Development of Polymer Acceptors for Organic Photovoltaic Cells
Abstract
:1. Introduction
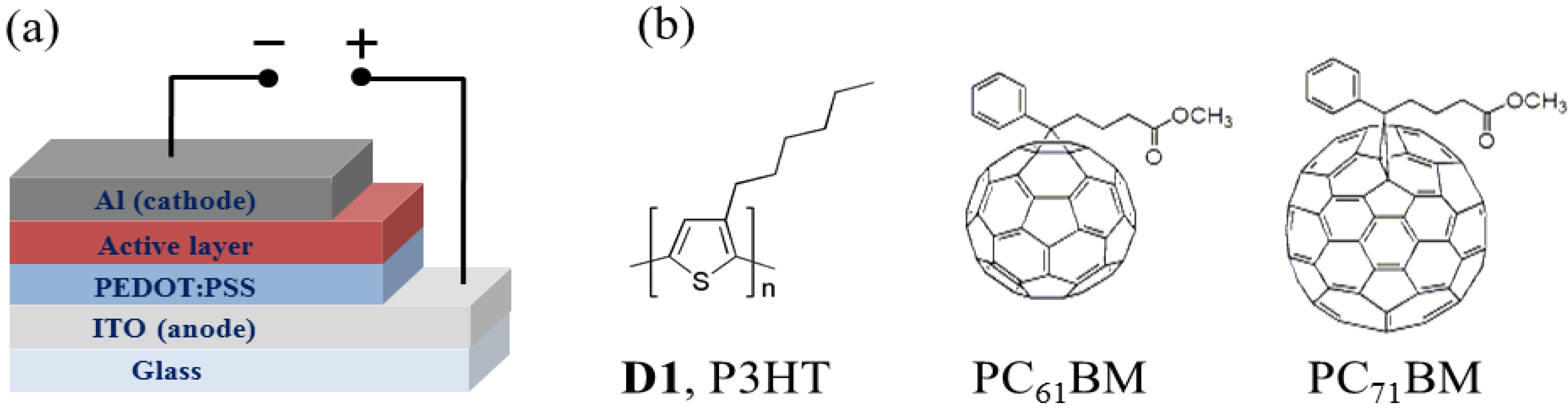
2. Rylene Diimide-Based Polymer Acceptors
2.1. PDI-Based Polymer Acceptors
| Acceptor [Ref] | Mn Mw | Mobility, μe [cm2V−1s−1] | HOMO/LUMO (Eg [eV]) | VOC [V] | JSC [mA/cm2] | FF | PCE [%] | |
|---|---|---|---|---|---|---|---|---|
| 1 | [41] | 10,000 15,000 | 1.3 × 10−2 b | −5.9/−3.9 (2.0) | 0.63 | 4.2 | 0.39 | 1 |
| (ITO/PEDOT:PSS/D2:1(1:1)/Al) | ||||||||
| [28] | – | 3.37 × 10−5 c | −5.9/−3.9 (2.0) | 0.75 | 8.55 | 0.52 | 3.45 | |
| (ITO/PEDOT:PSS/D6:1(1:1)/Ca/Al) | ||||||||
| 2 | [42] | 20,000 43,000 | – | −5.7/−3.8 (1.9) | 0.69 | 5.02 | 0.43 | 1.48 |
| (ITO/PEDOT:PSS/D3:2(3:1)/Ca/Al) | ||||||||
| 3 | [43] | 15,000 27,000 | – | −5.4/−4.0 (1.4) | 0.69 | 2.80 | 0.40 | 0.77 |
| (ITO/PEDOT:PSS/D3:3(1:1)/Ca/Al) | ||||||||
| 4 | [44] | – | – | −5.7/−3.8 (1.9) | 0.67 | 3.24 | 0.51 | 1.17 |
| (ITO/PEDOT:PSS/D4:4(2:1)/Ca/Al) | ||||||||
| 0.75 | 1.60 | 0.45 | 0.57 | |||||
| (ITO/PEDOT:PSS/D5:4(3:1)/Ca/Al) | ||||||||
| 5 | [45] | 6,300 8,500 | – | −5.49/−3.83 (1.66) | 0.66 | 3.05 | 0.46 | 0.93 |
| (ITO/PEDOT:PSS/D7:5(2:1)/Ca/Al) | ||||||||
| 0.42 | 1.86 | 0.53 | 0.41 | |||||
| (ITO/PEDOT:PSS/D8:5(1:1)/Ca/Al) | ||||||||
| [46] | 6,300 8,500 | 2.3 × 10−4 b | −5.49/−3.83 (1.67) | 0.46 | 0.76 | 0.50 | 0.17 | |
| (ITO/PEDOT:PSS/D1:5(2:1)/Ca/Al) | ||||||||
| 6 | [46] | 12,100 19,600 | 1.7 × 10−4 b | −5.83/−3.66 (2.17) | 0.70 | 6.35 | 0.50 | 2.23 |
| (ITO/PEDOT:PSS/D7:6(2:1)/Ca/Al) | ||||||||
| 0.58 | 0.91 | 0.55 | 0.29 | |||||
| (ITO/PEDOT:PSS/D1:6(2:1)/Ca/Al) | ||||||||
| 9 | [48] | 7,800 19,000 | 8.5 × 10−3 c | −5.75/−3.95 (1.76) | 0.6 | 2.98 | 0.39 | 2.32 |
| (ITO/D9:9(1:1)/Al) e | ||||||||
| 10 | [49] | 6,000 – | 5 × 10−4 d | – | 0.33 | 0.60 | 0.46 | 0.1 |
| (ITO/PEDOT:PSS/D1:10(2:1)/Al) | ||||||||
| 11 | [35] | 13,600 – | – | – | 0.51 | 2.57 | 0.37 | 0.49 |
| (ITO/PEDOT:PSS/11/LiF/Al) | ||||||||
| 12 | [36] | 29,500 33,900 | – | – (1.93) | 0.44 | 1.5 | 0.25 | 0.2 |
