Effect of Mannitol on Hyaluronic Acid Stability in Two in Vitro Models of Oxidative Stress
Abstract
:1. Introduction
2. Experimental Section
2.1. Induction of an Oxidative Stress
2.1.1. X/XO Model
2.1.2. H2O2 Model
2.2. Rheology
2.3. Molecular Weight Measurement
3. Results and Discussion
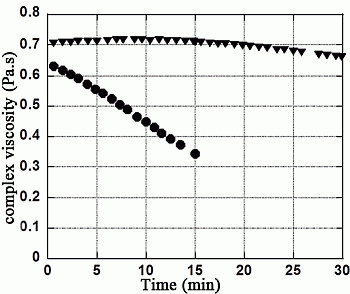
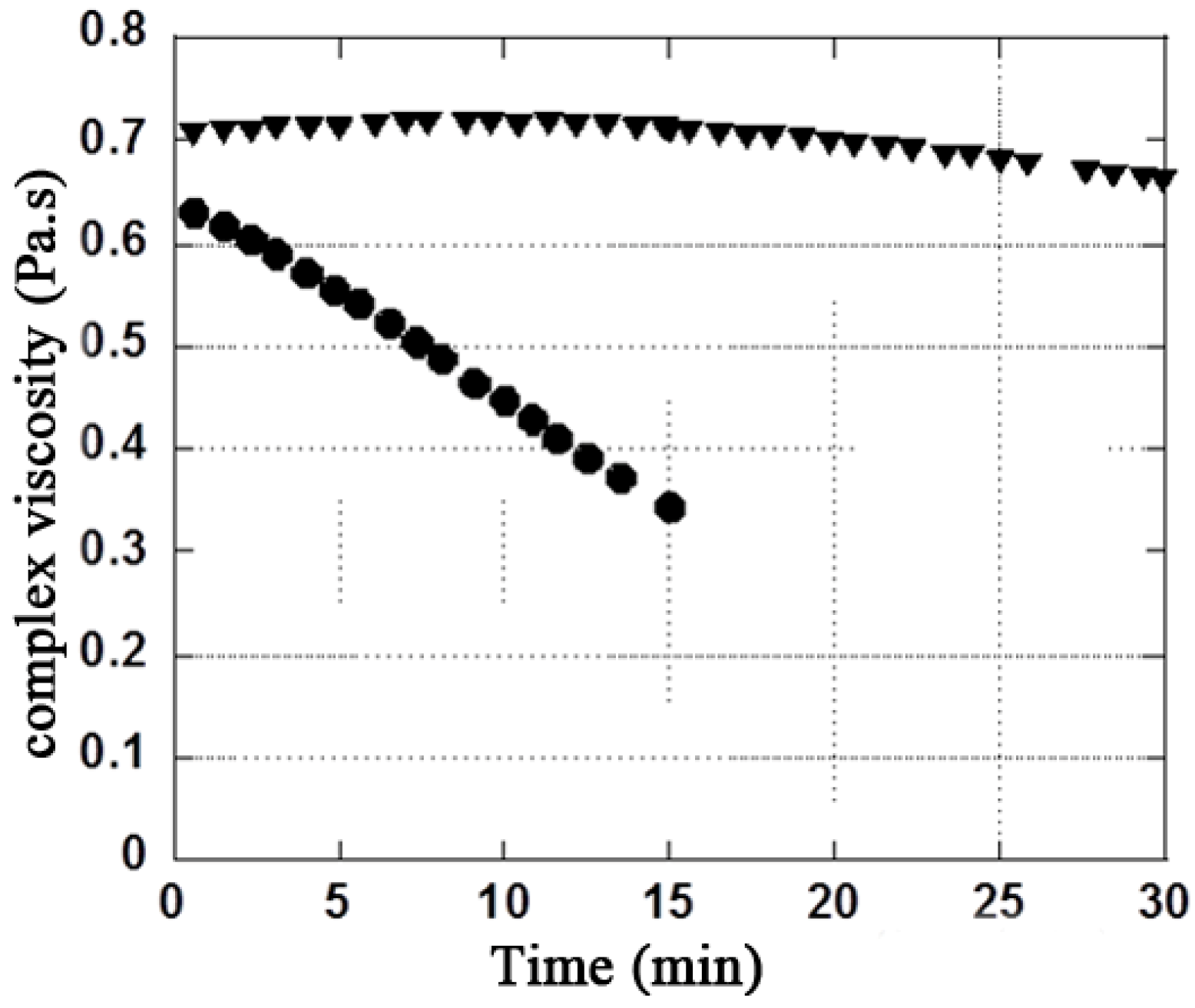
3.1. X/XO Stress Model
| Solution | Initial MW (g·mol−1) | Final MW (g·mol−1) +16 μL XO | Final Mw (g·mol−1) +32 μL XO | Difference (%) between initial and final MW with 32 μL XO |
|---|---|---|---|---|
| Hyaluronic acid (HA) | 787,000 | 621,450 | 498,900 | −36.6% |
| HA + Mannitol 35g·L−1 | 768,900 | 717,750 | 677,150 | −11.9% |
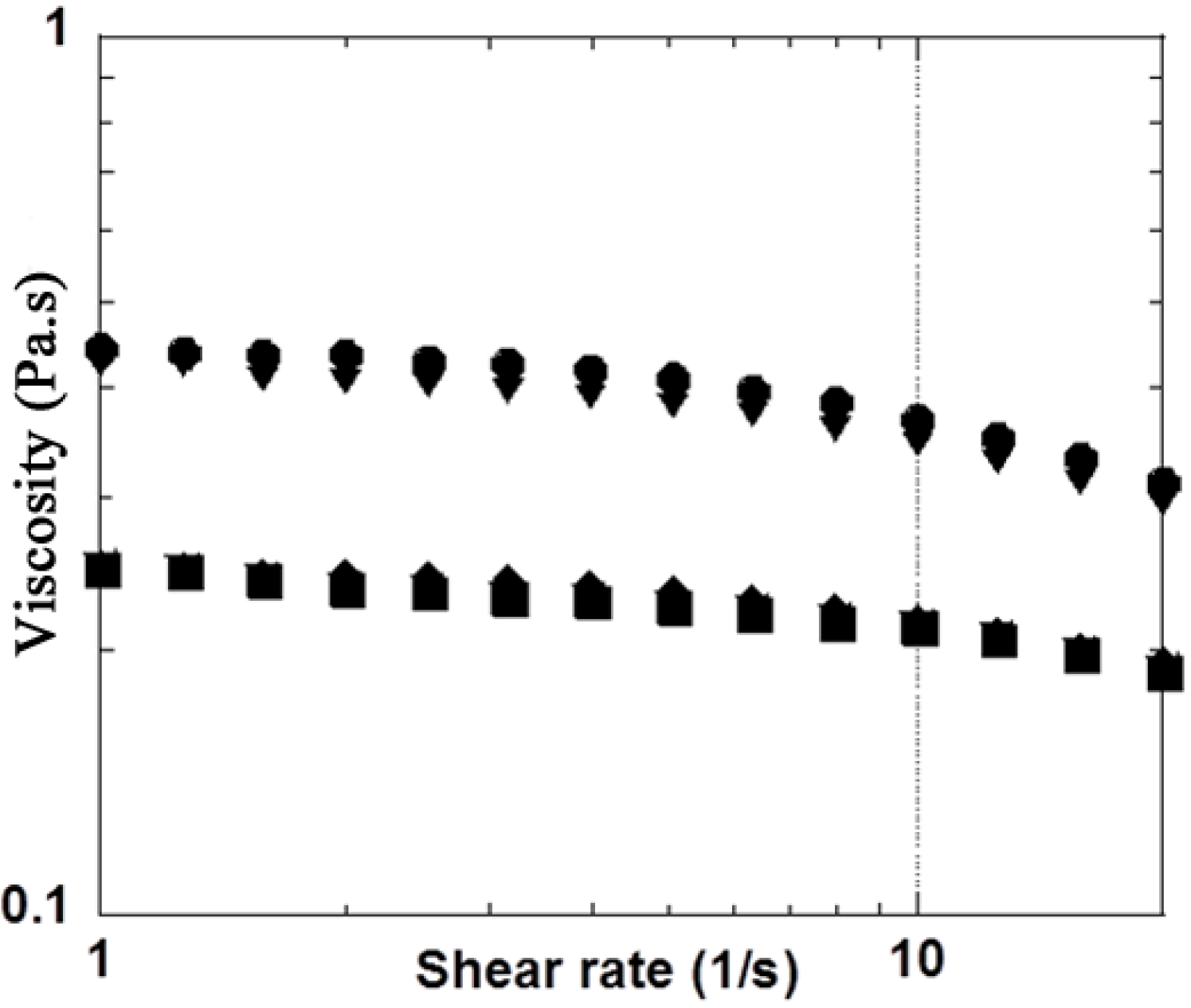
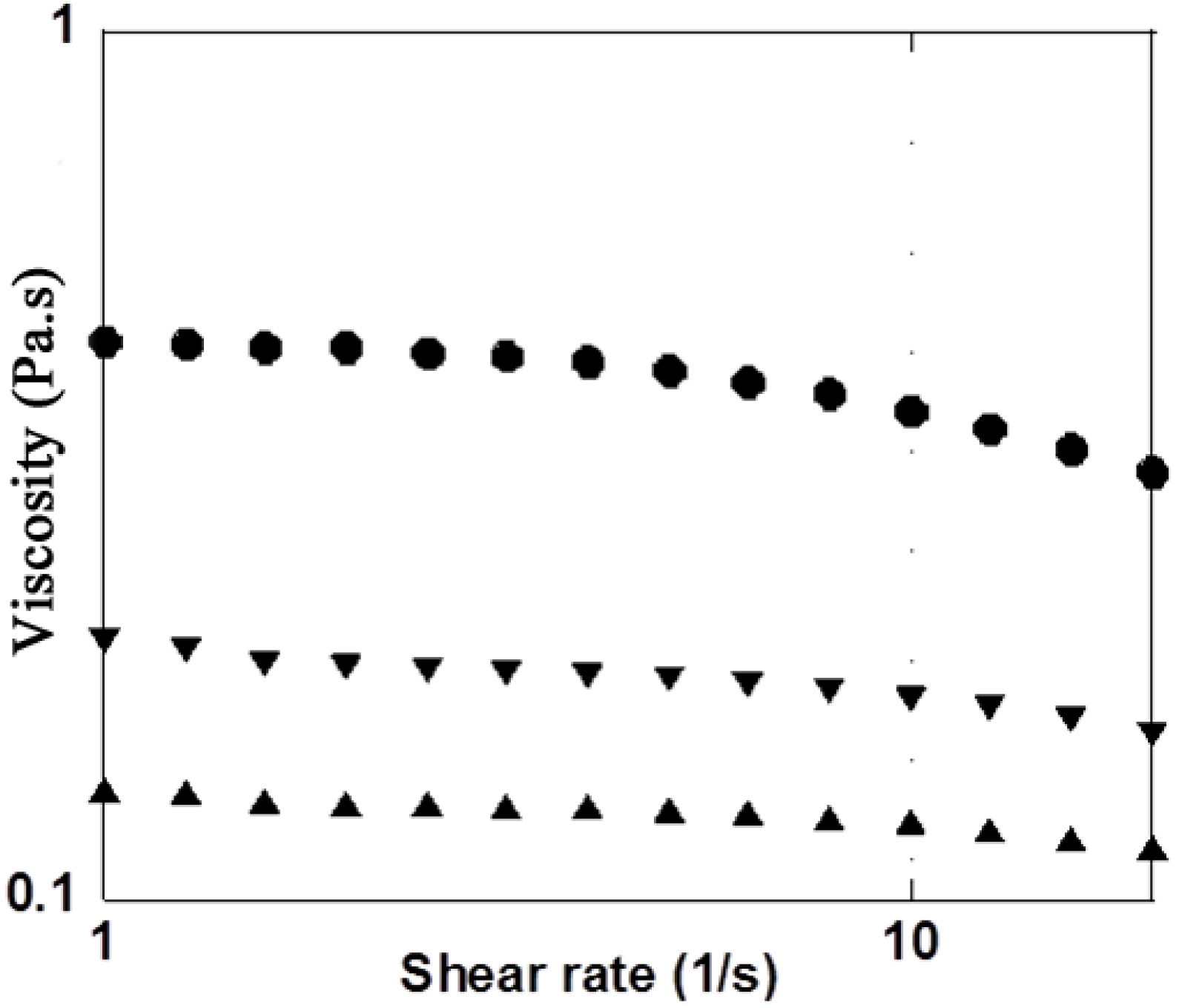
3.2. H2O2 Model
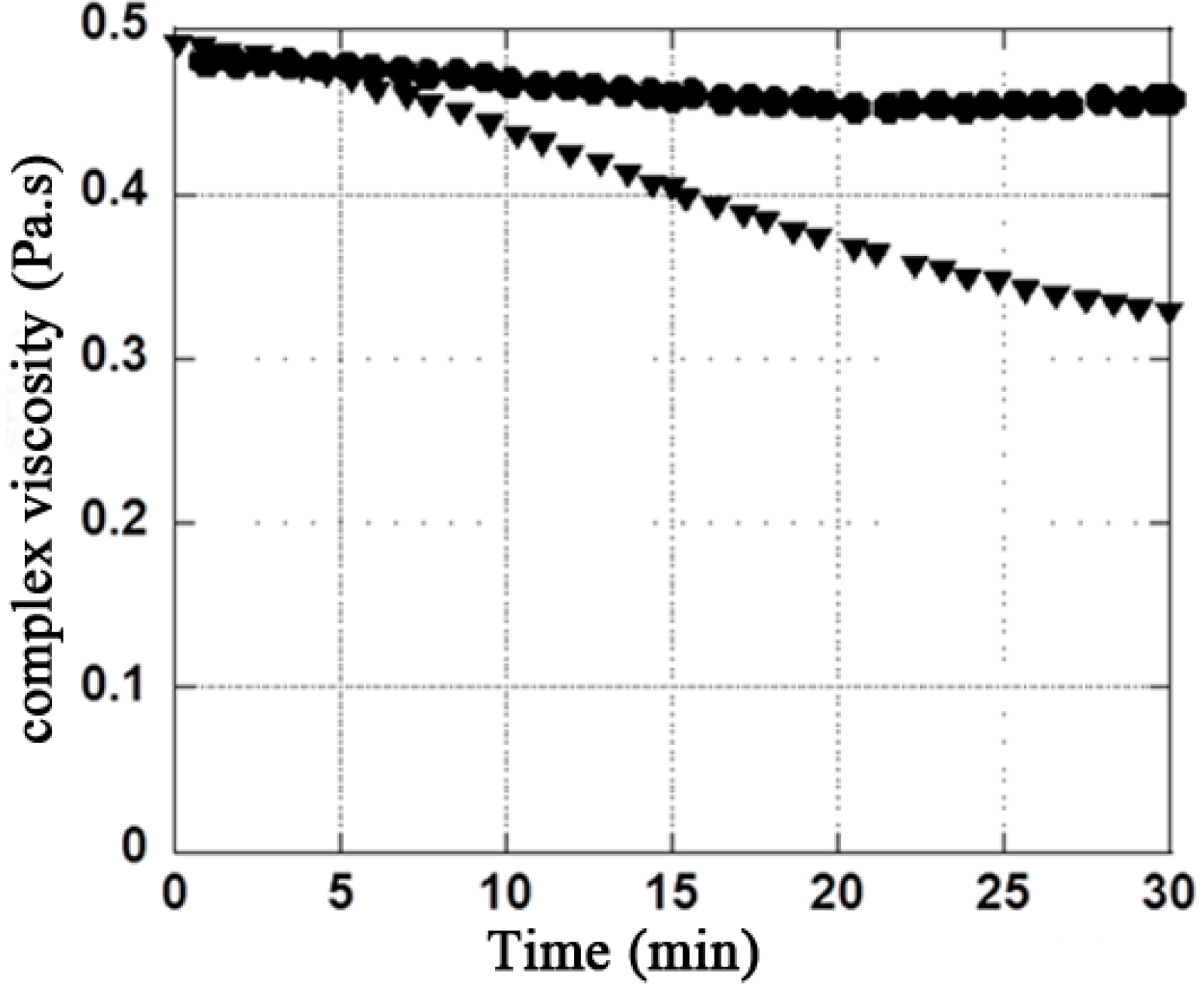
| Mannitol Concentration (g·L−1) | Rheological properties | Initial values | Final values in presence of H2O2 (2.7%) | Ratio (difference in %) Initial / Final |
|---|---|---|---|---|
