Stimuli-Responsive Polymers and Colloids under Electric and Magnetic Fields
Abstract
:1. Introduction
2. Materials
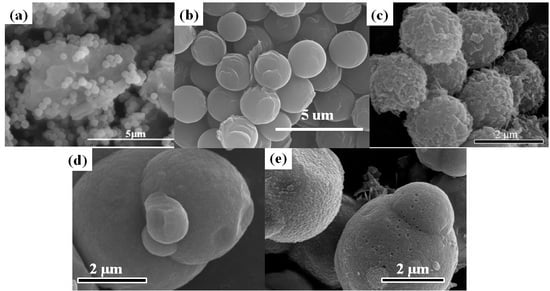
3. Morphology


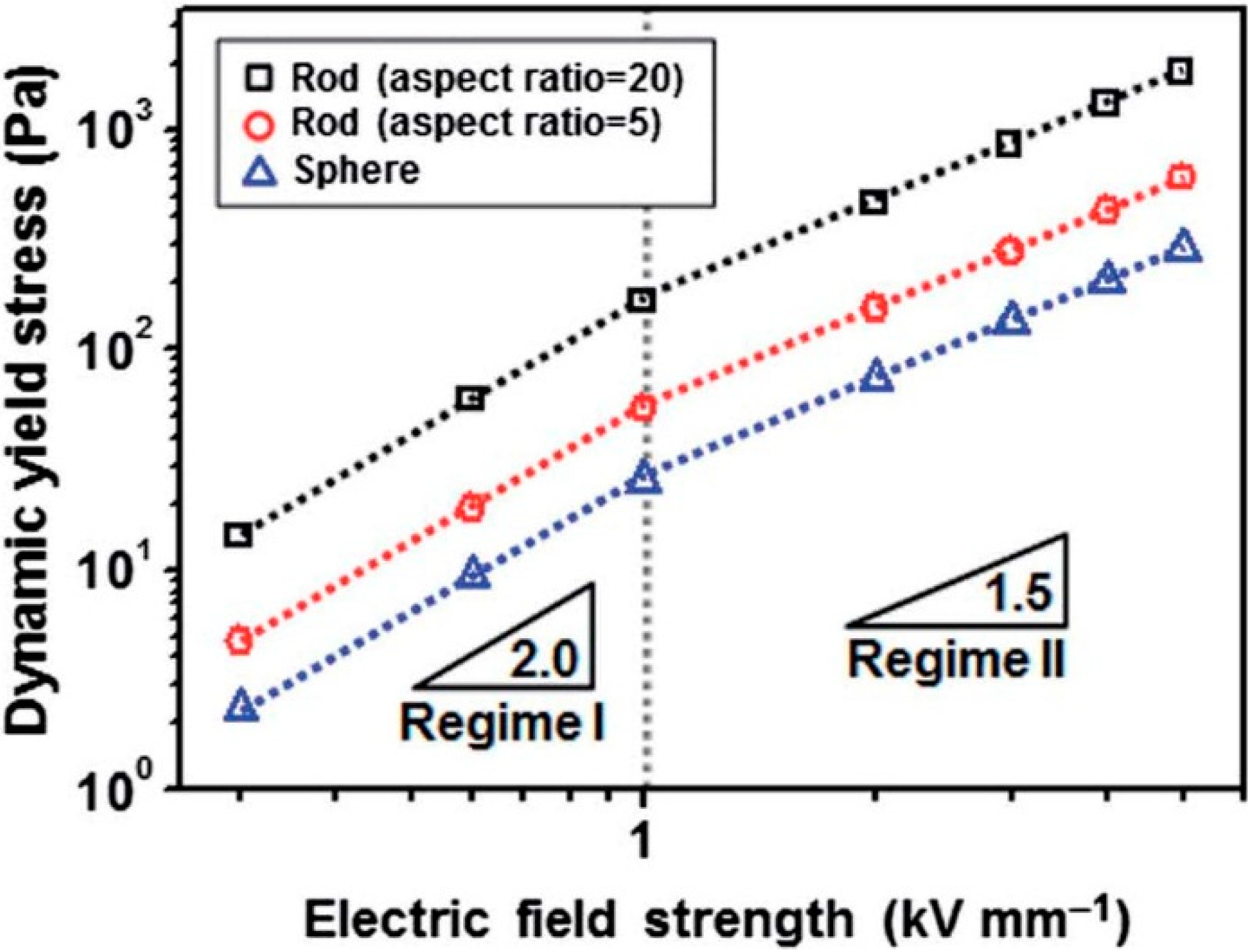
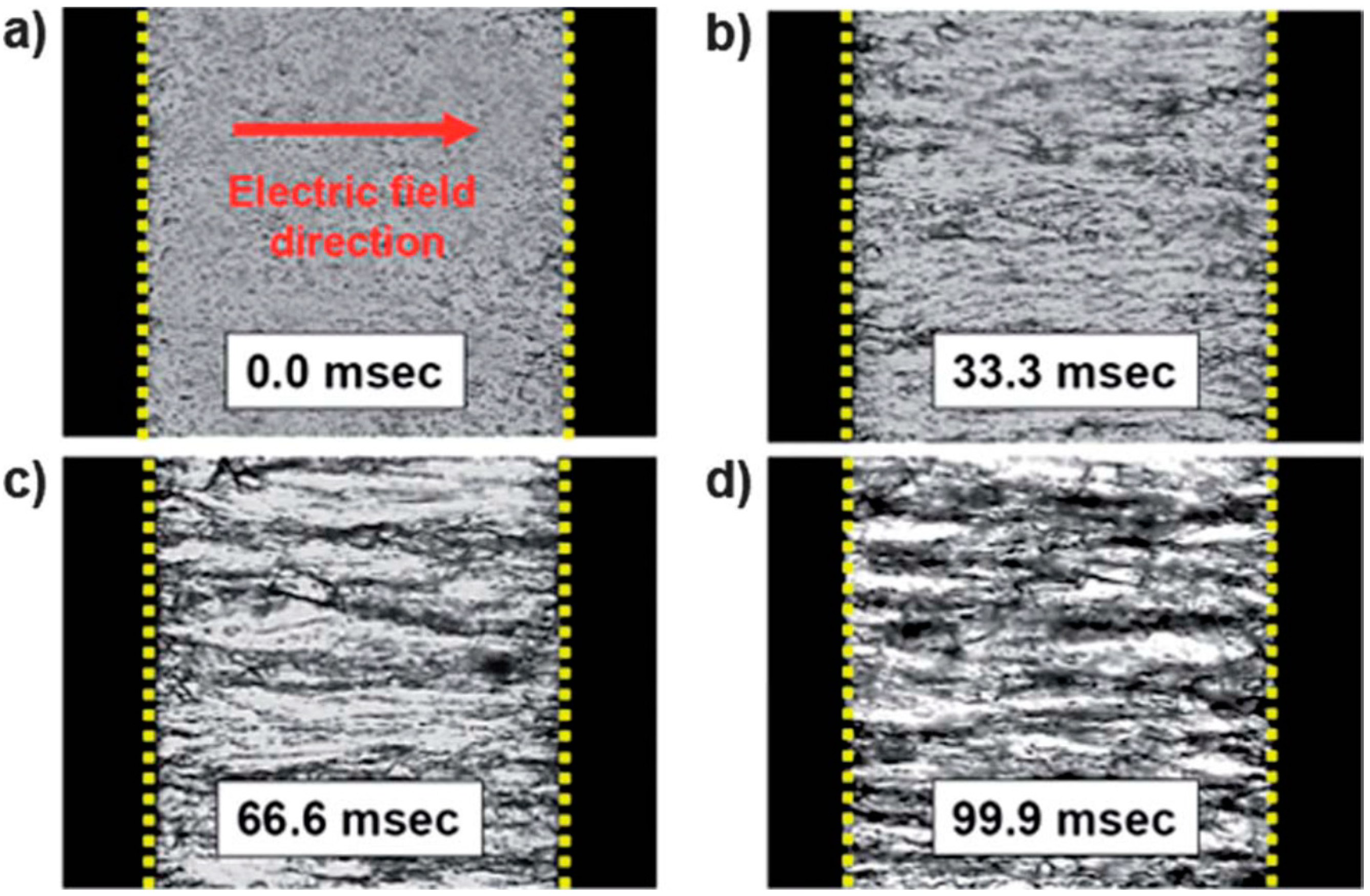
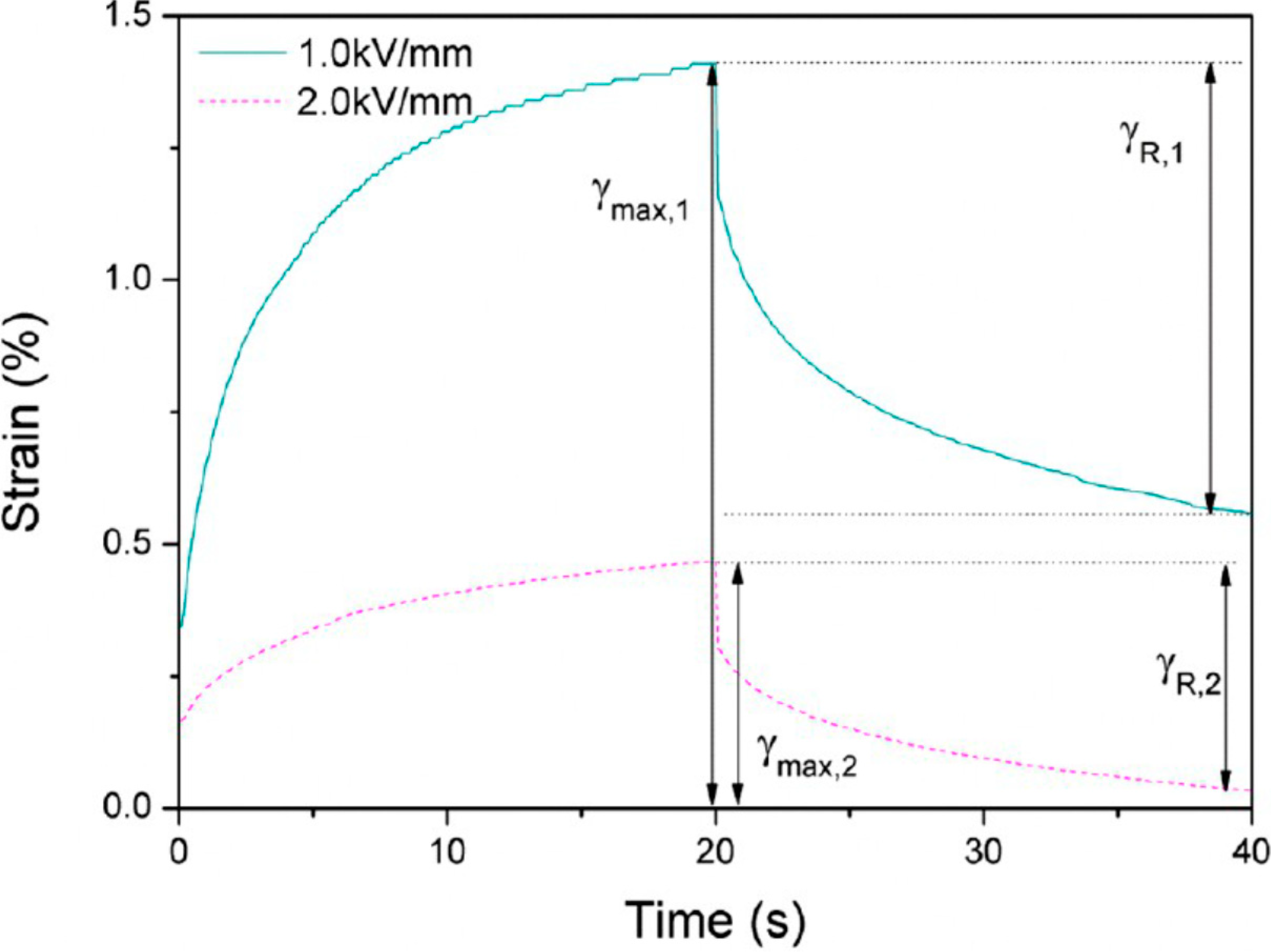
4. Typical Characteristics and Rheological Analysis

 is the shear rate. The simple Bingham fluid model cannot cover the shear stress curves well. A suggested model called the Cho–Choi–Jhon (CCJ) model [83] with six parameters has been adopted:
is the shear rate. The simple Bingham fluid model cannot cover the shear stress curves well. A suggested model called the Cho–Choi–Jhon (CCJ) model [83] with six parameters has been adopted:

 [84]. Note that the CCJ model has been also adopted into various ER materials [85,86].
[84]. Note that the CCJ model has been also adopted into various ER materials [85,86].
 H2. With increasing H, the local saturation becomes prominent, and τy can be expressed as follows:
H2. With increasing H, the local saturation becomes prominent, and τy can be expressed as follows:





 vs.
vs.  for MWCNT/PANI/CI-based MR suspension (Reprinted with permission from [76], copyright 2004 American Chemical Society).
for MWCNT/PANI/CI-based MR suspension (Reprinted with permission from [76], copyright 2004 American Chemical Society).
 vs.
vs.  for MWCNT/PANI/CI-based MR suspension (Reprinted with permission from [76], copyright 2004 American Chemical Society).
for MWCNT/PANI/CI-based MR suspension (Reprinted with permission from [76], copyright 2004 American Chemical Society).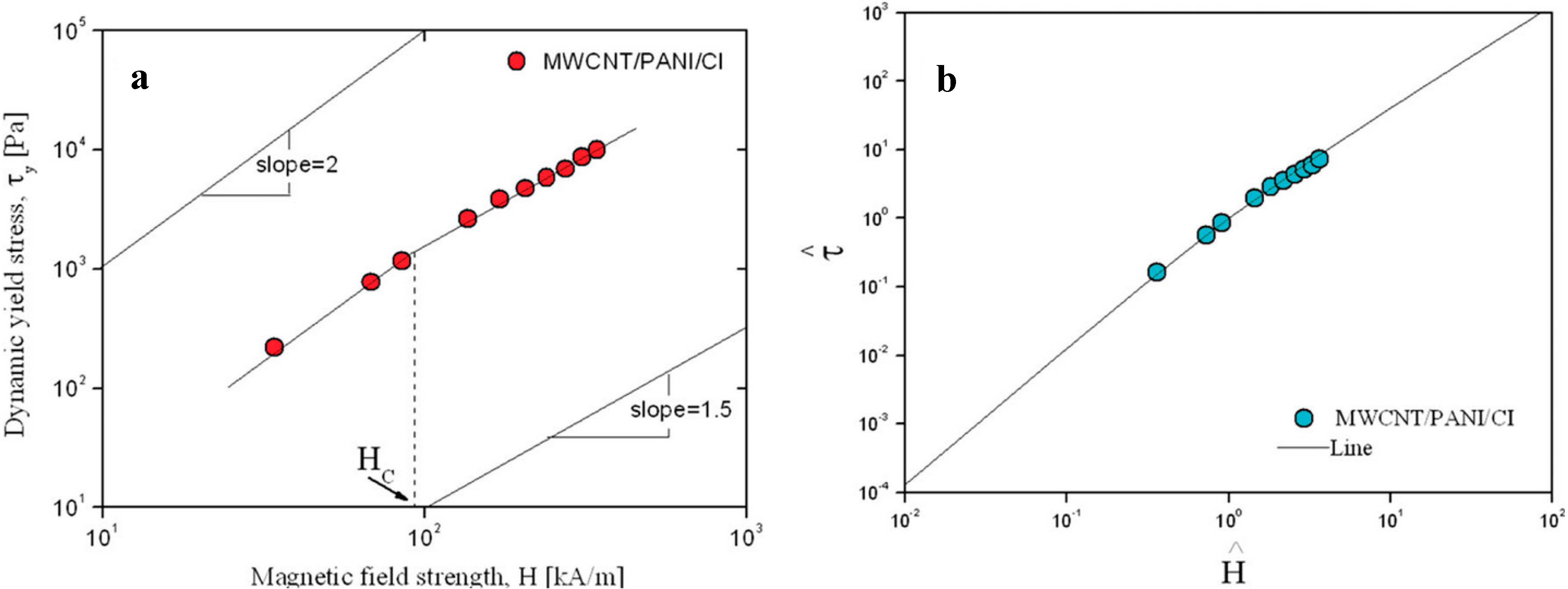
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Dai, S.; Ravi, P.; Tam, K.C. Thermo- and photo-responsive polymeric systems. Soft Matter 2009, 5, 2513–2533. [Google Scholar]
- Wu, X.; Huang, W.M.; Zhao, Y.; Ding, Z.; Tang, C.; Zhang, J. Mechanisms of the shape memory effect in polymeric materials. Polymers 2013, 5, 1169–1202. [Google Scholar]
- Choi, H.J.; Lee, Y.H.; Kim, C.A.; Jhon, M.S. Microencapsulated polyaniline particles for electrorheological materials. J. Mater. Sci. Lett. 2000, 19, 533–535. [Google Scholar]
- Aucoin, H.R.; Wilson, A.N.; Wilson, A.M.; Ishihara, K.; Guiseppi-Elie, A. Release of potassium ion and calcium ion from phosphorylcholine group bearing hydrogels. Polymers 2013, 5, 1241–1257. [Google Scholar]
