Morphological Control Agent in Ternary Blend Bulk Heterojunction Solar Cells
Abstract
:1. Introduction
2. Polymers
2.1. Copolymer
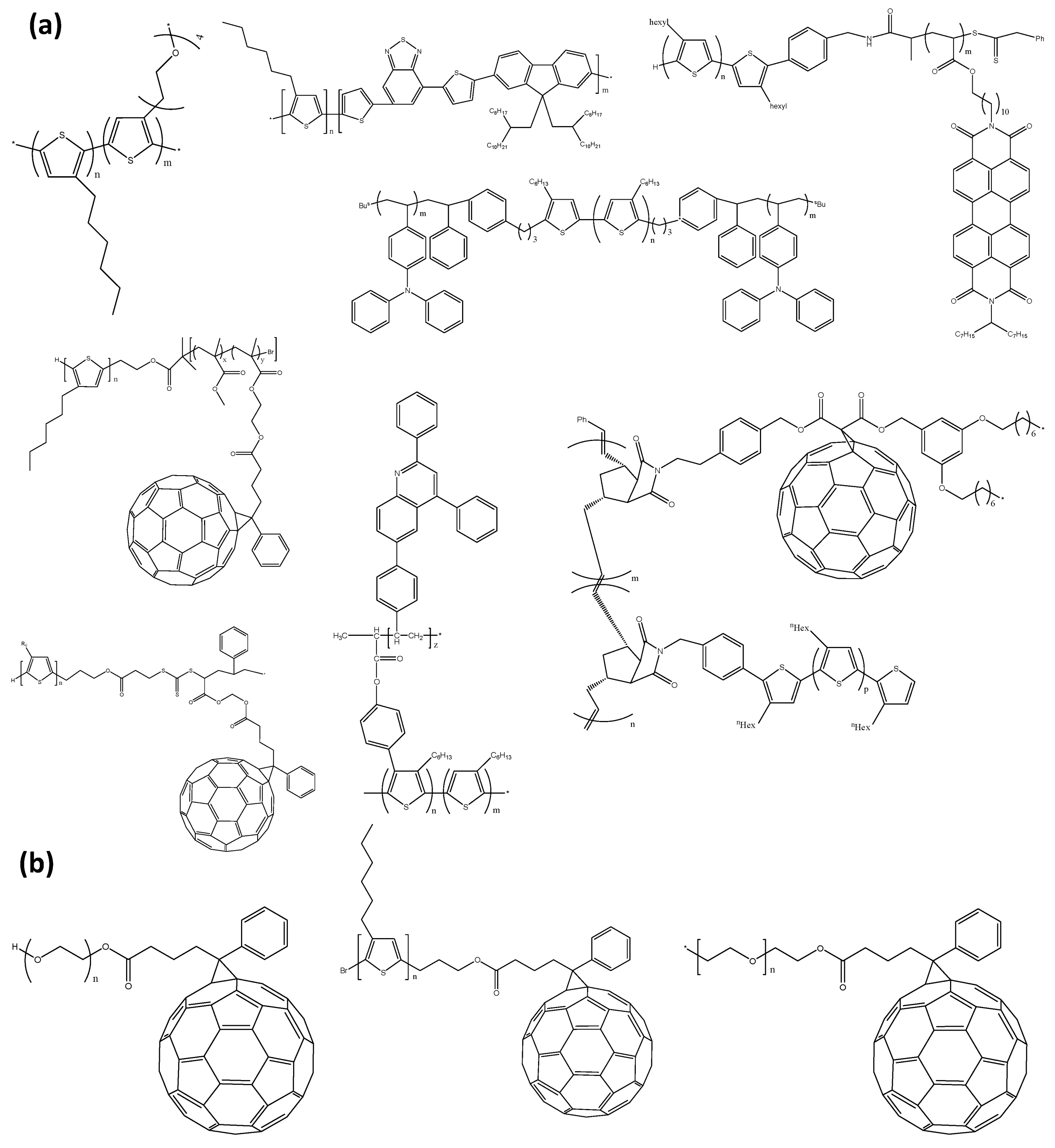


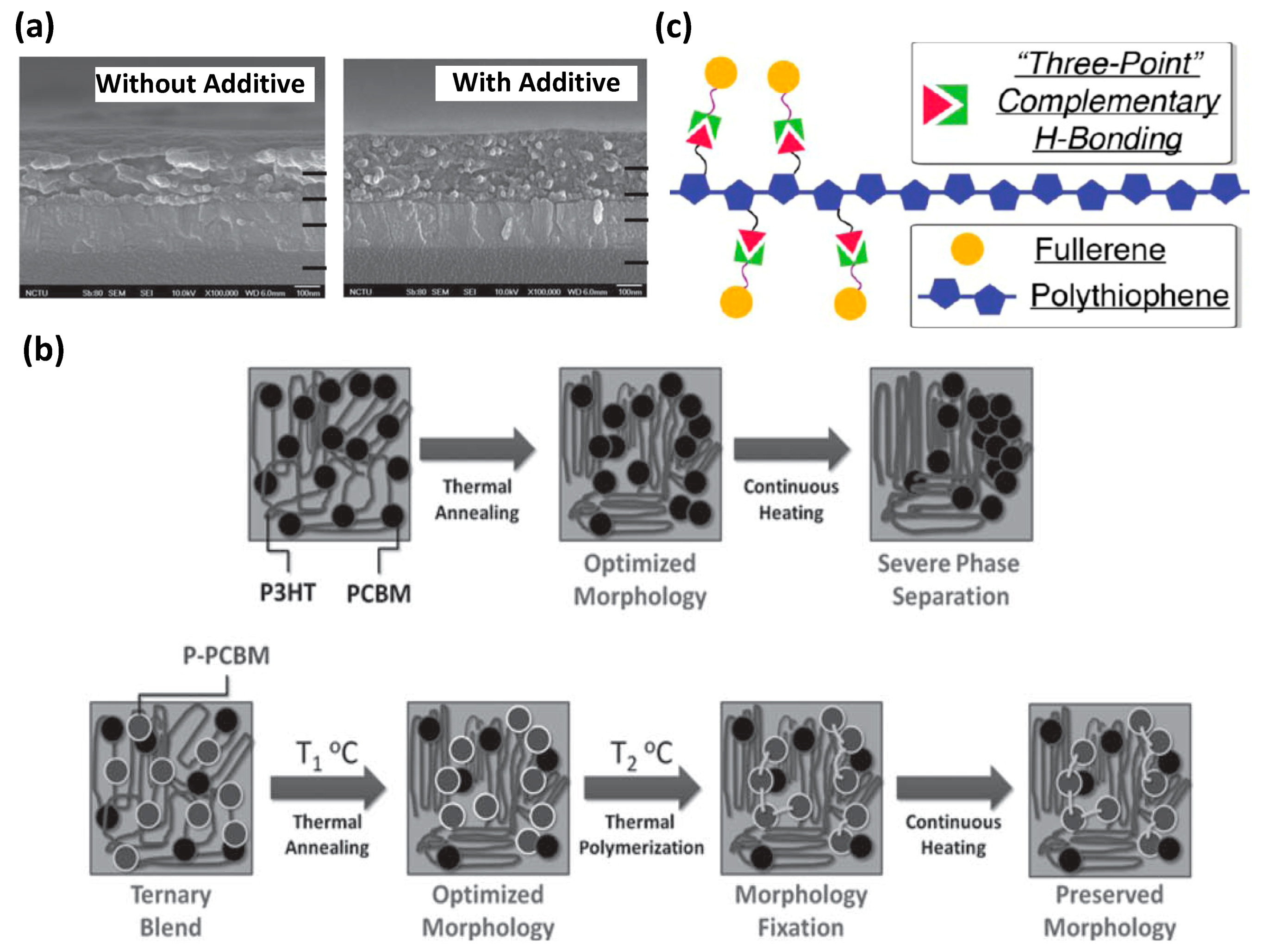
2.2. Homopolymer
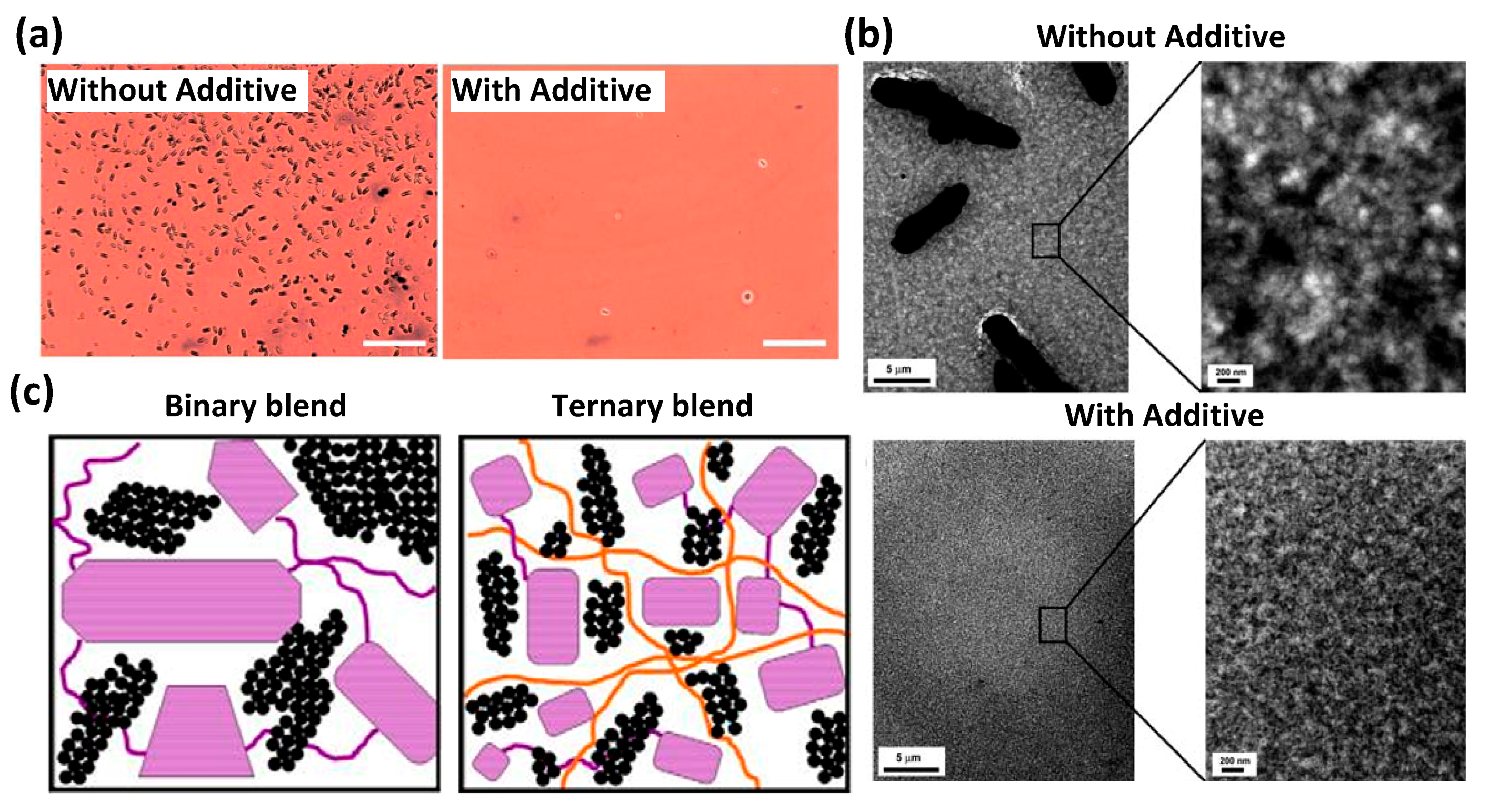
3. Small Molecules
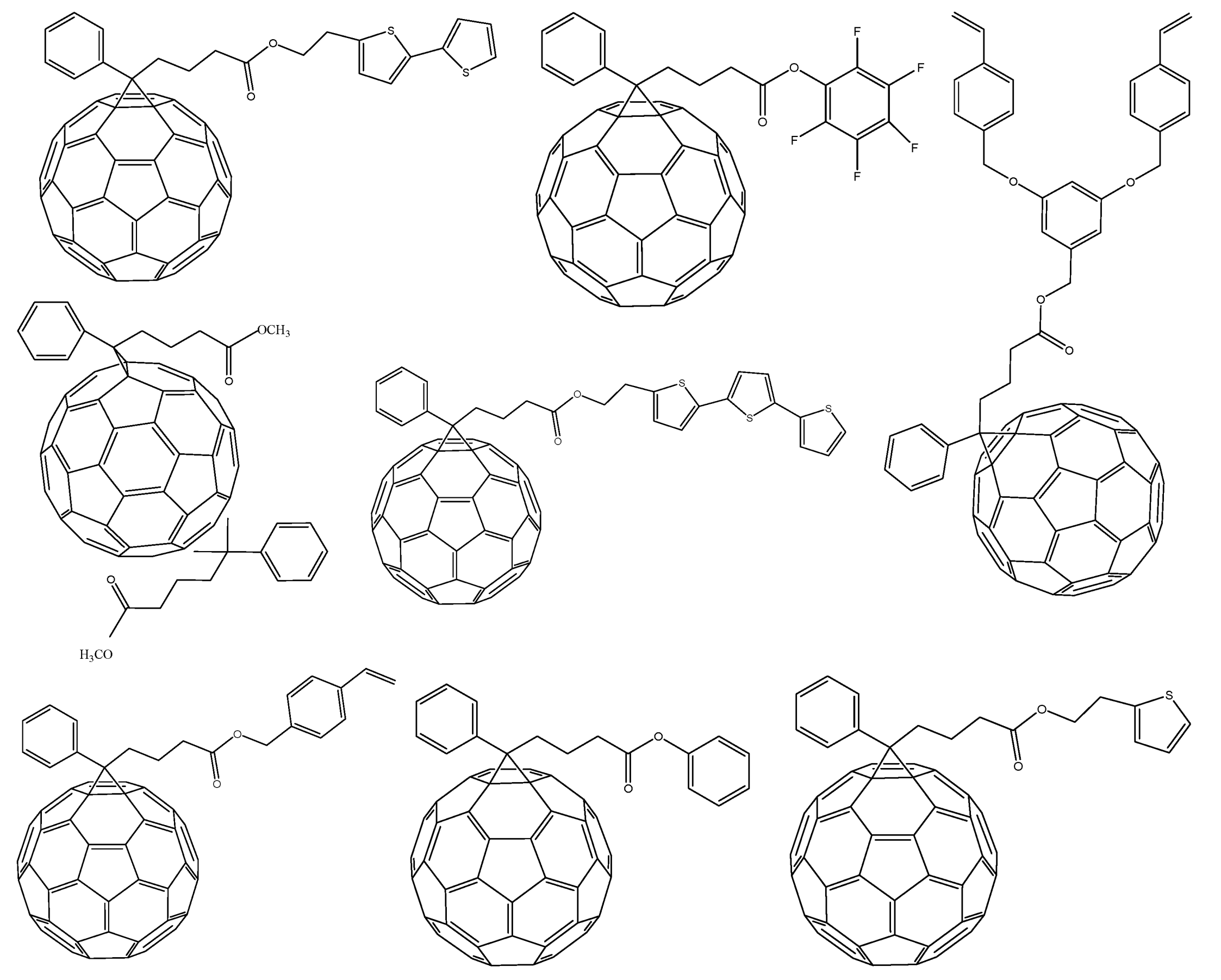
4. Inorganic Nanocrystals
5. Summary and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liao, H.C.; Ho, C.C.; Chang, C.Y.; Jao, M.H.; Darling, S.B.; Su, W.F. Additives for morphology control in high-efficiency organic solar cells. Mater. Today 2013, 16, 326–336. [Google Scholar]
- Cheng, Y.J.; Yang, S.H.; Hsu, C.S. Synthesis of conjugated polymers for organic solar cell applications. Chem. Rev. 2009, 109, 5868–5923. [Google Scholar] [PubMed]
- Kozycz, L.M.; Gao, D.; Hollinger, J.; Seferos, D.S. Donor–donor block copolymers for ternary organic solar cells. Macromolecules 2012, 45, 5823–5832. [Google Scholar] [CrossRef]
- Bian, L.; Zhu, E.; Tang, J.; Tang, W.; Zhang, F. Recent progress in the design of narrow bandgap conjugated polymers for high-efficiency organic solar cells. Prog. Polym. Sci. 2012, 37, 1292–1331. [Google Scholar] [CrossRef]
- Su, Y.W.; Lan, S.C.; Wei, K.H. Organic photovoltaics. Mater. Today 2012, 15, 554–562. [Google Scholar] [CrossRef]
- Brabec, C.J.; Heeney, M.; McCulloch, I.; Nelson, J. Influence of blend microstructure on bulk heterojunction organic photovoltaic performance. Chem. Soc. Rev. 2011, 40, 1185–1199. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Nikiforov, M.P.; Darling, S.B. Morphology characterization in organic and hybrid solar cells. Energy Environ. Sci. 2012, 5, 8045–8074. [Google Scholar] [CrossRef]
- Ruderer, M.A.; Müller-Buschbaum, P. Morphology of polymer-based bulk heterojunction films for organic photovoltaics. Soft Matter 2011, 7, 5482–5493. [Google Scholar] [CrossRef]
- Brady, M.A.; Su, G.M.; Chabinyc, M.L. Recent progress in the morphology of bulk heterojunction photovoltaics. Soft Matter 2011, 7, 11065–11077. [Google Scholar] [CrossRef]
- Liu, F.; Gu, Y.; Jung, J.W.; Jo, W.H.; Russell, T.P. On the morphology of polymer-based photovoltaics. J. Poly. Sci. B Poly. Phys. 2012, 50, 1018–1044. [Google Scholar] [CrossRef]
