscillatory Belousov-Zhabotinsky Reaction. J. Phys. Chem. 1990, 94, 7162–7170. [Google Scholar] [CrossRef]
- Scott, S.K. Chemical Chaos, 1st ed.; Oxford University Press: Oxford, UK, 1991. [Google Scholar]
- Field, R.J.; Burger, M. Oscillations and Traveling Waves in Chemical Systems; John Wiley & Sons: New York, NY, USA, 1985. [Google Scholar]
- Nicolis, G.; Prigogine, I. Self-Organization in Nonequilibrium. Systems; John Wiley & Sons: New York, NY, USA, 1977. [Google Scholar]
- Murray, J.D. Mathematical Biology; Springer-Verlag: Berlin, Germany, 1990. [Google Scholar]
- Hara, Y.; Yoshida, R. Self-Oscillation of Polymer Chains Induced by the Belousov-Zhabotinsky Reaction under Acid-Free Conditions. J. Phys. Chem. B 2005, 109, 9451–9454. [Google Scholar] [CrossRef]
- Hara, Y.; Yoshida, R. Damping Behavior of Aggregation-Disaggregation Self-Oscillation for a Polymer Chain. Macromol. Rapid Commun. 2009, 30, 1656–1662. [Google Scholar] [CrossRef]
- Hara, Y.; Sakai, T.; Maeda, S.; Hashimoto, S.; Yoshida, R. Self-Oscillating Soluble−Insoluble Changes of a Polymer Chain Including an Oxidizing Agent Induced by the Belousov−Zhabotinsky Reaction. J. Phys. Chem. B 2005, 109, 23316–23319. [Google Scholar] [CrossRef]
- Hara, Y.; Yoshida, R. Self-Oscillating Polymer Fueled by Organic Acid. J. Phys. Chem. B 2008, 112, 8427–8429. [Google Scholar] [CrossRef]
- Hara, Y.; Yamaguchi, Y.; Mayama, H. Switching the BZ Reaction with a Strong-Acid-Free Gel. J. Phys. Chem. B 2014, 118, 634–638. [Google Scholar] [CrossRef]
- Hara, Y.; Yoshida, R. Control of Oscillating Behavior for the Self-Oscillating Polymer with pH-Control Site. Langmuir 2005, 21, 9773–9776. [Google Scholar] [CrossRef]
- Hara, Y.; Yoshida, R. A Viscosity Self-Oscillation of Polymer Solution Induced by the BZ Reaction under Acid-Free Conditions. J. Chem. Phys. 2008, 128, 224904. [Google Scholar] [CrossRef]
- Maeda, S.; Hara, Y.; Yoshida, R.; Hashimoto, S. Control of the Dynamic Motion of a Gel Actuator Driven by the Belousov–Zhabotinsky Reaction. Macromol. Rapid Commun. 2008, 29, 401–405. [Google Scholar] [CrossRef]
- Maeda, S.; Hara, Y.; Sakai, T.; Yoshida, R.; Hashimoto, S. Self-Walking Gel. Adv. Mater. 2007, 19, 3480–3484. [Google Scholar] [CrossRef]
- Maeda, S.; Hara, Y.; Yoshida, R.; Hashimoto, S. Peristaltic Motion of Polymer Gels. Angew. Chem. Int. Ed. 2008, 120, 6792–6795. [Google Scholar] [CrossRef]
- Ito, Y.; Hara, T.; Uetsuka, H.; Hasuda, H.; Onishi, H.; Arakawa, H.; Ikai, A.; Yoshida, R. AFM Observation of Immobilized Self-Oscillating Polymer. J. Phys. Chem. B 2006, 110, 5170–5173. [Google Scholar] [CrossRef]
- Hara, Y.; Mayama, H.; Yamaguchi, Y.; Takenaka, Y.; Fukuda, F. Direct Observation of Periodic Swelling and Collapse of Polymer Chains Induced by the Belousov–Zhabotinsky Reaction. J. Phys. Chem. B 2013, 117, 14351–14357. [Google Scholar]
- Hara, Y.; Rumana, J.A. Activation Energy of Aggregation-Disaggregation Self-Oscillation of Polymer Chains. Int. J. Mol. Sci. 2012, 13, 16281–16290. [Google Scholar] [CrossRef]
- Miyakawa, K.; Sakamoto, F.; Yoshida, R.; Yamaguchi, T.; Kokufuta, E. Chemical Waves in Self-Oscillating Gels. Phys. Rev. E 2000, 62, 793–798. [Google Scholar] [CrossRef]
- Hara, Y.; Takenaka, Y. Autonomous Oscillation of Polymer Chains Induced by the Belousov–Zhabotinsky Reaction. Sensors 2014, 14, 1497–1510. [Google Scholar] [CrossRef]
- Field, R.J.; Koros, E.; Noyes, R.M. Oscillations in Chemical Systems. II. Thorough Analysis of Temporal Oscillation in the Bromate-Cerium-Malonic Acid System. J. Am. Chem. Soc. 1972, 94, 8649–8664. [Google Scholar] [CrossRef]
- Field, R.J.; Noyes, R.M. Oscillations in Chemical Systems. IV. Limit Cycle Behavior in a Model of a Real Chemical Reaction. J. Chem. Phys. 1974, 60, 1877–1884. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, G. Periodic Swelling and Collapse of Polyelectrolyte Brushes Driven by Chemical Oscillation. J. Phys. Chem. B 2008, 112, 10137–10141. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hara, Y. Function and Autonomous Behavior of Self-Oscillating Polymer Systems. Polymers 2014, 6, 1958-1971. https://doi.org/10.3390/polym6071958
Hara Y. Function and Autonomous Behavior of Self-Oscillating Polymer Systems. Polymers. 2014; 6(7):1958-1971. https://doi.org/10.3390/polym6071958
Chicago/Turabian StyleHara, Yusuke. 2014. "Function and Autonomous Behavior of Self-Oscillating Polymer Systems" Polymers 6, no. 7: 1958-1971. https://doi.org/10.3390/polym6071958