- Wu, J.; Jin, T.; Liu, E.; Guo, J.; Cheng, Y.; Xu, G. Formamide-modified titanium oxide nanoparticles with high electrorheological activity. RSC Adv. 2014, 4, 29622–29628. [Google Scholar]
- Parthasarathy, M.; Klingenberg, D.J. Electrorheology: Mechanisms and models. Mater. Sci. Eng. R 1996, 17, 57–103. [Google Scholar]
- Choi, H.J.; Jhon, M.S. Electrorheology of polymers and nanocomposites. Soft Matter 2009, 5, 1562–1567. [Google Scholar]
- Marins, J.A.; Dahmouche, K.; Soares, B.G. New electrorheological fluid obtained from mercaptosilsesquioxane-modified silicate suspensions. Mater. Sci. Eng. C 2013, 33, 133–139. [Google Scholar]
- Li, C.L.; Chen, J.K.; Fan, S.K.; Ko, F.H.; Chang, F.C. Electrorheological operation of low-/high-permittivity core/shell sio2/au nanoparticle microspheres for display media. ACS Appl. Mater. Interface 2012, 4, 5650–5661. [Google Scholar]
- Kontopoulou, M.; Kaufman, M.; Docoslis, A. Electrorheological properties of PDMS/carbon black suspensions under shear flow. Rheol. Acta 2009, 48, 409–421. [Google Scholar]
- Song, X.; Song, K.; Ding, S.; Chen, Y.; Lin, Y. Electrorheological properties of poly[N,N'-(2-amino-5-carboxybutyl-1,3-phenylenedimethylene)-2,2'-diamino-4,4'-bithiazole]. J. Ind. Eng. Chem. 2013, 19, 416–420. [Google Scholar]
- Hiamtup, P.; Sirivat, A.; Jamieson, A.M. Hysteresis and strain hardening in the creep response of a polyaniline ER fluid. J. Colloid Interface Sci. 2008, 325, 122–129. [Google Scholar]
- Yilmaz, H.; Zengin, H.; Unal, H.I. Synthesis and electrorheological properties of polyaniline/silicon dioxide composites. J. Mater. Sci. 2012, 47, 5276–5286. [Google Scholar]
- Orihara, H.; Nishimoto, Y.; Aida, K.; Na, Y.H. Three-dimensional observation of an immiscible polymer blend subjected to a step electric field under shear flow. Phys. Rev. E 2011, 83. [Google Scholar] [CrossRef]
- Rodrıguez-Arco, L.; Lopez-Lopez, M.T.; Kuzhir, P.; Duran, J.D.G. Steady state rheological behaviour of multi-component magnetic suspensions. Soft Matter 2013, 9, 5726–5737. [Google Scholar]
- Chen, K.; Tian, Y.; Shan, L.; Zhang, X.; Meng, Y. The rheological properties of magnetic field excited magnetic powders sheared between two parallel plates. Smart Mater. Struct. 2013, 22. [Google Scholar] [CrossRef]