| (ITO/PEDOT:PSS/12/LiF/Al) | ||||||||
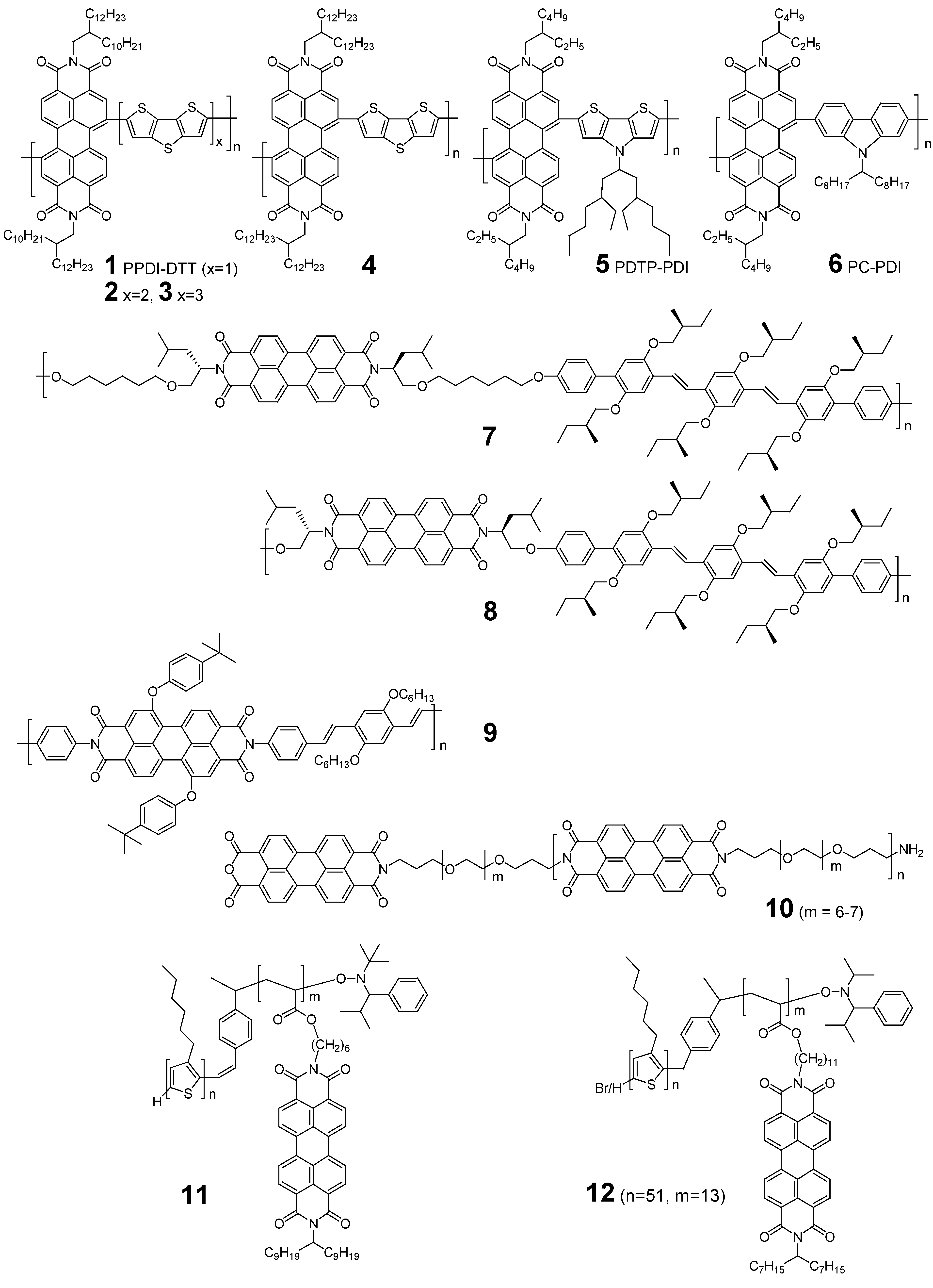
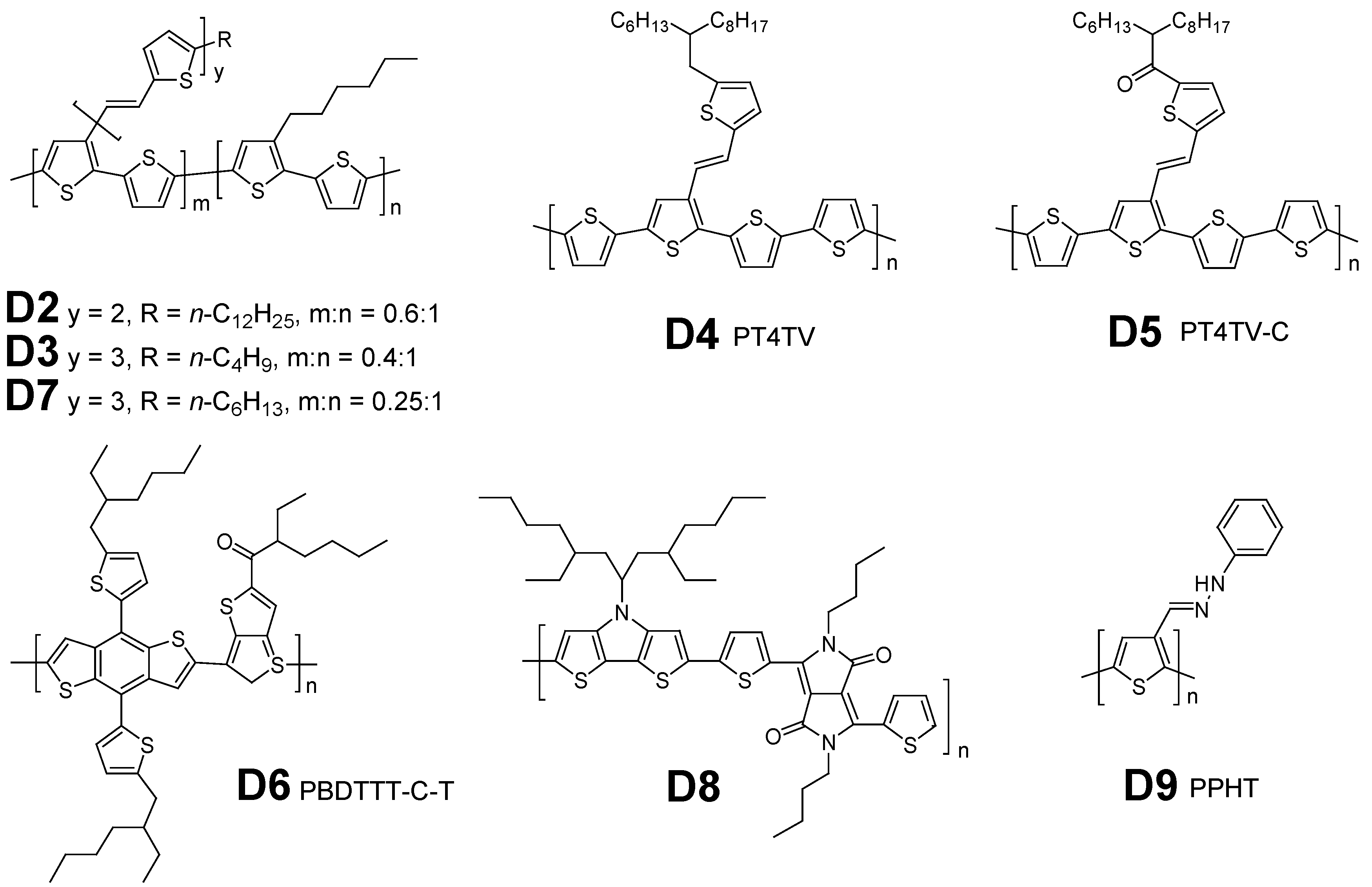
2.2. NDI-Based Polymer Acceptors
| Acceptor [Ref] | Mn Mw | Mobility, μe [cm2V−1s−1] b | HOMO/LUMO (Eg [eV]) | VOC [V] | JSC [mA/cm2] | FF | PCE [%] | |
|---|---|---|---|---|---|---|---|---|
| 13 | [14] | – | – | – | 1.2 | 1.2 | 0.43 | 0.7 |
| (ITO/D10(50nm)/13(50nm)/Al) | ||||||||
| [50] | – | – | – | 1.10 | 2.15 | 0.50 | 1.5 | |
| (ITO/D10(60nm)/13(60nm)/Al) c | ||||||||
| 14 | [51] | 50,000250,000 | ~5 × 10−3 | −5.6/−4.0(1.6) | 0.48 | 2.39 | 0.54 | 0.62 |
| (ITO/PEDOT:PSS/D1:14(1:2)/LiF/Al) | ||||||||
| [52] | – | 0.8 | −5.45/−4(1.45) | 0.52 | 1.41 | 0.29 | 0.21 | |
| (ITO/PEDOT:PSS/D1:14(1:1)/Al) | ||||||||
| [53] | 26,20085,200 | 0.85 | −5.8/−4.35(1.45) | 0.56 | 3.77 | 0.65 | 1.4 | |
| (ITO/PEDOT:PSS/D1:14(4:3)/Sm/Al) | ||||||||
| 15 | [53] | 36,60059,300 | – | −5.35/−4.15(1.2) | 0.63 | 2.43 | 0.70 | 1.1 |
| (ITO/PEDOT:PSS/D1:14(4:3)/Sm/Al) | ||||||||
| 16 | [54] | 22,20040,300 | 0.07 | −5.95/−4.55(1.4) | 0.53 | 3.79 | 0.44 | 0.9 |
| (ITO/PEDOT:PSS/D1:16(1:3)/LiF/Al) | ||||||||
| 17 | [55] | 23,90031,500 | 2 × 10−4 | −5.77/−4.0(1.77) | 0.61 | 3.80 | 0.56 | 1.30 |
| (ITO/ZnO/D11:17(1:1)/MoO3/Ag) | ||||||||
| 18 | [55] | 26,10031,600 | 2 × 10−3 | −5.70/−4.0(1.70) | 0.75 | 6.53 | 0.60 | 2.96 |
| (ITO/ZnO/D11:18(1:1)/MoO3/Ag) | ||||||||
| 19 | [55] | 79,000177,900 | 7 × 10−3 | −5.65/−4.0(1.65) | 0.76 | 7.78 | 0.55 | 3.26 |
| (ITO/ZnO/D11:19(1:1)/ MoO3/Ag) | ||||||||
| 20 | [37] | 26,00041,600 | – | −5.60/−4.22(1.38) | 0.56 | 4.57 | 0.50 | 1.28 |
| (ITO/PEDOT:PSS/D1:20(1:1)/Ca/Al) | ||||||||
| 21 | [56] | 62,500206,300 | 7.0 × 10−3 | −5.45/−3.88(1.57) | 0.51 | 0.46 | 0.39 | 0.11 |
| (ITO/PEDOT:PSS/ D1:21(1:1)/Al) | ||||||||
| 22 | [56] | 21,30061,800 | 4.8 × 10−3 | −5.31/−3.91(1.40) | 0.48 | 0.19 | 0.48 | 0.045 |
| (ITO/PEDOT:PSS/ D1:22(1:1)/Al) | ||||||||
| 23 | [56] | 92,400332,600 | 1.2 × 10−2 | −5.29/−3.92(1.37) | 0.47 | 0.57 | 0.55 | 0.13 |