- Janssen, R.A.; Nelson, J. Factors limiting device efficiency in organic photovoltaics. Adv. Mater. 2013, 25, 1847–1858. [Google Scholar] [CrossRef] [PubMed]
- Braun, S.; Salaneck, W.R.; Fahlman, M. Energy-level alignment at organic/metal and organic/organic interfaces. Adv. Mater. 2009, 21, 1450–1472. [Google Scholar] [CrossRef]
- Deibel, C.; Strobel, T.; Dyakonov, V. Role of the charge transfer state in organic donor-acceptor solar cells. Adv. Mater. 2010, 22, 4097–4111. [Google Scholar] [CrossRef] [PubMed]
- Piliego, C.; Loi, M.A. Charge transfer state in highly efficient polymer–fullerene bulk heterojunction solar cells. J. Mater. Chem. 2012, 22, 4141–4150. [Google Scholar] [CrossRef]
- Sista, S.; Hong, Z.; Chen, L.M.; Yang, Y. Tandem polymer photovoltaic cells—Current status, challenges and future outlook. Energy Environ. Sci. 2011, 4, 1606–1620. [Google Scholar] [CrossRef]
- Ma, W.L.; Yang, C.Y.; Gong, X.; Lee, K.; Heeger, A.J. Thermally stable, efficient polymer solar cells with nanoscale control of the interpenetrating network morphology. Adv. Funct. Mater. 2005, 15, 1617–1622. [Google Scholar] [CrossRef]
- Li, G.; Shrotriya, V.; Huang, J.; Yao, Y.; Moriarty, T.; Emery, K.; Yang, Y. High-efficiency solution processable polymer photovoltaic cells by self-organization of polymer blends. Nat. Mater. 2005, 4, 864–868. [Google Scholar] [CrossRef]
- Kim, Y.; Cook, S.; Tuladhar, S.M.; Choulis, S.A.; Nelson, J.; Durrant, J.R.; Bradley, D.D.C.; Giles, M.; McCulloch, I.; Ha, C.S.; et al. A strong regioregularity effect in self-organizing conjugated polymer films and high-efficiency polythiophene:fullerene solar cells. Nat. Mater. 2006, 5, 197–203. [Google Scholar] [CrossRef]
- Yang, X.N.; Loos, J.; Veenstra, S.C.; Verhees, W.J.H.; Wienk, M.M.; Kroon, J.M.; Michels, M.A.J.; Janssen, R.A.J. Nanoscale morphology of high-performance polymer solar cells. Nano Lett. 2005, 5, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yao, Y.; Yang, H.; Shrotriya, V.; Yang, G.; Yang, Y. “Solvent annealing” effect in polymer solar cells based on poly(3-hexylthiophene) and methanofullerenes. Adv. Funct. Mater. 2007, 17, 1636–1644. [Google Scholar] [CrossRef]