- Seo, Y.P.; Seo, Y. Modeling and analysis of electrorheological suspensions in shear flow. Langmuir 2012, 28, 3077–3084. [Google Scholar]
- Kim, Y.D.; Kim, H.S. Negative electrorheological responses of mono-dispersed polypyrrole-san copolymer suspensions. Macromol. Res. 2013, 21, 1153–1158. [Google Scholar]
- Schwarz, G.; Haßlauer, I.; Kurth, D.G. From terpyridine-based assemblies to metallo-supramolecular polyelectrolytes (MEPEs). Adv. Colloid Interface Sci. 2014, 207, 107–120. [Google Scholar]
- Cheng, Q.; Pavlinek, V.; He, Y.; Yan, Y.; Li, C.; Saha, P. Synthesis and electrorheological characteristics of sea urchin-like TiO2 hollow spheres. Colloid Polym. Sci. 2011, 289, 799–805. [Google Scholar]
- Seo, Y. A new yield stress scaling function for electrorheological fluids. J. Non-Newton. Fluid Mech. 2011, 166, 241–243. [Google Scholar]
- Ginder, J.M.; Davis, L.C.; Elie, L.D. Rheology of magnetorheological fluids: Models and measurements. Int. J. Mod. Phys. B 1996, 10, 3293–3303. [Google Scholar]
- De Vicente, J.; Klingenberg, D.J.; Hidalgo-Alvarez, R. Magnetorheological fluids: A review. Soft Matter 2011, 7, 3701–3710. [Google Scholar]
- Guerrero-Sanchez, C.; Lara-Ceniceros, T.; Jimenez-Legalado, E.; Rasa, M.; Schubert, U.S. Magnetorheological fluids based on ionic liquids. Adv. Mater. 2007, 19, 1740–1747. [Google Scholar]
- Bossis, G.; Lacis, S.; Meunier, A.; Volkova, O. Magnetorheological fluids. J. Magn. Magn. Mater. 2002, 252, 224–228. [Google Scholar]
- Tang, X.; Zhang, X.; Tao, R.; Rong, Y.M. Structure-enhanced yield stress of magnetorheological fluids. J. Appl. Phys. 2000, 87, 2634–2638. [Google Scholar]
- Wen, W.J.; Huang, X.X.; Sheng, P. Electrorheological fluids: Structures and mechanisms. Soft Matter 2008, 4, 200–210. [Google Scholar]
- Qian, B.; McKinley, G.H.; Hosoi, A.E. Structure evolution in electrorheological fluids flowing through microchannels. Soft Matter 2013, 9, 2889–2898. [Google Scholar]
- Tao, R.; Du, E.; Tang, H.; Xu, X. Neutron scattering studies of crude oil viscosity reduction with electric field. Fuel 2014, 134, 493–498. [Google Scholar]
- Goncalves, J.L.; Bombard, A.J.F.; Soares, D.A.W.; Alcantara, G.B. Reduction of paraffin precipitation and viscosity of brazilian crude oil exposed to magnetic fields. Energy Fuels 2010, 24, 3144–3149. [Google Scholar]