| 10 | G′ (Pa) | 0.88 | 0.64 | 1.37 / (−27.2%) |
| 10 | G″ (Pa) | 2.90 | 2.40 | 1.21 / (−17.2%) |
| 10 | |η*| (Pa·s) | 0.49 | 0.40 | 1.22 / (−18.4%) |
| 35 | G′ (Pa) | 0.87 | 0.82 | 1.06 / (−5.7%) |
| 35 | G″ (Pa) | 2.90 | 2.77 | 1.05 / (−4.4 %) |
| 35 | |η*| (Pa·s) | 0.48 | 0.46 | 1.04 / (−4.2%) |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Balazs, E.A.; Denlinger, J.L. Viscosupplementation: A new concept in the treatment of osteoarthritis. J. Rheumatol. Suppl. 1993, 39, 3–9. [Google Scholar]
- Balazs, E.A. Viscosupplementation for treatment of osteoarthritis: From initial discovery to current status and results. Surg. Technol. Int. 2004, 12, 278–289. [Google Scholar]
- Pelletier, J.P.; Martel-Pelletier, J. The pathophysiology of osteoarthritis and the implication of the use of hyaluronan and hylan as therapeutic agents in viscosupplementation. J. Rheumatol. 1993, 20, 19–23. [Google Scholar]
- Bagga, H.; Burkhardt, D.; Sambrook, P.; March, L. Long term effects of intra-particular hyaluronan on synovial fluid in osteoarthritis of the knee. J. Rheumatol. 2006, 33, 946–950. [Google Scholar]