- Ruderer, M.A.; Guo, S.; Meier, R.; Chiang, H.Y.; Körstgens, V.; Wiedersich, J.; Perlich, J.; Roth, S.V.; Müller-Buschbaum, P. Solvent-induced morphology in polymer-based systems for organic photovoltaics. Adv. Funct. Mater. 2011, 21, 3382–3391. [Google Scholar] [CrossRef]
- Peet, J.; Kim, J.Y.; Coates, N.E.; Ma, W.L.; Moses, D.; Heeger, A.J.; Bazan, G.C. Efficiency enhancement in low-bandgap polymer solar cells by processing with alkane dithiols. Nat. Mater. 2007, 6, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Dang, M.T.; Hirsch, L.; Wantz, G.; Wuest, J.D. Controlling the morphology and performance of bulk heterojunctions in solar cells. Lessons learned from the benchmark poly(3-hexylthiophene): [6,6]-phenyl-C61-butyric acid methyl ester system. Chem. Rev. 2013, 113, 3734–3765. [Google Scholar] [CrossRef] [PubMed]
- Rivnay, J.; Mannsfeld, S.C.; Miller, C.E.; Salleo, A.; Toney, M.F. Quantitative determination of organic semiconductor microstructure from the molecular to device scale. Chem. Rev. 2012, 112, 5488–5519. [Google Scholar] [CrossRef] [PubMed]
- Ameri, T.; Khoram, P.; Min, J.; Brabec, C.J. Organic ternary solar cells: A review. Adv. Mater. 2013, 25, 4245–4266. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Hsu, C.Y.; Lin, R.Y.; Ho, K.C.; Lin, J.T. Materials for the active layer of organic photovoltaics: ternary solar cell approach. ChemSusChem 2013, 6, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yan, L.; You, W. Organic solar cells beyond one pair of donor–acceptor: Ternary blends and more. J. Phys. Chem. Lett. 2013, 4, 1802–1810. [Google Scholar] [CrossRef]
- Meier, R.; Schindler, M.; Muller-Buschbaum, P.; Watts, B. Residual solvent content in conducting polymer-blend films mapped with scanning transmission X-ray microscopy. Phys. Rev. B 2011, 84. [Google Scholar] [CrossRef]
- Brabec, C.J.; Padinger, F.; Sariciftci, N.S.; Hummelen, J.C. Photovoltaic properties of conjugated polymer/methanofullerene composites embedded in a polystyrene matrix. J. Appl. Phys. 1999, 85. [Google Scholar] [CrossRef]
- Brabec, C.J.; Johannson, H.; Padinger, F.; Neugebauer, H.; Hummelen, J.C.; Sariciftci, N.S. Photoinduced FT-IR spectroscopy and CW-photocurrent measurements of onjugated polymers and fullerenes blended into a conventional polymer matrix. Sol. Energy Mater. Sol. Cells 2000, 61, 19–33. [Google Scholar] [CrossRef]