- Bica, I. Damper with magnetorheological suspension. J. Magn. Magn. Mater. 2002, 241, 196–200. [Google Scholar]
- Kemmetmuller, W.; Holzmann, K.; Kugi, A.; Stork, M. Electrorheological semiactive shock isolation platform for naval applications. IEEE-ASME Trans. Mechatron. 2013, 18, 1437–1447. [Google Scholar]
- Sidpara, A. Magnetorheological finishing: A perfect solution to nanofinishing requirements. Opt. Eng. 2014, 53. [Google Scholar] [CrossRef]
- Seong, M.S.; Choi, S.B.; Kim, C.H. Design and performance evaluation of MR damper for integrated Isolation mount. J. Intel. Mater. Sys. Struct. 2011, 22, 1729–1738. [Google Scholar]
- Rossa, C.; Eck, L.; Micaelli, A.; Lozada, J. On a novel torque detection technique for magnetorheological actuators. IEEE Sens. J. 2014, 14, 1223–1231. [Google Scholar]
- Wang, B.X.; Zhao, Y.; Zhao, X.P. The wettability, size effect and electrorheological activity of modified titanium oxide nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2007, 295, 27–33. [Google Scholar]
- See, H.; Kawai, A.; Ikazaki, F. The effect of mixing particles of different size on the electrorheological response under steady shear flow. Rheol. Acta 2002, 41, 55–60. [Google Scholar]
- Lengalova, A.; Pavlinek, V.; Saha, P.; Quadrat, O.; Kitano, T.; Steiskal, J. Influence of particle concentration on the electrorheological efficiency of polyaniline suspensions. Eur. Polym. J. 2003, 39, 641–645. [Google Scholar]
- El Wahed, A.K. The influence of solid-phase concentration on the performance of electrorheological fluids in dynamic squeeze flow. Mater. Des. 2011, 32, 1420–1426. [Google Scholar]
- Yin, J.B.; Zhao, X.P. Preparation and electrorheological activity of mesoporous rare-earth-doped TiO2. Chem. Mater. 2002, 14, 4633–4640. [Google Scholar]
- Yin, J.B.; Shui, Y.J.; Chang, R.T.; Zhao, X.P. Graphene-supported carbonaceous dielectric sheets and their electrorheology. Carbon 2012, 50, 5247–5255. [Google Scholar]
- Cheng, Y.C.; Guo, J.J.; Liu, X.H.; Sun, A.H.; Xu, G.J.; Cui, P. Preparation of uniform titania microspheres with good electrorheological performance and their size effect. J. Mater. Chem. 2011, 21, 5051–5056. [Google Scholar]
- Tian, Y.; Meng, Y.G.; Wen, S.Z. Electrorheology of a zeolite/silicone oil suspension under DC fields. J. Appl. Phys. 2001, 90, 493–496. [Google Scholar]