- Mathieu, P.; Conrozier, T.; Vignon, E; Rozand, Y.; Rinaudo, M. Rheologic behavior of osteoarthritic synovial fluid after addition of hyaluronic acid: A pilot study. Clin. Orthop. Relat. Res. 2009, 467, 3002–3009. [Google Scholar]
- Conrozier, T.; Mathieu, P.; Vignon, E.; Piperno, M.; Rinaudo, M. Differences in the osteoarthritic synovial fluid composition and rheology between patients with or without flare: A pilot study. Clin. Exp. Rheumatol. 2012, 30, 729–734. [Google Scholar]
- Bellamy, N.; Campbell, J.; Robinson, V.; Gee, T.; Bourne, R.; Wells, G.A. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst. Rev. 2006, 2. [Google Scholar] [CrossRef]
- Miller, L.E.; Block, J.E. US-approved intra-articular hyaluronic acid injections are safe and effective in patients with knee osteoarthritis: Systematic review and meta-analysis of randomized, saline-controlled trials. Clin. Med. Insight. Arthr. Musculoskelet. Disord. 2013, 6, 57–63. [Google Scholar]
- Bannuru, R.R.; Vaysbrot, E.E.; Sullivan, M.C.; McAlindon, T.E. Relative efficacy of hyaluronic acid in comparison with NSAIDs for knee osteoarthritis: A systematic review and meta-analysis. Semin. Arthr. Rheum. 2013, 43, 593–599. [Google Scholar]
- Rutjes, A.W.; Jüni, P.; da Costa, B.R.; Trelle, S.; Nüesch, E.; Reichenbach, S. Viscosupplementation for osteoarthritis of the knee: A systematic review and meta-analysis. Ann. Intern. Med. 2012, 157, 180–191. [Google Scholar] [CrossRef]
- Hochberg, M.C.; Altman, R.D.; April, K.T.; Benkhalti, M.; Guyatt, G.; McGowan, J.; Towheed, T.; Welch, V.; Wells, G.; Tugwell, P. American college of rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthr. Care Res. 2012, 64, 465–474. [Google Scholar]
- Colen, S.; van den Bekerom, M.P.; Mulier, M.; Haverkamp, D. Hyaluronic acid in the treatment of knee osteoarthritis: A systematic review and meta-analysis with emphasis on the efficacy of different products. Bio. Drugs 2012, 26, 257–268. [Google Scholar]