- Camaioni, N.; Catellani, M.; Luzzati, S.; Migliori, A. Morphological characterization of poly(3-octylthiophene):plasticizer:C60 blends. Thin Solid Films 2002, 403–404, 489–494. [Google Scholar] [CrossRef]
- Lee, M.; Cho, B.K.; Zin, W.C. Supramolecular structures from rod-coil block copolymers. Chem. Rev. 2001, 101, 3869–3892. [Google Scholar] [CrossRef] [PubMed]
- Klok, H.A. Supramolecular materials via block copolymer self-assembly. Adv. Mater. 2001, 13, 1217–1229. [Google Scholar] [CrossRef]
- Jenekhe, S.A.; Chen, X.L. Self-assembled aggregates of rod-coil block copolymers and their solubilization and encapsulation of fullerenes. Science 1998, 279, 1903–1907. [Google Scholar] [CrossRef] [PubMed]
- Jenekhe, S.A.; Chen, X.L. Self-assembly of ordered microporous materials from rod-coil block copolymers. Science 1999, 283, 372–375. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Qiu, F.; Lin, Z. Conjugated rod–coil and rod–rod block copolymers for photovoltaic applications. J. Mater. Chem. 2011, 21, 17039–17048. [Google Scholar] [CrossRef]
- Tsai, J.H.; Lai, Y.C.; Higashihara, T.; Lin, C.J.; Ueda, M.; Chen, W.C. Enhancement of P3HT/PCBM photovoltaic efficiency using the surfactant of triblock copolymer containing poly(3-hexylthiophene) and poly(4-vinyltriphenylamine) segments. Macromolecules 2010, 43, 6085–6091. [Google Scholar] [CrossRef]
- Sivula, K.; Ball, Z.T.; Watanabe, N.; Fréchet, J.M.J. Amphiphilic diblock copolymer compatibilizers and their effect on the morphology and performance of polythiophene:fullerene solar cells. Adv. Mater. 2006, 18, 206–210. [Google Scholar] [CrossRef]
- Yang, C.; Lee, J.K.; Heeger, A.J.; Wudl, F. Well-defined donor–acceptor rod–coil diblock copolymers based on P3HT containing C60: The morphology and role as a surfactant in bulk-heterojunction solar cells. J. Mater. Chem. 2009, 19, 5416–5423. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.U.; Jung, J.W.; Emrick, T.; Russell, T.P.; Jo, W.H. Morphology control of a polythiophene-fullerene bulk heterojunction for enhancement of the high-temperature stability of solar cell performance by a new donor-acceptor diblock copolymer. Nanotechnology 2010, 21. [Google Scholar] [CrossRef] [PubMed]