- Clercx, H.J.H.; Bossis, G. Many-body electrostatic interactions in electrorheological fluids. Phys. Rev. E 1993, 48, 2721–2738. [Google Scholar]
- Martin, J.E.; Anderson, R.A.; Williamson, R.L. Generating strange interactions in particle suspensions. Compos. Sci. Technol. 2003, 63, 1097–1103. [Google Scholar]
- Anderson, R.A.; Martin, J.E. Energy balance problems in systems of induced and permanent electric and magnetic dipoles. Am. J. Phys. 2002, 70, 1194–1204. [Google Scholar]
- Martin, J.E.; Anderson, R.A. Chain model of electrorheology. J. Chem. Phys. 1996, 104, 4814–4827. [Google Scholar]
- Martin, J.E.; Odinek, J.; Halsey, T.C.; Kamien, R. Structure and dynamics of electrorheological fluids. Phys. Rev. E 1998, 57, 756–775. [Google Scholar]
- Espin, M.J.; Delgado, A.V.; Martin, J.E. Effects of electric fields and volume fraction on the rheology of hematite/silicone oil suspensions. Rheol. Acta 2004, 44, 71–79. [Google Scholar]
- Cho, M.S.; Lim, S.T.; Jang, I.B.; Choi, H.J.; Jhon, M.S. Encapsulation of spherical iron-particle with PMMA and its magnetorheological particles. IEEE Trans. Magn. 2004, 40, 3036–3038. [Google Scholar]
- Margida, A.J.; Weiss, K.D.; Carlson, J.D. Magnetorheological materials based on iron alloy particles. Int. J. Mod. Phys. B 1996, 10, 3335–3341. [Google Scholar]
- Kim, Y.J.; Liu, Y.D.; Seo, Y.; Choi, H.J. Pickering-emulsion-polymerized polystyrene/Fe2O3 composite particles and their magnetoresponsive characteristics. Langmuir 2013, 29, 4959–4965. [Google Scholar]
- Pu, H.T.; Jiang, F.J. Towards high sedimentation stability: Magnetorheological fluids based on CNT/Fe3O4 nanocomposites. Nanotechnology 2005, 16, 1486–1489. [Google Scholar]
- Lopez-Lopez, M.T.; Gomez-Ramirez, A.; Duran, J.D.G.; Gonzalez-Caballero, F. Preparation and characterization of iron-based magnetorheological fluids stabilized by addition of organoclay particles. Langmuir 2008, 24, 7076–7084. [Google Scholar]
- Yin, J.; Shui, Y.; Dong, Y.; Zhao, X. Enhanced dielectric polarization and electro-responsive characteristic of graphene oxide-wrapped titania microspheres. Nanotechnology 2014, 25. [Google Scholar] [CrossRef]
- Zhang, W.L.; Choi, H.J. Silica-graphene oxide hybrid composite particles and their electroresponsive characteristics. Langmuir 2012, 28, 7055–7062. [Google Scholar]