| (ITO/PEDOT:PSS/ D1:23(1:1)/Al) | ||||||||
| 24 | [57] | 18,70033,600 | 2.15 × 10−6 | −5.98/−3.77(2.21) | 0.82 | 1.09 | 0.36 | 0.32 |
| (ITO/PEDOT:PSS/ D1:24(1:1)/Al) | ||||||||
| 25 | [39] | 9,80016,500 | – | −5.70/−3.51(2.19) | 0.92 | 2.14 | 0.43 | 0.84 |
| (ITO/PEDOT:PSS/D12:25(1:1)/Ca/Al) | ||||||||

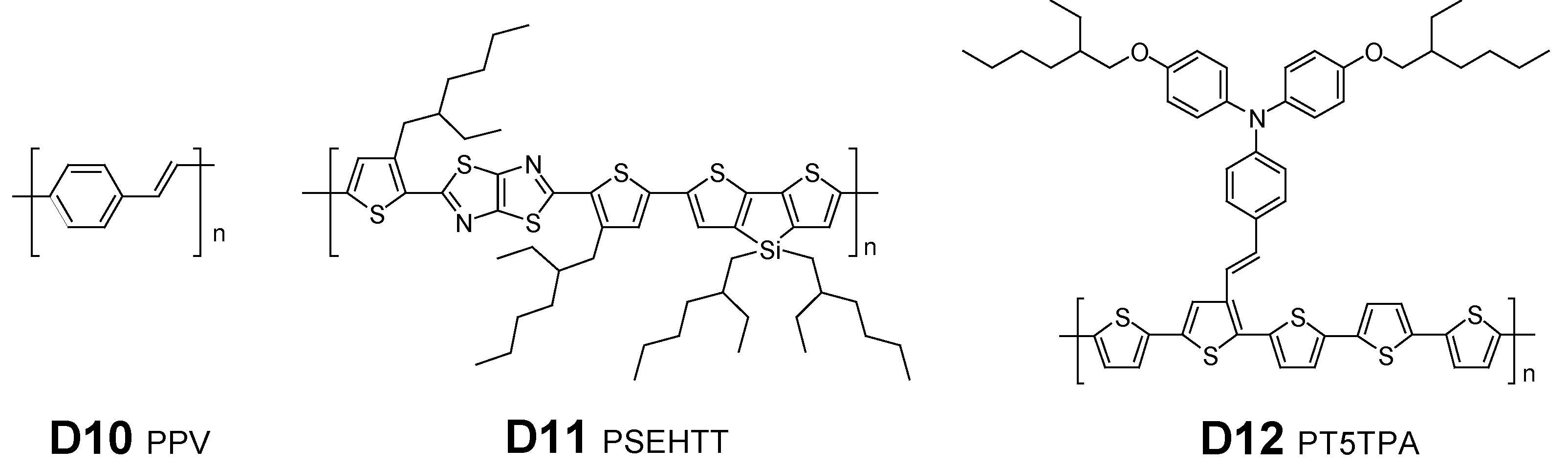
2.3. DTCDI-Based Polymer Acceptors
3. Fluorene and BT-Based Polymer Acceptors

| Acceptor [Ref] | Mn/Mw | HOMO/LUMO (Eg [eV]) | VOC [V] | JSC [mA/cm2] | FF | PCE [%] | |
|---|---|---|---|---|---|---|---|
| 26 | [61] | – | – | – | – | 0.36 | 0.13 |
| (ITO/PEDOT:PSS/D1:26/LiF/Al) | |||||||
| 27 | [62] | – | −5.37/−3.15 (2.22) | – | – | – | 1.8 |
| (ITO/PEDOT:PSS/D1:27(1:1)/LiF/Al) | |||||||
| [63] | – | −5.37/−3.15 (2.22) | 1.15 | 3.6 | 0.34 | 1.2 | |
| (ITO/PEDOT:PSS/D1:27(1:1)/Al) | |||||||
| [65] | – | −5.4/−3.2 (2.2) | 1.14 | 3.30 | 0.49 | 1.85 | |
| (ITO/PEDOT:PSS/D1/27/Al) | |||||||
| 28 | [66] | 10,000/20,000 | −5.5/−3.5 (2.0) | 1.19 | 3.94 | 0.42 | 2.0 |
| (ITO/PEDOT:PSS/D1:28(1:1)/LiF/Al) | |||||||
| [67] | 28,000/78,000 | – | 1.26 | 3.88 | 0.55 | 2.7 | |
| (ITO/PEDOT:PSS/ D1:28(1:1)/Ca/Al) | |||||||
| 29 | [38] | 22,000/28,500 | – | 1.23 | 5.2 | 0.47 | 3.1 |
| (ITO/PEDOT:PSS/29/Al) b | |||||||
4. CN-substituted Polymer Acceptors
| Acceptor [Ref] | Mn/Mw | HOMO/LUMO (Eg [eV]) | VOC [V] | JSC [mA/cm2] | FF | PCE [%] | |
|---|---|---|---|---|---|---|---|
| 31 | [71] | – | – (~2) | – | – | – | 0.9 |
| (ITO/D14:31(1:1)/Ca) b | |||||||
| [15] | – | – | 2.2 | – | – | 1.9 | |
| (Au/PEDOT:PSS/D15/31/Ca or Al) c | |||||||
| [72] | 16,000/72,000 | −/−3.7– | – | – | – | 2.0 | |
| (ITO/PEDOT:PSS/ D15/31/LiF/Al) | |||||||
| 32 | [73] | 8,000/19,000 | −5.97/−3.48 (2.49) | 0.9 | 0.86 | 0.44 | 0.34 |
| (Inverted structure) | |||||||
| 33 | [74] | 35,100/119,000 | −5.7/−3.35 (2.35) | 1.52 | 1.4 | 0.27 | 0.65 |
| (ITO/PEDOT:PSS/D16/33/Ca/Al) | |||||||
| 34 | [75] | – | – | 1.0 | 3.2 | 0.25 | 1.0 |
| (ITO/PEDOT/D16:34(1:1)/Ca) d | |||||||