- Im, M.J.; Son, S.Y.; Moon, B.J.; Lee, G.Y.; Kim, J.H.; Park, T. Improved photovoltaic performance by enhanced crystallinity of poly(3-hexyl)thiophene. Org. Electron. 2013, 14, 3046–3051. [Google Scholar] [CrossRef]
- Mulherin, R.C.; Jung, S.; Huettner, S.; Johnson, K.; Kohn, P.; Sommer, M.; Allard, S.; Scherf, U.; Greenham, N.C. Ternary photovoltaic blends incorporating an all-conjugated donor-acceptor diblock copolymer. Nano Lett. 2011, 11, 4846–4851. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Armstrong, P.B.; Kim, B.J.; Frechet, J.M.J. Effect of addition of a diblock copolymer on blend morphology and performance of poly(3-hexylthiophene):perylene diimide solar cells. Chem. Mater. 2009, 21, 1775–1777. [Google Scholar] [CrossRef]
- Economopoulo, S.P.; Chochos, C.L.; Gregoriou, V.G.; Kallitsis, J.K.; Barrau, S.; Hadziioannou, G. Novel brush-type copolymers bearing thiophene backbone and side chain quinoline blocks. Synthesis and their use as a compatibilizer in thiophene-quinoline polymer blends. Macromolecules 2007, 40, 921–927. [Google Scholar] [CrossRef]
- Jung, J.W.; Jo, J.W.; Jo, W.H. Enhanced performance and air stability of polymer solar cells by formation of a self-assembled buffer layer from fullerene-end-capped poly(ethylene glycol). Adv. Mater. 2011, 23, 1782–1787. [Google Scholar] [CrossRef] [PubMed]
- Tai, Q.; Li, J.; Liu, Z.; Sun, Z.; Zhao, X.; Yan, F. Enhanced photovoltaic performance of polymer solar cells by adding fullerene end-capped polyethylene glycol. J. Mater. Chem. 2011, 21, 6848–6853. [Google Scholar] [CrossRef]
- Lee, J.U.; Jung, J.W.; Emrick, T.; Russell, T.P.; Jo, W.H. Synthesis of C60-end capped P3HT and its application for high performance of P3HT/PCBM bulk heterojunction solar cells. J. Mater. Chem. 2010, 20, 3287–3294. [Google Scholar] [CrossRef]
- Chen, J.; Yu, X.; Hong, K.; Messman, J.M.; Pickel, D.L.; Xiao, K.; Dadmun, M.D.; Mays, J.W.; Rondinone, A.J.; Sumpter, B.G.; et al. Ternary behavior and systematic nanoscale manipulation of domain structures in P3HT/PCBM/P3HT-b-PEO films. J. Mater. Chem. 2012, 22, 13013–13022. [Google Scholar] [CrossRef]