- Yin, J.B.; Wang, X.X.; Chang, R.T.; Zhao, X.P. Polyaniline decorated graphene sheet suspension with enhanced electrorheology. Soft Matter 2012, 8, 294–297. [Google Scholar]
- Dong, Y.Z.; Yin, J.B.; Zhao, X.P. Microwave-synthesized poly(ionic liquid) particles: A new material with high electrorheological activity. J. Mater. Chem. A 2014, 2, 9812–9819. [Google Scholar]
- Zhang, W.L.; Liu, Y.D.; Choi, H.J. Graphene oxide coated core-shell structured polystyrene microspheres and their electrorheological characteristics under applied electric field. J. Mater. Chem. 2011, 21, 6916–6921. [Google Scholar]
- Hong, J.Y.; Kwon, E.; Jang, J. Fabrication of silica/polythiophene core/shell nanospheres and their electrorheological fluid application. Soft Matter 2009, 5, 951–953. [Google Scholar]
- Zhang, W.L.; Piao, S.H.; Choi, H.J. Facile and fast synthesis of polyaniline-coated poly(glycidyl methacrylate) core-shell microspheres and their electro-responsive characteristics. J. Colloid Interface Sci. 2013, 402, 100–106. [Google Scholar]
- Tsuda, K.; Takeda, Y.; Ogura, H.; Otsubo, Y. Electrorheological behavior of whisker suspensions under oscillatory shear. Colloids Surf. A Physicochem. Eng. Asp. 2007, 299, 262–267. [Google Scholar]
- Yin, J.B.; Zhao, X.P.; Xia, X.; Xiang, L.Q.; Qiao, Y.P. Electrorheological fluids based on nano-fibrous polyaniline. Polymer 2008, 49, 4413–4419. [Google Scholar]
- Liu, Y.D.; Fang, F.F.; Choi, H.J. Silica nanoparticle decorated polyaniline nanofiber and its electrorheological response. Soft Matter 2011, 7, 2782–2789. [Google Scholar]
- Geist, M.F.; Boussois, K.; Smith, A.; Peyratout, C.S.; Kurth, D.G. Nanocomposites derived from montmorillonite and metallosupramolecular polyelectrolytes: Modular compounds for electrorheological fluids. Langmuir 2013, 29, 1743–1747. [Google Scholar]
- Schwarz, G.; Maisch, S.; Ullrich, S.; Wagenhofer, J.; Kurth, D.G. Electrorheological fluids based on metallo-supramolecular polyelectrolyte-silicate composites. ACS Appl. Mater. Interfaces 2013, 5, 4031–4034. [Google Scholar]
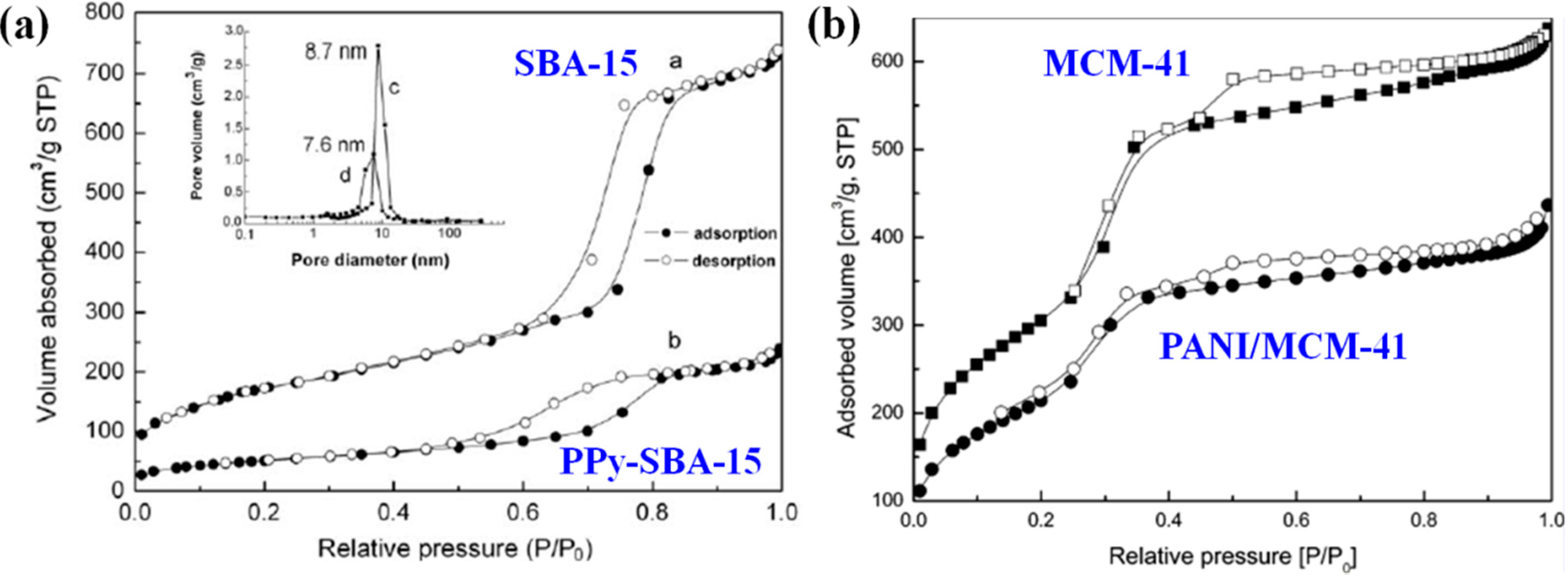
- Cheng, Q.L.; Pavlinek, V.; Lengalova, A.; Li, C.Z.; He, Y.; Saha, P. Conducting polypyrrole confined in ordered mesoporous silica SBA-15 channels: Preparation and its electrorheology. Microporous Mesoporous Mater. 2006, 93, 263–269. [Google Scholar]