| [76] | – | – | 1.36 | 3.57 | 0.35 | 1.7 | |
| (ITO/PEDOT/D16:34(1:1)/Ca/Al) | |||||||
| [77] | –/20,600 | – | 1.31 | 2.5 | 0.44 | 1.42 | |
| (ITO/PEDOT/D16:34(1:1)/Ca/Al) e | |||||||
| 35 | [79] | – | – | 1.40 | 3.0 | 0.37 | 1.5 |
| (ITO/PEDOT:PSS/D17:35/LiF/Al) | |||||||
| 36 | [80] | – | −5.75/−3.65 (2.1) | 0.85 | 3.14 | 0.29 | 0.8 |
| (ITO/PEDOT:PSS/D18:36(1:1)/LiF/Al) | |||||||
| 37 | [81] | 26,900/61,800 | −6.1/−3.6 (2.5) | 0.62 | 0.09 | 0.26 | 0.014 |
| (ITO/PEDOT:PSS/D17:37(1:2)/CsF:Al) | |||||||
| 0.59 | 0.02 | 0.27 | 0.003 | ||||
| (ITO/PEDOT:PSS/D19:37(2:1)/CsF:Al) | |||||||
| 38 | [82] | – | – | 0.74 | 0.28 | 0.33 | 0.07 |
| (ITO/PEDOT:PSS/D1:38(1:1)/Ca/Al) | |||||||
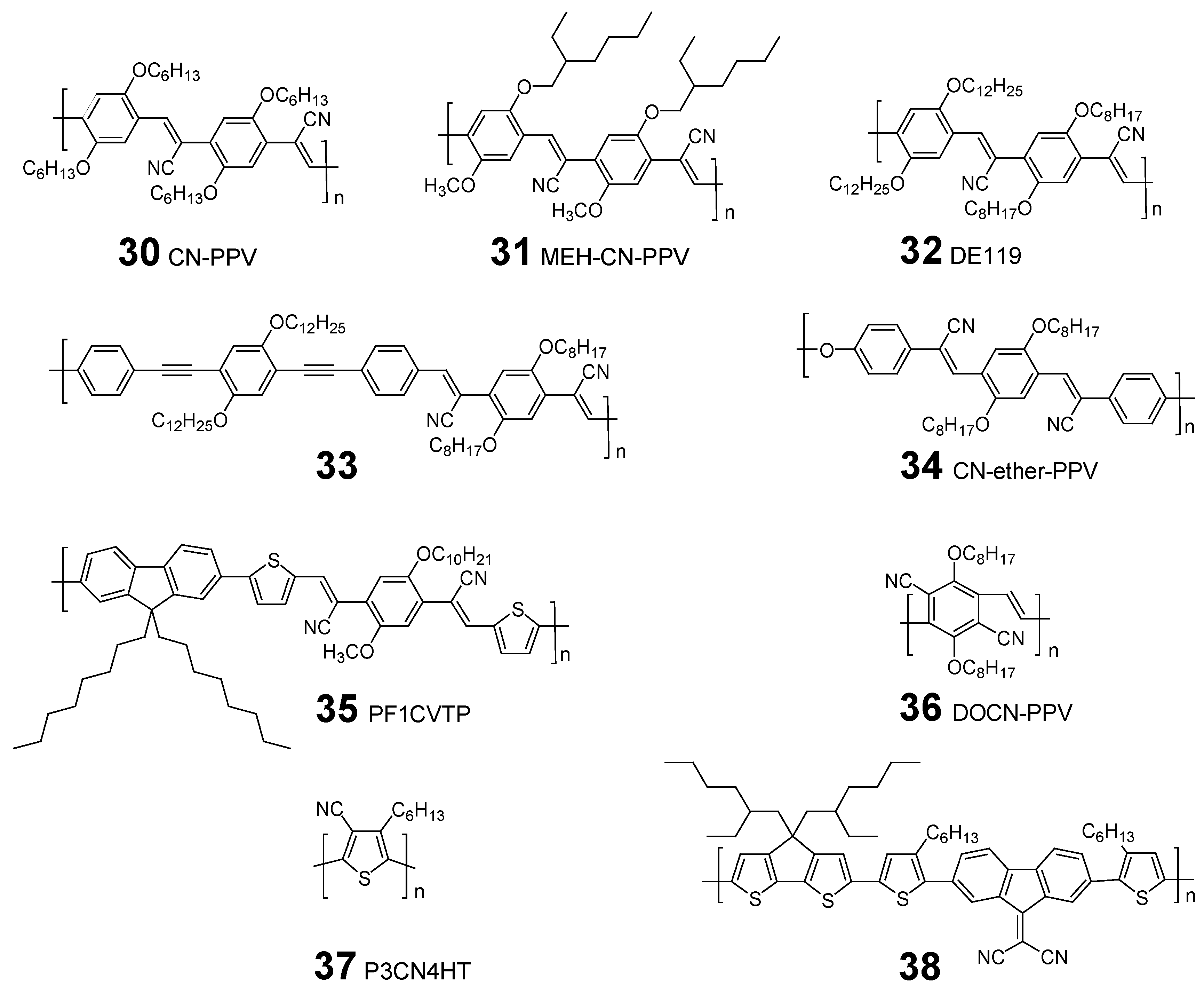
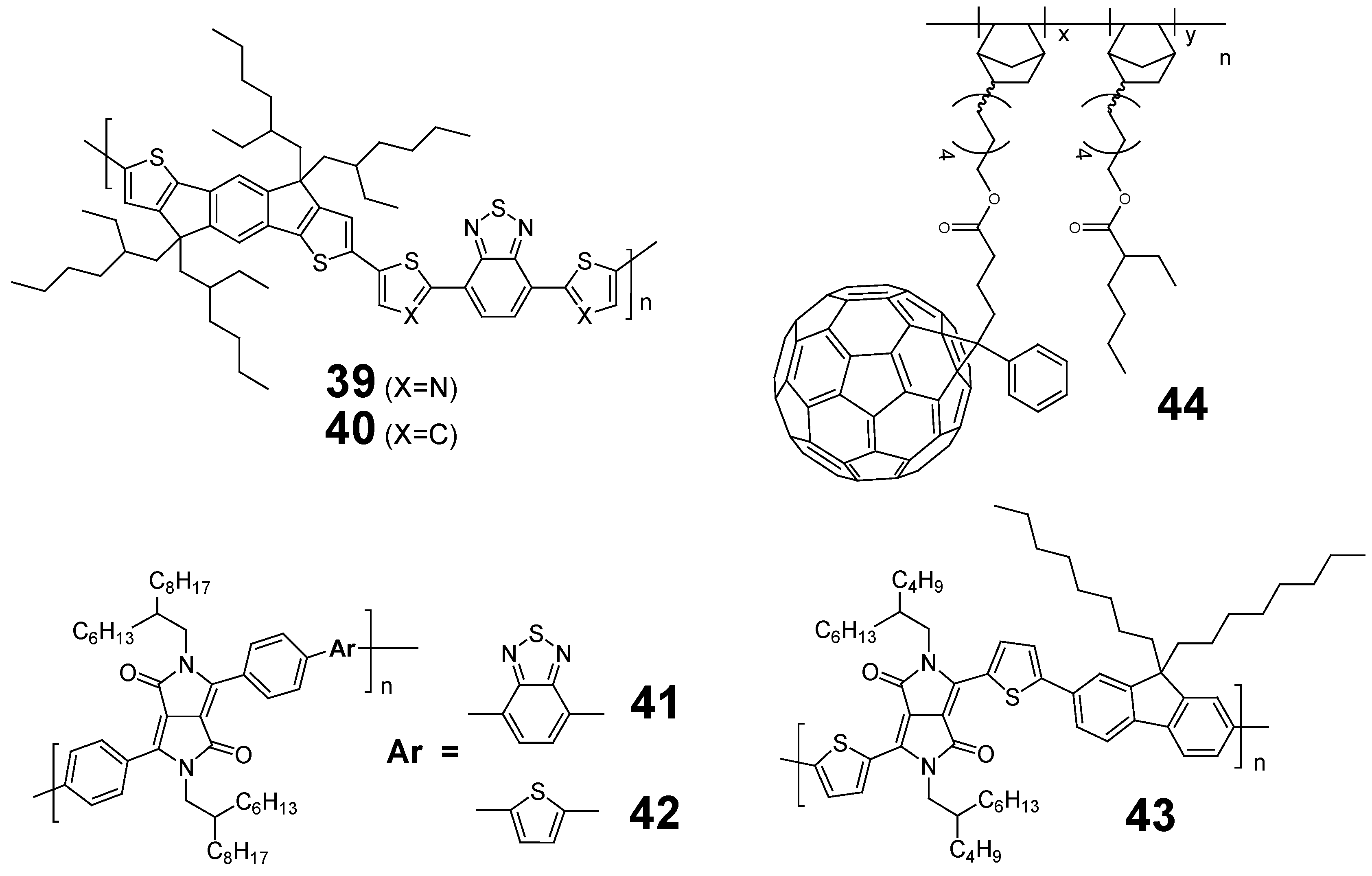
5. Other Polymer Acceptors Containing Electron-Withdrawing Units
| Acceptor [ref] | Mn Mw | mobility, μe [cm2V−1s−1] | HOMO/LUMO (Eg [eV]) | VOC [V] | JSC [mA/cm2] | FF | PCE [%] | |
|---|---|---|---|---|---|---|---|---|
| 39 | [84] | 14,300 26,000 | 1.1 × 10−2 b | −5.43/−3.45 (1.98) | 1.00 | 2.60 | 0.45 | 1.18 |
| (ITO/ZnO/D1:39(1.5:1)/MoO3/Ag) | ||||||||
| 40 | [84] | 26,100 39,200 | 2.9 × 10−4 b | −5.28/−3.21 (2.07) | 0.9 | 1.5 | 0.43 | 0.58 |
| (ITO/ZnO/D1:40(1.5:1)/MoO3/Ag) | ||||||||
| 41 | [85] | 16,600 41,500 | 3 × 10−9 c | −5.66/−3.61 (2.1) | 0.94 | 0.68 | 0.22 | 0.14 |
| (ITO/PEDOT:PSS/D1:41(1:1)/LiF/Al) | ||||||||
| 42 | [85] | 11,800 23,600 | 1 × 10−11 c | −5.58/−3.58 (2.0) | 0.90 | 0.44 | 0.27 | 0.11 |
| (ITO/PEDOT:PSS/D1:42(1:1)/LiF/Al) | ||||||||
| 43 | [85] | 10,500 23,100 | 5 × 10−10 c | −5.43/−3.71 (1.75) | 0.90 | 1.63 | 0.25 | 0.37 |
| (ITO/PEDOT:PSS/D1:43(1:1)/LiF/Al) | ||||||||
| 44 | [86] | 8,700 12,100 | – | −/−3.67 | 0.63 | 4.45 | 0.54 | 1.5 |
| (ITO/PEDOT:PSS/D1:44(1:0.45)/Ca/Al) | ||||||||
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Parida, B.; Iniyan, S.; Goic, R. A review of solar photovoltaic technologies. Renew. Sust. Energ. Rev. 2011, 15, 1625–1636. [Google Scholar] [CrossRef]