- Sun, Z.; Xiao, K.; Keum, J.K.; Yu, X.; Hong, K.; Browning, J.; Ivanov, I.N.; Chen, J.; Alonzo, J.; Li, D.; et al. PS-b-P3HT copolymers as P3HT/PCBM interfacial compatibilizers for high efficiency photovoltaics. Adv. Mater. 2011, 23, 5529–5535. [Google Scholar] [CrossRef] [PubMed]
- Campoy-Quiles, M.; Kanai, Y.; El-Basaty, A.; Sakai, H.; Murata, H. Ternary mixing: A simple method to tailor the morphology of organic solar cells. Org. Electron. 2009, 10, 1120–1132. [Google Scholar] [CrossRef]
- Lee, J.K.; Ma, W.L.; Brabec, C.J.; Yuen, J.; Moon, J.S.; Kim, J.K.; Lee, K.; Bazan, G.C.; Heeger, A.J. Processing additives for improved efficiency from bulk heterojunction solar cells. J. Am. Chem. Soc. 2008, 130, 3619–3623. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.Y.; Liao, H.C.; Shao, Y.T.; Sung, Y.M.; Hsu, S.H.; Ho, C.C.; Su, W.F.; Chen, Y.F. Enhancing the efficiency of low bandgap conducting polymer bulk heterojunction solar cells using P3HT as a morphology control agent. J. Mater. Chem. A 2013, 1, 2447–2452. [Google Scholar] [CrossRef]
- Kan, Z.; Colella, L.; Canesi, E.V.; Lerario, G.; Kumar, R.S.S.; Bonometti, V.; Mussini, P.R.; Cavallo, G.; Terraneo, G.; Pattanasattayavong, P.; et al. Triple bulk heterojunctions as means for recovering the microstructure of photoactive layers in organic solar cell devices. Sol. Energy Mater. Sol. Cells 2014, 120, 37–47. [Google Scholar] [CrossRef]
- Cha, H.; Chung, D.S.; Bae, S.Y.; Lee, M.J.; An, T.K.; Hwang, J.; Kim, K.H.; Kim, Y.H.; Choi, D.H.; Park, C.E. Complementary absorbing star-shaped small molecules for the preparation of ternary cascade energy structures in organic photovoltaic cells. Adv. Funct. Mater. 2013, 23, 1556–1565. [Google Scholar] [CrossRef]
- Kim, C.S.; Tinker, L.L.; DiSalle, B.F.; Gomez, E.D.; Lee, S.; Bernhard, S.; Loo, Y.L. Altering the thermodynamics of phase separation in inverted bulk-heterojunction organic solar cells. Adv. Mater. 2009, 21, 3110–3115. [Google Scholar] [CrossRef]
- Brabec, C.J.; Sariciftc, S.; Hummelen, J.C. Plastic solar cells. Adv. Funct. Mater. 2011, 11, 15–26. [Google Scholar] [CrossRef]
- Li, C.Z.; Yip, H.L.; Jen, A.K.Y. Functional fullerenes for organic photovoltaics. J. Mater. Chem. 2012, 22, 4161–4177. [Google Scholar] [CrossRef]