- Cheng, Q.L.; Pavlinek, V.; He, Y.; Lengalova, A.; Li, C.Z.; Saha, P. Structural and electrorheological properties of mesoporous silica modified with triethanolamine. Colloids Surf. A Physicochem. Eng. Asp. 2008, 318, 169–174. [Google Scholar]
- Cheng, Q.L.; Pavlinek, V.; Lengalova, A.; Li, C.Z.; Belza, T.; Saha, P. Electrorheological properties of new mesoporous material with conducting polypyrrole in mesoporous silica. Microporous Mesoporous Mater. 2006, 94, 193–199. [Google Scholar]
- Cheng, Q.L.; He, Y.; Pavlinek, V.; Lengalova, A.; Li, C.Z.; Saha, P. Preparation and electrorheology of new mesoporous polypyrrole/MCM-41 suspensions. J. Mater. Sci. 2006, 41, 5047–5049. [Google Scholar]
- Cho, M.S.; Choi, H.J.; Ahn, W.S. Enhanced electrorheology of conducting polyaniline confined in MCM-41 channels. Langmuir 2004, 20, 202–207. [Google Scholar]
- Zhang, W.L.; Choi, H.J. Self-assembly of graphene oxide coated soft magnetic carbonyl iron particles and their magnetorheology. J. Appl. Phys. 2014, 115. [Google Scholar] [CrossRef]
- Fang, F.F.; Choi, H.J.; Seo, Y. Sequential coating of magnetic carbonyliron particles with polystyrene and multiwalled carbon nanotubes and its effect on their magnetorheology. ACS Appl. Mater. Interfaces 2010, 2, 54–60. [Google Scholar]
- Liu, Y.D.; Fang, F.F.; Choi, H.J. Core-shell-structured silica-coated magnetic carbonyl iron microbead and its magnetorheology with anti-acidic characteristics. Colloid Polym. Sci. 2011, 289, 1295–1298. [Google Scholar]
- Fang, F.F.; Liu, Y.D.; Choi, H.J. Fabrication of carbonyl iron embedded polycarbonate composite particles and magnetorheological characterization. IEEE Trans. Magn. 2009, 45, 2507–2510. [Google Scholar]
- Fang, F.F.; Liu, Y.D.; Choi, H.J.; Seo, Y. Core-shell structured carbonyl iron microspheres prepared via dual-step functionality coatings and their magnetorheological response. ACS Appl. Mater. Interfaces 2011, 3, 3487–3495. [Google Scholar]
- Fang, F.F.; Kim, J.H.; Choi, H.J. Synthesis of core-shell structured PS/Fe3O4 microbeads and their magnetorheology. Polymer 2009, 50, 2290–2293. [Google Scholar]
- Cao, Z.; Jiang, W.Q.; Ye, X.Z.; Gong, X.L. Preparation of superparamagnetic Fe3O4/PMMA nano composites and their magnetorheological characteristics. J. Magn. Magn. Mater. 2008, 320, 1499–1502. [Google Scholar]
- Lopez-Lopez, M.T.; Kuzhir, P.; Bossis, G. Magnetorheology of fiber suspensions. I. Experimental. J. Rheol. 2009, 53, 115–126. [Google Scholar]