- Dou, L.; You, J.; Hong, Z.; Xu, Z.; Li, G.; Street, R.A.; Yang, Y. 25th Anniversary Article: A Decade of Organic/Polymeric Photovoltaic Research. Adv. Mater. 2013, 25, 6642–6671. [Google Scholar] [CrossRef]
- Yue, D.; Khatav, P.; You, F.; Darling, S.B. Deciphering the uncertainties in life cycle energy and environmental analysis of organic photovoltaics. Energ. Environ. Sci. 2012, 5, 9163–9172. [Google Scholar] [CrossRef]
- Darling, S.B.; You, F. The case for organic photovoltaics. RSC Adv. 2013, 3, 17633–17648. [Google Scholar] [CrossRef]
- Boudreault, P.-L.T.; Najari, A.; Leclerc, M. Processable Low-Bandgap Polymers for Photovoltaic Applications. Chem. Mater. 2010, 23, 456–469. [Google Scholar] [CrossRef]
- Liao, H.-C.; Ho, C.-C.; Chang, C.-Y.; Jao, M.-H.; Darling, S.B.; Su, W.-F. Additives for morphology control in high-efficiency organic solar cells. Mater. Today 2013, 16, 326–336. [Google Scholar] [CrossRef]
- You, J.; Dou, L.; Hong, Z.; Li, G.; Yang, Y. Recent trends in polymer tandem solar cells research. Prog. Polym. Sci. 2013, 38, 1909–1928. [Google Scholar] [CrossRef]
- Chen, L.-M.; Hong, Z.; Li, G.; Yang, Y. Recent Progress in Polymer Solar Cells: Manipulation of Polymer: Fullerene Morphology and the Formation of Efficient Inverted Polymer Solar Cells. Adv. Mater. 2009, 21, 1434–1449. [Google Scholar] [CrossRef]
- Yi, C.; Gong, X. Towards high performance inverted polymer solar cells. Curr. Opin. Chem. Eng. 2013, 2, 125–131. [Google Scholar] [CrossRef]
- Chen, W.; Nikiforov, M.P.; Darling, S.B. Morphology characterization in organic and hybrid solar cells. Energ. Environ. Sci. 2012, 5, 8045–8074. [Google Scholar] [CrossRef]
- Nikiforov, M.P.; Lai, B.; Chen, W.; Chen, S.; Schaller, R.D.; Strzalka, J.; Maser, J.; Darling, S.B. Detection and role of trace impurities in high-performance organic solar cells. Energ. Environ. Sci. 2013, 6, 1513–1520. [Google Scholar] [CrossRef]
- Heliatek. Available online: http://www.heliatek.com/ (accessed on 7 February 2014).