- Lai, Y.C.; Higashihara, T.; Hsu, J.C.; Ueda, M.; Chen, W.C. Enhancement of power conversion efficiency and long-term stability of P3HT/PCBM solar cells using C60 derivatives with thiophene units as surfactants. Sol. Energy Mater. Sol. Cells 2012, 97, 164–170. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Hsieh, C.H.; Li, P.J.; Hsu, C.S. Morphological stabilization by in situ polymerization of fullerene derivatives leading to efficient, thermally stable organic photovoltaics. Adv. Funct. Mater. 2011, 21, 1723–1732. [Google Scholar] [CrossRef]
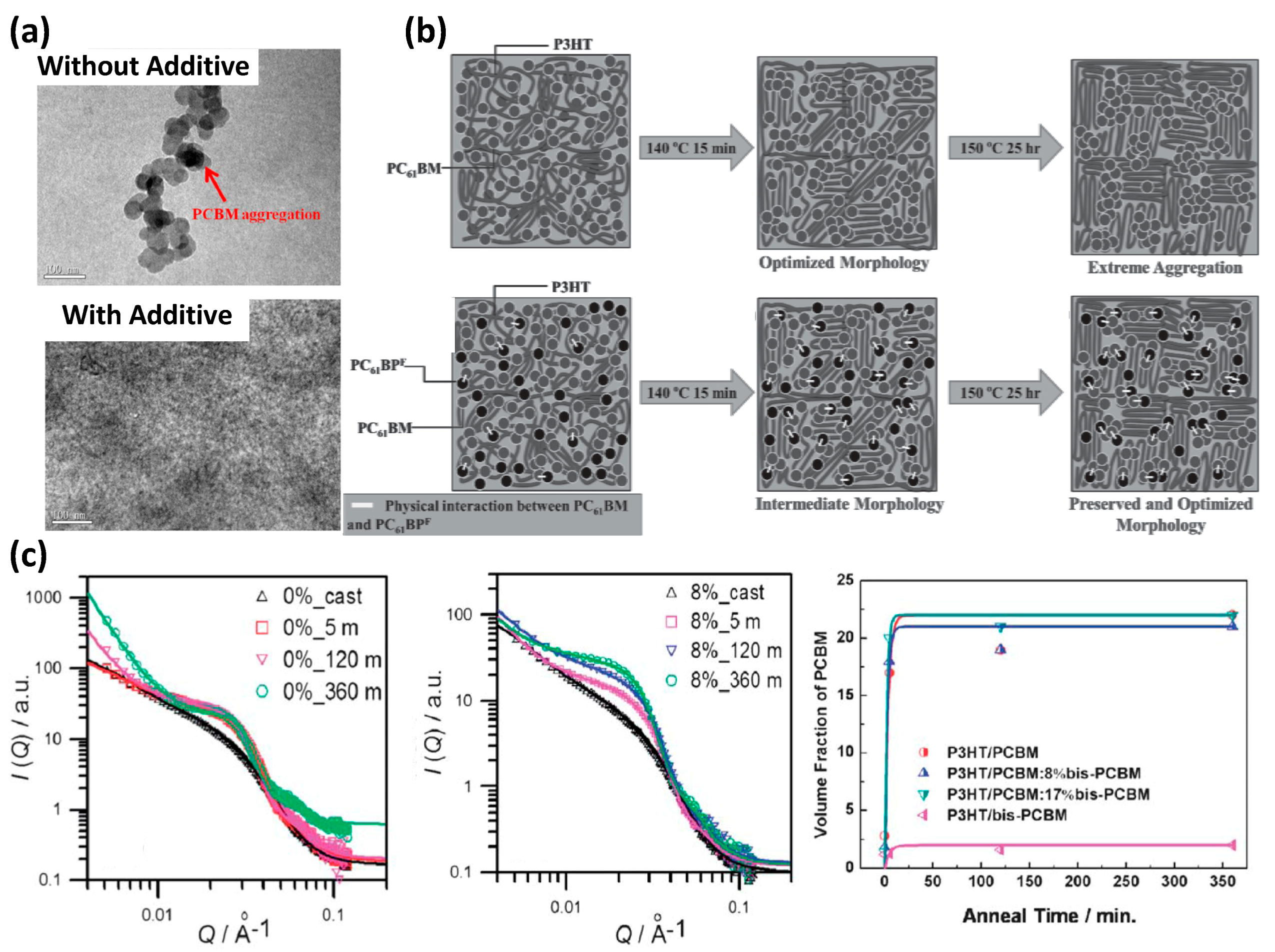
- Liao, M.H.; Tsai, C.E.; Lai, Y.Y.; Cao, F.Y.; Wu, J.S.; Wang, C.L.; Hsu, C.S.; Liau, I.; Cheng, Y.J. Morphological stabilization by supramolecular perfluorophenyl-C60 interactions leading to efficient and thermally stable organic photovoltaics. Adv. Funct. Mater. 2014, 24, 1418–1429. [Google Scholar] [CrossRef]
- Liu, H.W.; Chang, D.Y.; Chiu, W.Y.; Rwei, S.P.; Wang, L. Fullerene bisadduct as an effective phase-separation inhibitor in preparing poly(3-hexylthiophene)-[6,6]-phenyl-C61-butyric acid methyl ester blends with highly stable morphology. J. Mater. Chem. 2012, 22, 15586–15591. [Google Scholar] [CrossRef]
- Li, F.; Yager, K.G.; Dawson, N.M.; Yang, J.; Malloy, K.J.; Qin, Y. Complementary hydrogen bonding and block copolymer self-assembly in cooperation toward stable solar cells with tunable morphologies. Macromolecules 2013, 46, 9021–9031. [Google Scholar] [CrossRef]
- Chen, C.Y.; Tsao, C.S.; Huang, Y.C.; Liu, H.W.; Chiu, W.Y.; Chuang, C.M.; Jeng, U.S.; Su, C.J.; Wu, W.R.; Su, W.F.; et al. Mechanism and control of the structural evolution of a polymer solar cell from a bulk heterojunction to a thermally unstable hierarchical structure. Nanoscale 2013, 5, 7629–7638. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.C.; Tsao, C.S.; Lin, T.H.; Chuang, C.M.; Chen, C.Y.; Jeng, U.S.; Su, C.H.; Chen, Y.F.; Su, W.F. Quantitative nanoorganized structural evolution for a high efficiency bulk heterojunction polymer solar cell. J. Am. Chem. Soc. 2011, 133, 13064–13073. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.C.; Tsao, C.S.; Lin, T.H.; Jao, M.H.; Chuang, C.M.; Chang, S.Y.; Huang, Y.C.; Shao, Y.T.; Chen, C.Y.; Su, C.J.; et al. Nanoparticle-tuned self-organization of a bulk heterojunction hybrid solar cell with enhanced performance. ACS Nano 2012, 6, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Choy, W.C.; Huo, L.; Xie, F.; Sha, W.E.; Ding, B.; Guo, X.; Li, Y.; Hou, J.; You, J.; et al. Dual plasmonic nanostructures for high performance inverted organic solar cells. Adv. Mater. 2012, 24, 3046–3052. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Choy, W.C.H.; Lu, H.; Sha, W.E.I.; Ho, A.H.P. Efficiency enhancement of organic solar cells by using shape-dependent broadband plasmonic absorption in metallic nanoparticles. Adv. Funct. Mater. 2013, 23, 2728–2735. [Google Scholar] [CrossRef]
- Chen, H.C.; Chou, S.W.; Tseng, W.H.; Chen, I.W.P.; Liu, C.C.; Liu, C.; Liu, C.L.; Chen, C.H.; Wu, C.I.; Chou, P.T. Large AuAg alloy nanoparticles synthesized in organic media using a one-pot reaction: Their applications for high-performance bulk heterojunction solar cells. Adv. Funct. Mater. 2012, 22, 3975–3984. [Google Scholar] [CrossRef]
- Wang, D.H.; Kim, D.Y.; Choi, K.W.; Seo, J.H.; Im, S.H.; Park, J.H.; Park, O.O.; Heeger, A.J. Enhancement of donor–acceptor polymer bulk heterojunction solar cell power conversion efficiencies by addition of Au nanoparticles. Angew. Chem. 2011, 50, 5519–5523. [Google Scholar] [CrossRef]
- Taukeer Khan, M.; Kaur, A.; Dhawan, S.K.; Chand, S. In-situ growth of cadmium telluride nanocrystals in poly(3-hexylthiophene) matrix for photovoltaic application. J. Appl. Phys. 2011, 110. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, H.-C.; Chen, P.-H.; Chang, R.P.H.; Su, W.-F. Morphological Control Agent in Ternary Blend Bulk Heterojunction Solar Cells. Polymers 2014, 6, 2784-2802. https://doi.org/10.3390/polym6112784
Liao H-C, Chen P-H, Chang RPH, Su W-F. Morphological Control Agent in Ternary Blend Bulk Heterojunction Solar Cells. Polymers. 2014; 6(11):2784-2802. https://doi.org/10.3390/polym6112784
Chicago/Turabian StyleLiao, Hsueh-Chung, Po-Hsuen Chen, Robert P. H. Chang, and Wei-Fang Su. 2014. "Morphological Control Agent in Ternary Blend Bulk Heterojunction Solar Cells" Polymers 6, no. 11: 2784-2802. https://doi.org/10.3390/polym6112784