- Lindenhayn, K.; Heilmann, H.H.; Niederhausen, T.; Walther, H.U.; Pohlenz, K. Elimination of tritium-labelled hyaluronic acid from normal and osteoarthritic rabbit knee joints. Eur. J. Clin. Chem. Clin. Biochem. 1997, 35, 355–363. [Google Scholar]
- Lindqvist, U.; Tolmachev, V.; Kairemo, K.; Aström, G.; Jonsson, E.; Lundqvist, H. Elimination of stabilised hyaluronan from the knee joint in healthy men. Clin. Pharmacokinet. 2002, 4, 603–613. [Google Scholar]
- Larsen, N.E.; Dursema, H.D.; Pollak, C.T.; Skrabut, E.M. Clearance kinetics of a hylan-based viscosupplement after intra-articular and intravenous administration in animal models. J. Biomed. Mater. Res. B Appl. Biomater. 2011. [Google Scholar] [CrossRef]
- Stern, R.; Kogan, G.; Jedrzejas, M.J.; Soltés, L. The many ways to cleave hyaluronan. Biotechnol. Adv. 2007, 25, 537–557. [Google Scholar] [CrossRef]
- Hokputsa, S.; Jumel, K. A comparison of molecular mass determination of hyaluronic acid using SEC/MALLS and sedimentation equilibrium. Eur. Biophys. J. 2003, 32, 450–456. [Google Scholar] [CrossRef]