- Tang, C.W. Two-layer organic photovoltaic cell. Appl. Phys. Lett. 1986, 48, 183–185. [Google Scholar] [CrossRef]
- Jenekhe, S.A.; Yi, S. Efficient photovoltaic cells from semiconducting polymer heterojunctions. Appl. Phys. Lett. 2000, 77, 2635–2637. [Google Scholar] [CrossRef]
- Granstrom, M.; Petritsch, K.; Arias, A.C.; Lux, A.; Andersson, M.R.; Friend, R.H. Laminated fabrication of polymeric photovoltaic diodes. Nature 1998, 395, 257–260. [Google Scholar] [CrossRef]
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer Photovoltaic Cells: Enhanced Efficiencies via a Network of Internal Donor-Acceptor Heterojunctions. Science 1995, 270, 1789–1791. [Google Scholar]
- Lee, J.K.; Ma, W.L.; Brabec, C.J.; Yuen, J.; Moon, J.S.; Kim, J.Y.; Lee, K.; Bazan, G.C.; Heeger, A.J. Processing Additives for Improved Efficiency from Bulk Heterojunction Solar Cells. J. Am. Chem. Soc. 2008, 130, 3619–3623. [Google Scholar] [CrossRef]
- Zhicai, H.; Chengmei, Z.; Shijian, S.; Miao, X.; Hongbin, W.; Yong, C. Enhanced power-conversion efficiency in polymer solar cells using an inverted device structure. Nat. Photonics 2012, 6, 591–595. [Google Scholar]
- Guo, X.; Baumgarten, M.; Müllen, K. Designing π-conjugated polymers for organic electronics. Prog. Polym. Sci. 2013, 38, 1832–1908. [Google Scholar] [CrossRef]
- Zhu, Z.; Waller, D.; Gaudiana, R.; Morana, M.; Mühlbacher, D.; Scharber, M.; Brabec, C. Panchromatic Conjugated Polymers Containing Alternating Donor/Acceptor Units for Photovoltaic Applications. Macromolecules 2007, 40, 1981–1986. [Google Scholar] [CrossRef]
- Hou, J.; Chen, H.-Y.; Zhang, S.; Li, G.; Yang, Y. Synthesis, Characterization, and Photovoltaic Properties of a Low Band Gap Polymer Based on Silole-Containing Polythiophenes and 2,1,3-Benzothiadiazole. J. Am. Chem. Soc. 2008, 130, 16144–16145. [Google Scholar] [CrossRef]
- Dou, L.; Chen, C.-C.; Yoshimura, K.; Ohya, K.; Chang, W.-H.; Gao, J.; Liu, Y.; Richard, E.; Yang, Y. Synthesis of 5H-Dithieno[3,2-b:2′,3′-d]pyran as an Electron-Rich Building Block for Donor–Acceptor Type Low-Bandgap Polymers. Macromolecules 2013, 46, 3384–3390. [Google Scholar]
- Zhou, J.; Zuo, Y.; Wan, X.; Long, G.; Zhang, Q.; Ni, W.; Liu, Y.; Li, Z.; He, G.; Li, C.; Kan, B.; Li, M.; Chen, Y. Solution-Processed and High-Performance Organic Solar Cells Using Small Molecules with a Benzodithiophene Unit. J. Am. Chem. Soc. 2013, 135, 8484–8487. [Google Scholar]
- Sonar, P.; Fong Lim, J.P.; Chan, K.L. Organic non-fullerene acceptors for organic photovoltaics. Energ. Environ. Sci. 2011, 4, 1558–1574. [Google Scholar]
- Lin, Y.; Li, Y.; Zhan, X. Small molecule semiconductors for high-efficiency organic photovoltaics. Chem. Soc. Rev. 2012, 41, 4245–4272. [Google Scholar]
- Kozma, E.; Catellani, M. Perylene diimides based materials for organic solar cells. Dyes Pigm. 2013, 98, 160–179. [Google Scholar]
- Qu, S.; Tian, H. Diketopyrrolopyrrole (DPP)-based materials for organic photovoltaics. Chem. Commun. 2012, 48, 3039–3051. [Google Scholar]
- Cheng, P.; Ye, L.; Zhao, X.; Hou, J.; Li, Y.; Zhan, X. Binary additives synergistically boost the efficiency of all-polymer solar cells up to 3.45%. Energ. Environ. Sci. 2014. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Z.; Ye, L.; Zhan, C.; Hou, J.; Zhang, S.; Jiang, B.; Zhao, Y.; Huang, J.; Zhang, S.; Liu, Y.; Shi, Q.; Liu, Y.; Yao, J. A Potential Perylene Diimide Dimer-Based Acceptor Material for Highly Efficient Solution-Processed Non-Fullerene Organic Solar Cells with 4.03% Efficiency. Adv. Mater. 2013, 25, 5791–5797. [Google Scholar] [CrossRef]
- Facchetti, A. Polymer donor–polymer acceptor (all-polymer) solar cells. Mater. Today 2013, 16, 123–132. [Google Scholar] [CrossRef]
- Darling, S.B. Block copolymers for photovoltaics. Energ. Environ. Sci. 2009, 2, 1266–1273. [Google Scholar] [CrossRef]
- Segalman, R.A.; McCulloch, B.; Kirmayer, S.; Urban, J.J. Block Copolymers for Organic Optoelectronics. Macromolecules 2009, 42, 9205–9216. [Google Scholar] [CrossRef]
- Bang, J.; Jeong, U.; Ryu, D.Y.; Russell, T.P.; Hawker, C.J. Block Copolymer Nanolithography: Translation of Molecular Level Control to Nanoscale Patterns. Adv. Mater. 2009, 21, 4769–4792. [Google Scholar] [CrossRef]
- Sommer, M.; Huettner, S.; Thelakkat, M. Donor-acceptor block copolymers for photovoltaic applications. J. Mater. Chem. 2010, 20, 10788–10797. [Google Scholar] [CrossRef]
- Zhang, Q.; Cirpan, A.; Russell, T.P.; Emrick, T. Donor−Acceptor Poly(thiophene-block-perylene diimide) Copolymers: Synthesis and Solar Cell Fabrication. Macromolecules 2009, 42, 1079–1082. [Google Scholar] [CrossRef]
- Sommer, M.; Hüttner, S.; Steiner, U.; Thelakkat, M. Influence of molecular weight on the solar cell performance of double-crystalline donor-acceptor block copolymers. Appl. Phys. Lett. 2009, 95, 183308:1–183308:3. [Google Scholar]
- Nakabayashi, K.; Mori, H. All-Polymer Solar Cells Based on Fully Conjugated Block Copolymers Composed of Poly(3-hexylthiophene) and Poly(naphthalene bisimide) Segments. Macromolecules 2012, 45, 9618–9625. [Google Scholar] [CrossRef]
- Guo, C.; Lin, Y.-H.; Witman, M.D.; Smith, K.A.; Wang, C.; Hexemer, A.; Strzalka, J.; Gomez, E.D.; Verduzco, R. Conjugated Block Copolymer Photovoltaics with near 3% Efficiency through Microphase Separation. Nano Lett. 2013, 13, 2957–2963. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, Z.-G.; Ma, L.; Li, Y.; Zhan, X. Dithienocoronene diimide based conjugated polymers as electron acceptors for all-polymer solar cells. Sol. Energ. Mat. Sol. C. 2013, 112, 13–19. [Google Scholar] [CrossRef]
- Sommer, M. Conjugated polymers based on naphthalene diimide for organic electronics. J. Mater. Chem. C 2014. [Google Scholar] [CrossRef]
- Zhan, X.; Tan, Z.; Domercq, B.; An, Z.; Zhang, X.; Barlow, S.; Li, Y.; Zhu, D.; Kippelen, B.; Marder, S.R. A High-Mobility Electron-Transport Polymer with Broad Absorption and Its Use in Field-Effect Transistors and All-Polymer Solar Cells. J. Am. Chem. Soc. 2007, 129, 7246–7247. [Google Scholar] [CrossRef]
- Tan, Z.; Zhou, E.; Zhan, X.; Wang, X.; Li, Y.; Barlow, S.; Marder, S.R. Efficient all-polymer solar cells based on blend of tris(thienylenevinylene)-substituted polythiophene and poly[perylene diimide-alt-bis(dithienothiophene)]. Appl. Phys. Lett. 2008, 93, 073309:1–073309:3. [Google Scholar]