- Kuzhir, P.; Lopez-Lopez, M.T.; Bossis, G. Magnetorheology of fiber suspensions. II. Theory. J. Rheol. 2009, 53, 127–151. [Google Scholar]
- Lee, S.; Yoon, C.M.; Hong, J.Y.; Jang, J. Enhanced electrorheological performance of a graphene oxide-wrapped silica rod with a high aspect ratio. J. Mater. Chem. C 2014, 2, 6010–6016. [Google Scholar]
- Hong, J.Y.; Jang, J. Highly stable, concentrated dispersions of graphene oxide sheets and their electro-responsive characteristics. Soft Matter 2012, 8, 7348–7350. [Google Scholar]
- Cho, M.S.; Choi, H.J.; Jhon, M.S. Shear stress analysis of a semiconducting polymer based electrorheological fluid system. Polymer 2005, 46, 11484–11488. [Google Scholar]
- Fang, F.F.; Liu, Y.D.; Lee, I.S.; Choi, H.J. Well controlled core/shell type polymeric microspherescoated with conducting polyaniline: fabrication and electrorheology. RSC Adv. 2011, 1, 1026–1032. [Google Scholar]
- Meheust, Y.; Parmar, K.P.S.; Schjelderupsen, B.; Fossum, J.O. The electrorheology of suspensions of Na-fluorohectorite clay in silicone oil. J. Rheol. 2011, 55, 809–833. [Google Scholar]
- Wang, B.; Rozynek, Z.; Fossum, J.O.; Knudsen, K.; Yu, Y. Guided self-assembly of nanostructured titanium oxide. Nanotechnology 2012, 23. [Google Scholar] [CrossRef]
- Liu, Y.D.; Quan, X.; Hwang, B.; Kwon, Y.K.; Choi, H.J. Core-shell-structured monodisperse copolymer/silica particle suspension and its electrorheological response. Langmuir 2014, 30, 1729–1734. [Google Scholar]
- Kim, J.W.; Kim, S.G.; Choi, H.J.; Suh, M.S.; Shin, M.J.; Jhon, M.S. Synthesis and electrorheological characterization of polyaniline and Na+-Montmorillonite clay nanocomposite. Int. J. Mod. Phys. B 2001, 15, 657–664. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.L.; Choi, H.J. Stimuli-Responsive Polymers and Colloids under Electric and Magnetic Fields. Polymers 2014, 6, 2803-2818. https://doi.org/10.3390/polym6112803
Zhang WL, Choi HJ. Stimuli-Responsive Polymers and Colloids under Electric and Magnetic Fields. Polymers. 2014; 6(11):2803-2818. https://doi.org/10.3390/polym6112803
Chicago/Turabian StyleZhang, Wen Ling, and Hyoung Jin Choi. 2014. "Stimuli-Responsive Polymers and Colloids under Electric and Magnetic Fields" Polymers 6, no. 11: 2803-2818. https://doi.org/10.3390/polym6112803