- Grange, L.; Nguyen, M.V.; Lardy, B.; Derouazi, M.; Campion, Y.; Trocme, C.; Paclet, M.H.; Gaudin, P.; Morel, F. NAD(P)H oxidase activity of Nox4 in chondrocytes is both inducible and involved in collagenase expression. Antioxid. Redox. Signal. 2006, 8, 1485–1496. [Google Scholar] [CrossRef]
- Rousset, F.; Nguyen, M.V.; Grange, L.; Morel, F.; Lardy, B. Heme oxygenase-1 regulates matrix metalloproteinase MMP-1 secretion and chondrocyte cell death via Nox4 NADPH oxidase activity in chondrocytes. PLoS One 2013, 8. [Google Scholar] [CrossRef]
- Rousset, F.; Lardy, B.; Grange, L.; Morel, F. Differential impact of glucosamine sulfate and cuivramine on the il-1β stimulated c-20/a4 chondrocyte cell line in vitro. Osteoarthr. Cartil. 2012, 20. [Google Scholar] [CrossRef]
- Mannitol. INCHEM International program for Chemical safety (IPCS). Available online: http://www.inchem.org/documents/jecfa/jecmono/v21je10.htm (accessed on 6 June 2014).
- VIDAL. Available online: http://www.vidal.fr/recherche/index/q:mannitol/sorbitol/classe_therapeutique (accessed on 6 June 2014).