- Zhan, X.; Tan, Z.; Zhou, E.; Li, Y.; Misra, R.; Grant, A.; Domercq, B.; Zhang, X.-H.; An, Z.; Zhang, X.; Barlow, S.; Kippelen, B.; Marder, S.R. Copolymers of perylene diimide with dithienothiophene and dithienopyrrole as electron-transport materials for all-polymer solar cells and field-effect transistors. J. Mater. Chem. 2009, 19, 5794–5803. [Google Scholar] [CrossRef]
- Liao, X.-X.; Zhao, X.; Zhang, Z.-G.; Wang, H.-Q.; Zhan, X.; Li, Y.; Wang, J.; Zheng, J.-C. All-polymer solar cells based on side-chain-isolated polythiophenes and poly(perylene diimide-alt-dithienothiophene). Sol. Energ. Mat. Sol. C. 2013, 117, 336–342. [Google Scholar]
- Zhou, E.; Tajima, K.; Yang, C.; Hashimoto, K. Band gap and molecular energy level control of perylene diimide-based donor-acceptor copolymers for all-polymer solar cells. J. Mater. Chem. 2010, 20, 2362–2368. [Google Scholar] [CrossRef]
- Zhou, E.; Cong, J.; Wei, Q.; Tajima, K.; Yang, C.; Hashimoto, K. All-Polymer Solar Cells from Perylene Diimide Based Copolymers: Material Design and Phase Separation Control. Angew. Chem. Int. Ed. 2011, 50, 2799–2803. [Google Scholar] [CrossRef]
- Neuteboom, E.E.; Meskers, S.C.; van Hal, P.A.; van Duren, J.K.; Meijer, E.W.; Janssen, R.A.; Dupin, H.; Pourtois, G.; Cornil, J.; Lazzaroni, R.; Brédas, J.-L.; Beljonne, D. Alternating Oligo(p-phenylene vinylene)—Perylene Bisimide Copolymers: Synthesis, Photophysics, and Photovoltaic Properties of a New Class of Donor—Acceptor Materials. J. Am. Chem. Soc. 2003, 125, 8625–8638. [Google Scholar] [CrossRef]
- Mikroyannidis, J.A.; Stylianakis, M.M.; Sharma, G.D.; Balraju, P.; Roy, M.S. A Novel Alternating Phenylenevinylene Copolymer with Perylene Bisimide Units: Synthesis, Photophysical, Electrochemical, and Photovoltaic Properties. J. Phys. Chem. C 2009, 113, 7904–7912. [Google Scholar] [CrossRef]
- Liang, Z.; Cormier, R.A.; Nardes, A.M.; Gregg, B.A. Developing perylene diimide based acceptor polymers for organic photovoltaics. Synt. Met. 2011, 161, 1014–1021. [Google Scholar]
- Alam, M.M.; Jenekhe, S.A. Efficient Solar Cells from Layered Nanostructures of Donor and Acceptor Conjugated Polymers. Chem. Mater. 2004, 16, 4647–4656. [Google Scholar] [CrossRef]
- Fabiano, S.; Chen, Z.; Vahedi, S.; Facchetti, A.; Pignataro, B.; Loi, M.A. Role of photoactive layer morphology in high fill factor all-polymer bulk heterojunction solar cells. J. Mater. Chem. 2011, 21, 5891–5896. [Google Scholar]
- Moore, J.R.; Albert-Seifried, S.; Rao, A.; Massip, S.; Watts, B.; Morgan, D.J.; Friend, R.H.; McNeill, C.R.; Sirringhaus, H. Polymer Blend Solar Cells Based on a High-Mobility Naphthalenediimide-Based Polymer Acceptor: Device Physics, Photophysics and Morphology. Adv. Energy Mater. 2011, 1, 230–240. [Google Scholar]
- Schubert, M.; Dolfen, D.; Frisch, J.; Roland, S.; Steyrleuthner, R.; Stiller, B.; Chen, Z.; Scherf, U.; Koch, N.; Facchetti, A.; Neher, D. Influence of Aggregation on the Performance of All-Polymer Solar Cells Containing Low-Bandgap Naphthalenediimide Copolymers. Adv. Energy Mater. 2012, 2, 369–380. [Google Scholar]
- Hwang, Y.-J.; Ren, G.; Murari, N.M.; Jenekhe, S.A. n-Type Naphthalene Diimide–Biselenophene Copolymer for All-Polymer Bulk Heterojunction Solar Cells. Macromolecules 2012, 45, 9056–9062. [Google Scholar] [CrossRef]
- Earmme, T.; Hwang, Y.-J.; Murari, N.M.; Subramaniyan, S.; Jenekhe, S.A. All-Polymer Solar Cells with 3.3% Efficiency Based on Naphthalene Diimide-Selenophene Copolymer Acceptor. J. Am. Chem. Soc. 2013, 135, 14960–14963. [Google Scholar]
- Yuan, M.; Durban, M.M.; Kazarinoff, P.D.; Zeigler, D.F.; Rice, A.H.; Segawa, Y.; Luscombe, C.K. Synthesis and characterization of fused-thiophene containing naphthalene diimide n-type copolymers for organic thin film transistor and all-polymer solar cell applications. J. Polym. Sci. A Polym. Chem. 2013, 51, 4061–4069. [Google Scholar]
- Chen, S.-C.; Zheng, Q.; Zhang, Q.; Cai, D.; Wang, J.; Yin, Z.; Tang, C. Tuning the frontier molecular orbital energy levels of n-type conjugated copolymers by using angular-shaped naphthalene tetracarboxylic diimides, and their use in all-polymer solar cells with high open-circuit voltages. J. Polym. Sci. A Polym. Chem. 2013, 51, 1999–2005. [Google Scholar]
- He, Y.; Gong, S.; Hattori, R.; Kanicki, J. High performance organic polymer light-emitting heterostructure devices. Appl. Phys. Lett. 1999, 74, 2265–2267. [Google Scholar]
- Arias, A.C.; MacKenzie, J.D.; Stevenson, R.; Halls, J.J.M.; Inbasekaran, M.; Woo, E.P.; Richards, D.; Friend, R.H. Photovoltaic Performance and Morphology of Polyfluorene Blends: A Combined Microscopic and Photovoltaic Investigation. Macromolecules 2001, 34, 6005–6013. [Google Scholar]
- Snaith, H.J.; Arias, A.C.; Morteani, A.C.; Silva, C.; Friend, R.H. Charge Generation Kinetics and Transport Mechanisms in Blended Polyfluorene Photovoltaic Devices. Nano Lett. 2002, 2, 1353–1357. [Google Scholar]
- Kim, Y.; Cook, S.; Choulis, S.A.; Nelson, J.; Durrant, J.R.; Bradley, D.D.C. Organic Photovoltaic Devices Based on Blends of Regioregular Poly(3-hexylthiophene) and Poly(9,9-dioctylfluorene-co-benzothiadiazole). Chem. Mater. 2004, 16, 4812–4818. [Google Scholar] [CrossRef]
- McNeill, C.R.; Abrusci, A.; Zaumseil, J.; Wilson, R.; McKiernan, M.J.; Burroughes, J.H.; Halls, J.J.M.; Greenham, N.C.; Friend, R.H. Dual electron donor/electron acceptor character of a conjugated polymer in efficient photovoltaic diodes. Appl. Phys. Lett. 2007, 90, 193506:1–193506:3. [Google Scholar]
- McNeill, C.R.; Halls, J.J.M.; Wilson, R.; Whiting, G.L.; Berkebile, S.; Ramsey, M.G.; Friend, R.H.; Greenham, N.C. Efficient Polythiophene/Polyfluorene Copolymer Bulk Heterojunction Photovoltaic Devices: Device Physics and Annealing Effects. Adv. Funct. Mater. 2008, 18, 2309–2321. [Google Scholar]
- McNeill, C.R.; Abrusci, A.; Hwang, I.; Ruderer, M.A.; Müller-Buschbaum, P.; Greenham, N.C. Photophysics and Photocurrent Generation in Polythiophene/Polyfluorene Copolymer Blends. Adv. Funct. Mater. 2009, 19, 3103–3111. [Google Scholar]
- He, X.; Gao, F.; Tu, G.; Hasko, D.; Hüttner, S.; Steiner, U.; Greenham, N.C.; Friend, R.H.; Huck, W.T.S. Formation of Nanopatterned Polymer Blends in Photovoltaic Devices. Nano Lett. 2010, 10, 1302–1307. [Google Scholar] [CrossRef]
- Mori, D.; Benten, H.; Kosaka, J.; Ohkita, H.; Ito, S.; Miyake, K. Polymer/Polymer Blend Solar Cells with 2.0% Efficiency Developed by Thermal Purification of Nanoscale-Phase-Separated Morphology. ACS Appl. Mater. Inter. 2011, 3, 2924–2927. [Google Scholar]
- Mori, D.; Benten, H.; Ohkita, H.; Ito, S.; Miyake, K. Polymer/Polymer Blend Solar Cells Improved by Using High-Molecular-Weight Fluorene-Based Copolymer as Electron Acceptor. ACS Appl. Mater. Inter. 2012, 4, 3325–3329. [Google Scholar]
- Moratti, S.C.; Cervini, R.; Holmes, A.B.; Baigent, D.R.; Friend, R.H.; Greenham, N.C.; Grüner, J.; Hamer, P.J. High electron affinity polymers for LEDs. Synt. Met. 1995, 71, 2117–2120. [Google Scholar]
- Greenham, N.C.; Moratti, S.C.; Bradley, D.D.C.; Friend, R.H.; Holmes, A.B. Efficient light-emitting diodes based on polymers with high electron affinities. Nature 1993, 365, 628–630. [Google Scholar]
- Halls, J.J.M.; Walsh, C.A.; Greenham, N.C.; Marseglia, E.A.; Friend, R.H.; Moratti, S.C.; Holmes, A.B. Efficient photodiodes from interpenetrating polymer networks. Nature 1995, 376, 498–500. [Google Scholar] [CrossRef]
- Yu, G.; Heeger, A.J. Charge separation and photovoltaic conversion in polymer composites with internal donor/acceptor heterojunctions. J. Appl. Phys. 1995, 78, 4510–4515. [Google Scholar] [CrossRef]
- Holcombe, T.W.; Woo, C.H.; Kavulak, D.F.J.; Thompson, B.C.; Fréchet, J.M.J. All-Polymer Photovoltaic Devices of Poly(3-(4-n-octyl)-phenylthiophene) from Grignard Metathesis (GRIM) Polymerization. J. Am. Chem. Soc. 2009, 131, 14160–14161. [Google Scholar]
- Cevik, E.; İlicali, D.; Egbe, D.A.M.; Günes, S. Bulk heterojunction and inverted type solar cells using a CN-PPV derivative. Sol. Energ. Mat. Sol. C. 2012, 98, 94–102. [Google Scholar]
- Egbe, D.A.M.; Kietzke, T.; Carbonnier, B.; Mühlbacher, D.; Hörhold, H.-H.; Neher, D.; Pakula, T. Synthesis, Characterization, and Photophysical, Electrochemical, Electroluminescent, and Photovoltaic Properties of Yne-Containing CN−PPVs. Macromolecules 2004, 37, 8863–8873. [Google Scholar] [CrossRef]
- Breeze, A.J.; Schlesinger, Z.; Carter, S.A.; Tillmann, H.; Hörhold, H.H. Improving power efficiencies in polymer—polymer blend photovoltaics. Solar Sol. Energ. Mat. Sol. C. 2004, 83, 263–271. [Google Scholar] [CrossRef]
- Kietzke, T.; Hörhold, H.-H.; Neher, D. Efficient Polymer Solar Cells Based on M3EH−PPV. Chem. Mater. 2005, 17, 6532–6537. [Google Scholar] [CrossRef]
- Yin, C.; Schubert, M.; Bange, S.; Stiller, B.; Castellani, M.; Neher, D.; Kumke, M.; Hörhold, H.-H. Tuning of the Excited-State Properties and Photovoltaic Performance in PPV-Based Polymer Blends. J. Phys. Chem. C 2008, 112, 14607–14617. [Google Scholar] [CrossRef]
- Cho, N.S.; Hwang, D.-H.; Jung, B.-J.; Lim, E.; Lee, J.; Shim, H.-K. Synthesis, Characterization, and Electroluminescene of New Conjugated Polyfluorene Derivatives Containing Various Dyes as Comonomers. Macromolecules 2004, 37, 5265–5273. [Google Scholar]
- Koetse, M.M.; Sweelssen, J.; Hoekerd, K.T.; Schoo, H.F.M.; Veenstra, S.C.; Kroon, J.M.; Yang, X.; Loos, J. Efficient polymer:polymer bulk heterojunction solar cells. Appl. Phys. Lett. 2006, 88, 83504:1–83504:3. [Google Scholar]
- Sang, G.; Zou, Y.; Huang, Y.; Zhao, G.; Yang, Y.; Li, Y. All-polymer solar cells based on a blend of poly 3-(10-n-octyl-3-phenothiazine-vinylene)thiophene-co-2,5-thiophene and poly 1,4-dioctyloxyl-p-2,5-dicyanophenylenevinylene. Appl. Phys. Lett. 2009, 94, 193302:1–193302:3. [Google Scholar]
- Chochos, C.L.; Economopoulos, S.P.; Deimede, V.; Gregoriou, V.G.; Lloyd, M.T.; Malliaras, G.G.; Kallitsis, J.K. Synthesis of a Soluble n-Type Cyano Substituted Polythiophene Derivative: A Potential Electron Acceptor in Polymeric Solar Cells. J. Phys. Chem. C 2007, 111, 10732–10740. [Google Scholar]
- Vijayakumar, C.; Saeki, A.; Seki, S. Optoelectronic Properties of Dicyanofluorene-Based n-Type Polymers. Chem. Asian J. 2012, 7, 1845–1852. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Yang, S.-H.; Hsu, C.-S. Synthesis of Conjugated Polymers for Organic Solar Cell Applications. Chem. Rev. 2009, 109, 5868–5923. [Google Scholar] [CrossRef]
- Cao, Y.; Lei, T.; Yuan, J.; Wang, J.-Y.; Pei, J. Dithiazolyl-benzothiadiazole-containing polymer acceptors: synthesis, characterization, and all-polymer solar cells. Polym. Chem. 2013, 4, 5228–5236. [Google Scholar] [CrossRef]
- Falzon, M.-F.; Zoombelt, A.P.; Wienk, M.M.; Janssen, R.A. Diketopyrrolopyrrole-based acceptor polymers for photovoltaic application. Phys. Chem. Chem. Phys. 2011, 13, 8931–8939. [Google Scholar] [CrossRef]
- Eo, M.; Lee, S.; Park, M.H.; Lee, M.H.; Yoo, S.; Do, Y. Vinyl-Type Polynorbornenes with Pendant PCBM: A Novel Acceptor for Organic Solar Cells. Macromol. Rapid Commun. 2012, 33, 1119–1125. [Google Scholar] [CrossRef]
- Eo, M.; Han, D.; Park, M.H.; Hong, M.; Do, Y.; Yoo, S.; Lee, M.H. Polynorbornenes with pendant PCBM as an acceptor for OPVs: Ring-opening metathesis versus vinyl-addition polymerization. Eur. Polym. J. 2014, 51, 37–44. [Google Scholar] [CrossRef]
- Chochos, C.L.; Govaris, G.K.; Kakali, F.; Yiannoulis, P.; Kallitsis, J.K.; Gregoriou, V.G. Synthesis, optical and morphological characterization of soluble main chain 1,3,4-oxadiazole copolyarylethers—Potential candidates for solar cells applications as electron acceptors. Polymer 2005, 46, 4654–4663. [Google Scholar] [CrossRef]
- Kymakis, E.; Koudoumas, E.; Franghiadakis, I. Bi-layer photovoltaic devices with PPQ as the electron acceptor layer. Sol. Energ. Mat. Sol. Cells 2006, 90, 1705–1714. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kim, Y.; Lim, E. Development of Polymer Acceptors for Organic Photovoltaic Cells. Polymers 2014, 6, 382-407. https://doi.org/10.3390/polym6020382
Kim Y, Lim E. Development of Polymer Acceptors for Organic Photovoltaic Cells. Polymers. 2014; 6(2):382-407. https://doi.org/10.3390/polym6020382
Chicago/Turabian StyleKim, Yujeong, and Eunhee Lim. 2014. "Development of Polymer Acceptors for Organic Photovoltaic Cells" Polymers 6, no. 2: 382-407. https://doi.org/10.3390/polym6020382