- Heisel, J.; Kipshoven, C. Safety and efficacy findings from a non-interventional study of a new hyaluronic acid/sorbitol formulation (GO-ON matrix) for intra-articular injection to relieve pain and disability in osteoarthritis patients. Drug Res. 2013, 63, 445–449. [Google Scholar] [CrossRef]
- Mendoza, G.; Alvarez, A.I.; Pulido, M.M.; Molina, A.J.; Merino, G.; Real, R.; Fernandes, P.; Prieto, J.G. Inhibitory effects of different antioxidants on hyaluronan depolymerization. Carbohydr. Res. 2007, 342, 96–102. [Google Scholar] [CrossRef]
- Belda, J.I.; Artola, A.; García-Manzanares, M.D.; Ferrer, C.; Haroun, H.E.; Hassanein, A.; Baeyens, V.; Munoz, G.; Alió, J.L. Hyaluronic acid combined with mannitol to improve protection against free-radical endothelial damage: Experimental model. J. Cataract. Refract. Surg. 2005, 31, 1213–1218. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Y.; Hu, M.L.; Ding, J.; Xu, C.; Wang, R. In vitro antioxidant activities of the polysaccharides from Tricholoma lobayense. Int. J. boil. Macromol. 2012, 50, 534–539. [Google Scholar] [CrossRef]
- Raza, W.; Yang, W.; Jun, Y.; Shakoor, F.; Huang, Q.; Shen, Q. Optimization and characterization of a polysaccharide produced by Pseudomonas fluorescens WR-1 and its antioxidant activity. Carbohydr. Polym. 2012, 90, 921–929. [Google Scholar] [CrossRef]
- Yao, X.C.; Cao, Y.; Pan, S.K.; Wu, S.J. Preparation of peach gum polysaccharides using hydrogen peroxide. Carbohydr. Polym. 2013, 94, 88–90. [Google Scholar]
- Tankovska, M.; Soltes, L.; Vikartovska, A.; Mendichi, R.; Lath, D.; Molnarova, M.; Gemeiner, P. Sudy of hyaluronan degradation by means of rotational viscometry: Contribution of the material of viscometer. Chem. Pap. 2004, 58, 348–352. [Google Scholar]
- Milas, M.; Rinaudo, M. Characterization and Properties of Hyaluronic Acid (Hyaluronan). In Polysaccharides: Structural Diversity and Functional Versatility; Dimitriu, S., Ed.; Marcel Dekker Inc.: New York, NY, USA, 2004. [Google Scholar]
- Magovern, G.J., Jr.; Bolling, S.F.; Casale, A.S.; Bulkley, B.H.; Gardner, T.J. The mechanism of mannitol in reducing ischemic injury: Hyperosmolarity or hydroxyl scavenger? Circulation 1984, 70, 91–95. [Google Scholar]
- Suzuki, J.; Imaizumi, S.; Kayama, T.; Yoshimoto, T. Chemiluminescence in hypoxic brain—The second report: Cerebral protective effect of mannitol, vitamin E and glucocorticoid. Stroke 1985, 16, 695–700. [Google Scholar] [CrossRef]
- Fu, W.; Jiao, X. The effect of mannitol and anisodim on the prevention of free radical injury to post-ischemia flaps: an experimental study. Br. J. Plast. Surg. 1995, 48, 218–221. [Google Scholar] [CrossRef]
- Frati, E.; Khatib, A.M.; Front, P.; Panasyuk, A.; Aprile, F.; Mitrovic, D.R. Degradation of HA by photosensitized riboflavin in vitro: Modulation of the effect by transition metals, radical quenchers, and metal chelators. Free Radic. Biol. Med. 1997, 22, 1139–1144. [Google Scholar] [CrossRef]
- Borras-Verdera, A.; Calcedo-Bernal, V.; Ojeda-Levenfeld, J.; Clavel-Sainz, C. Efficacy and safety of a single intra-articular injection of 2% hyaluronic acid plus mannitol in knee osteoarthritis over a 6-month period. Rev. Esp. Cir. Ortop. Traumatol. 2012, 56, 274–280. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rinaudo, M.; Lardy, B.; Grange, L.; Conrozier, T. Effect of Mannitol on Hyaluronic Acid Stability in Two in Vitro Models of Oxidative Stress. Polymers 2014, 6, 1948-1957. https://doi.org/10.3390/polym6071948
Rinaudo M, Lardy B, Grange L, Conrozier T. Effect of Mannitol on Hyaluronic Acid Stability in Two in Vitro Models of Oxidative Stress. Polymers. 2014; 6(7):1948-1957. https://doi.org/10.3390/polym6071948
Chicago/Turabian StyleRinaudo, Marguerite, Bernard Lardy, Laurent Grange, and Thierry Conrozier. 2014. "Effect of Mannitol on Hyaluronic Acid Stability in Two in Vitro Models of Oxidative Stress" Polymers 6, no. 7: 1948-1957. https://doi.org/10.3390/polym6071948
APA StyleRinaudo, M., Lardy, B., Grange, L., & Conrozier, T. (2014). Effect of Mannitol on Hyaluronic Acid Stability in Two in Vitro Models of Oxidative Stress. Polymers, 6(7), 1948-1957. https://doi.org/10.3390/polym6071948