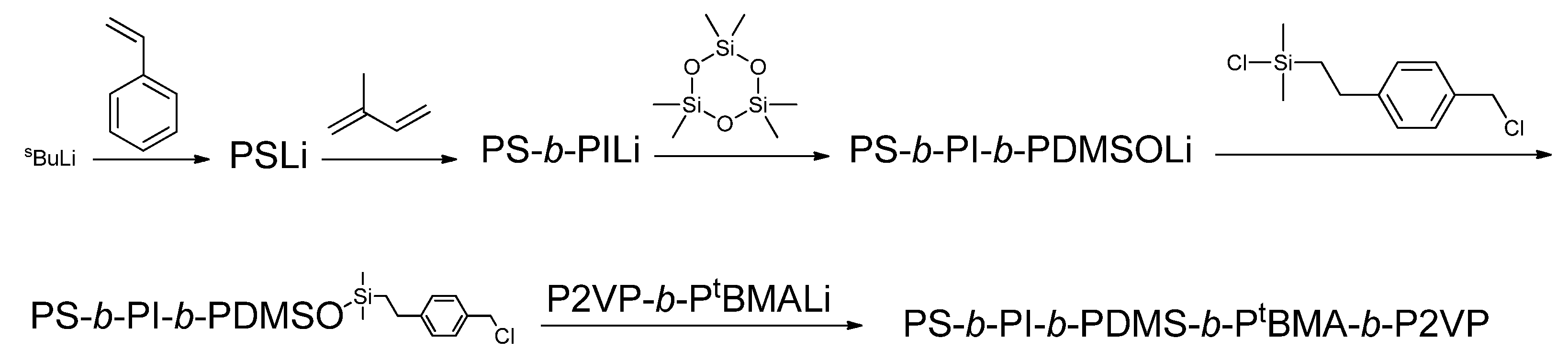
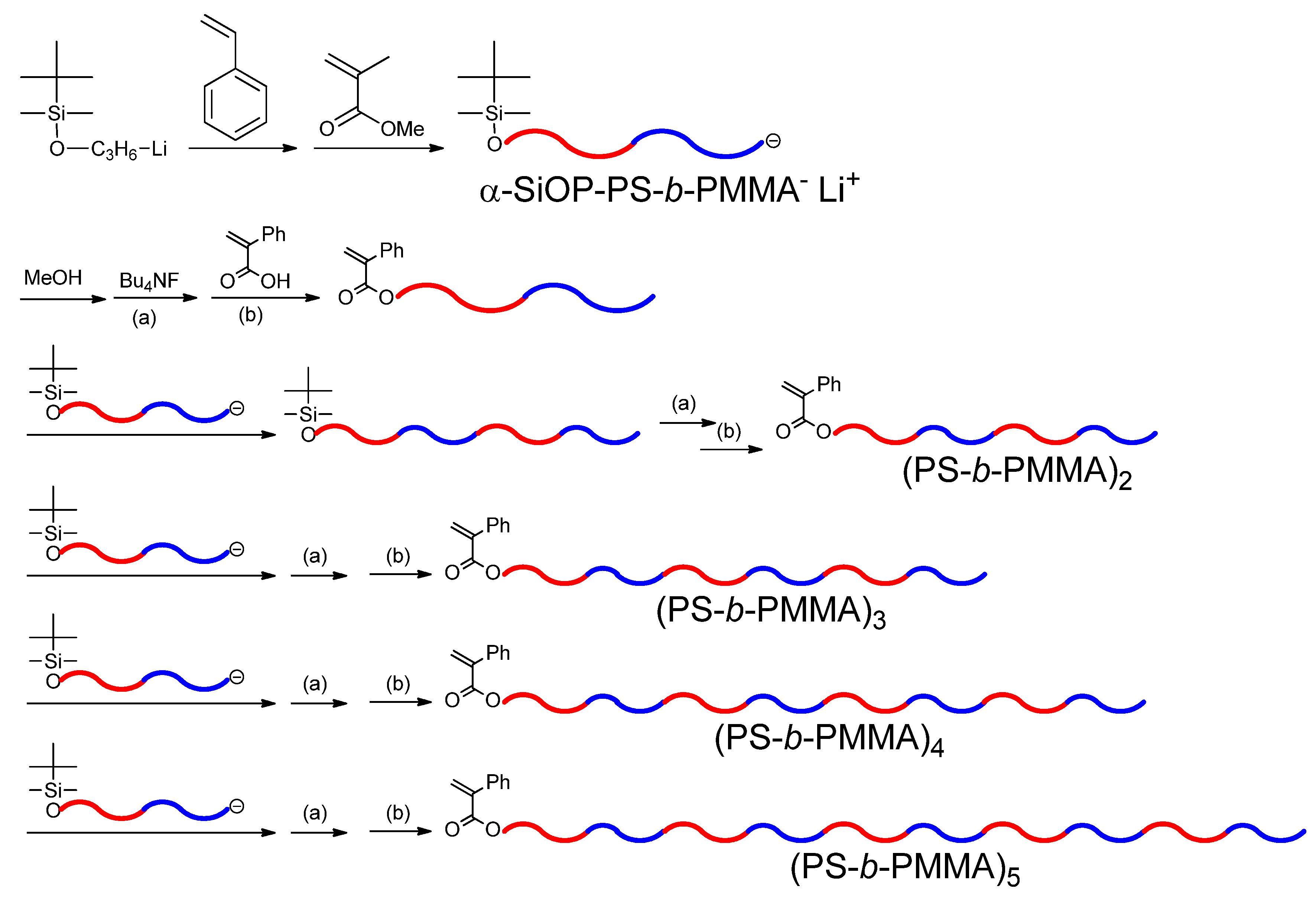
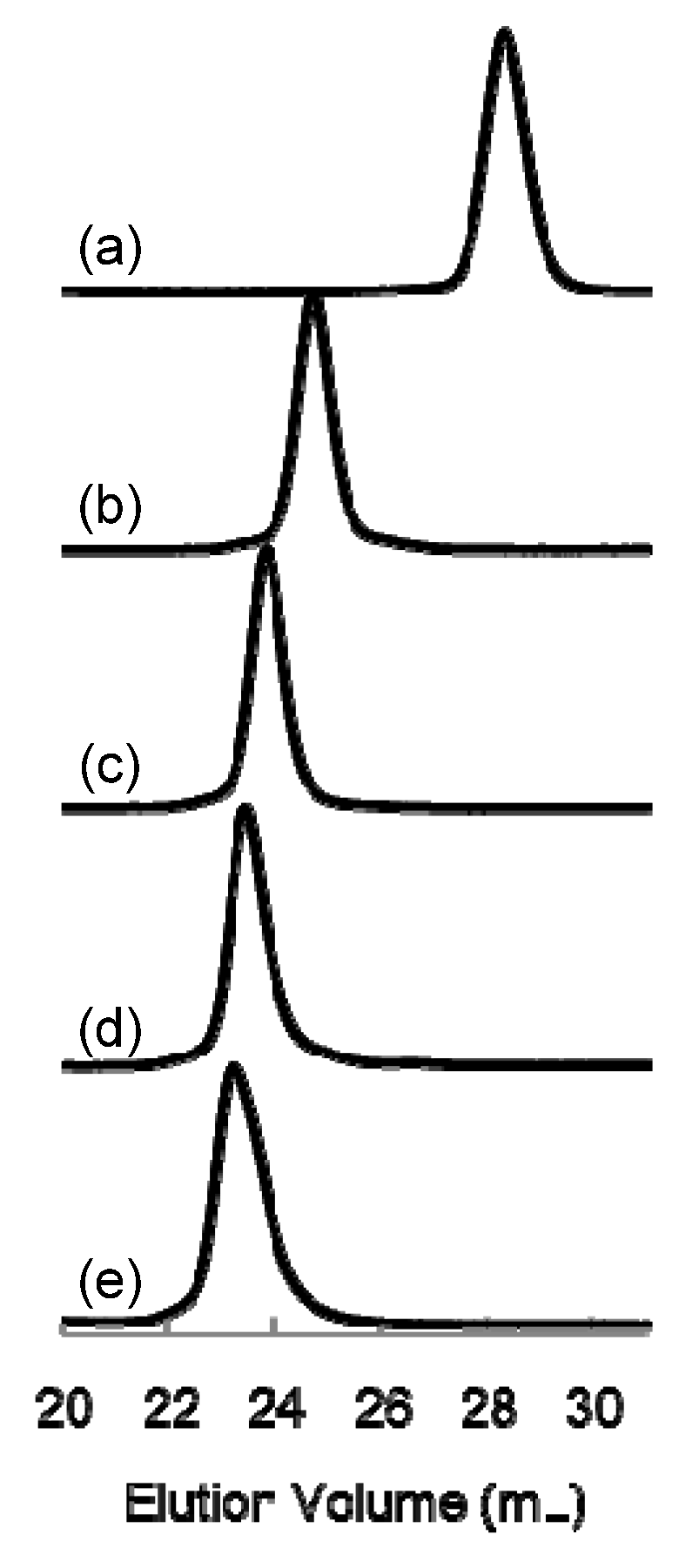
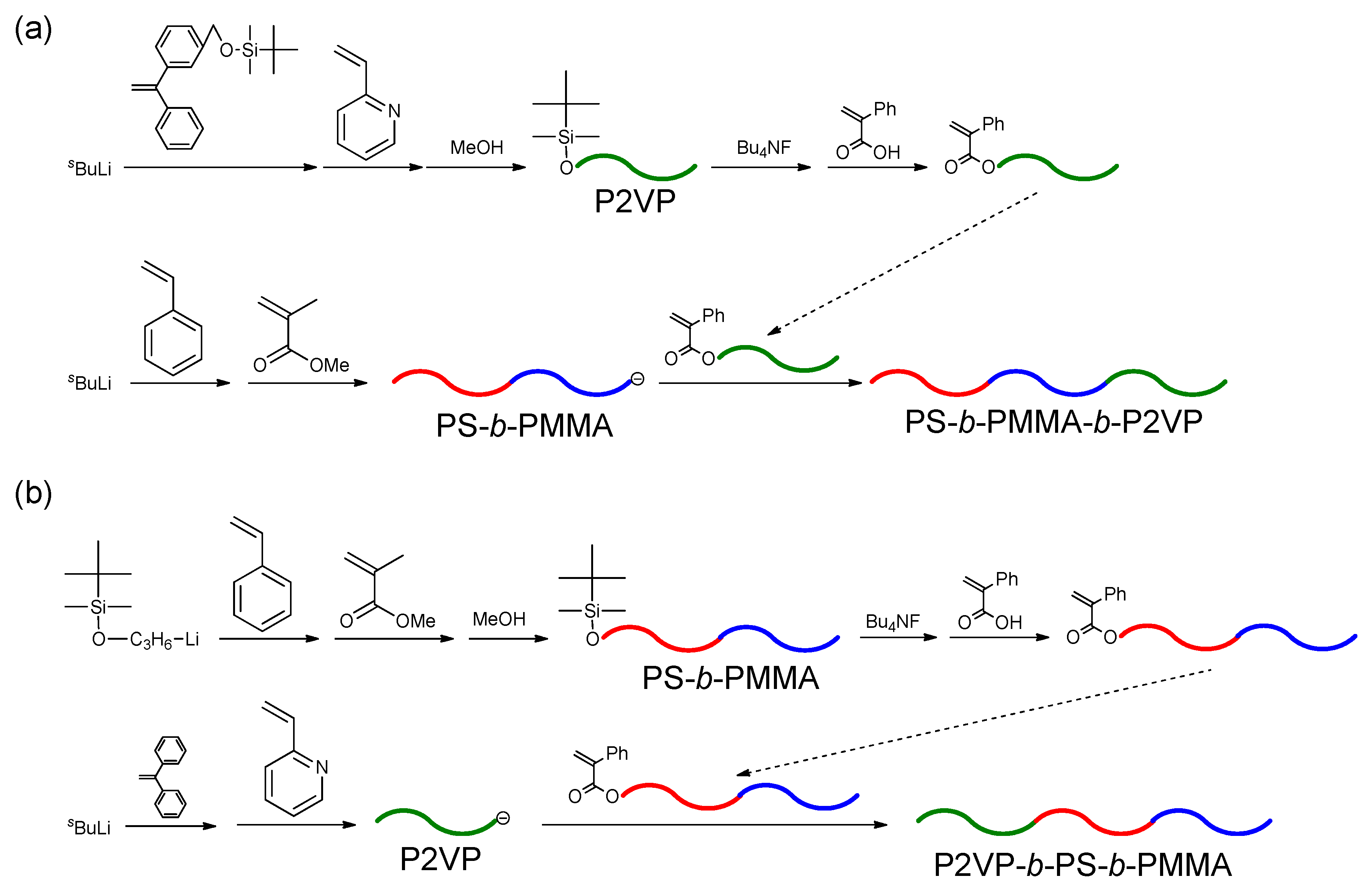
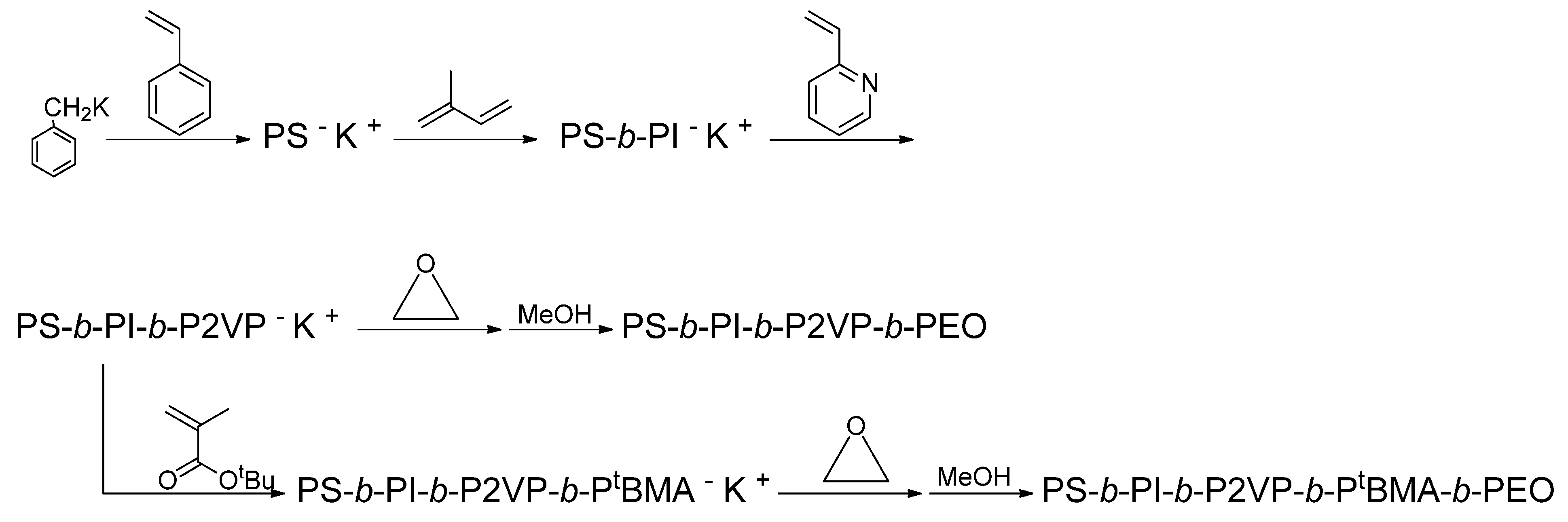1. Introduction
The recent development of living polymerization systems has allowed the successful synthesis of a variety of architectural polymers, such as block polymers, graft polymers, star-branched polymers, and hyperbranched polymers. Among them, block polymers have been the most widely investigated for a long time from the viewpoints of synthesis, properties, behavior, and morphology in solution, bulk, and melt states, as well as practical applications [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21]. Since block segments are generally thermodynamically incompatible, they are phase-separated at the molecular level, followed by self-organizing to assemble, to form three-dimensional periodic nanostructures and supramolecular assemblies. Such self-assemblies have attracted considerable attention due to the ability to arrange functional domains at the nanoscale, which are expected to play an important role in molecular devices with many potential applications in the fields of nanoscience and nanotechnology. Most block polymers utilized for this purpose are currently limited to AB-type diblock copolymers. Although both ABA and BAB triblock, and (AB)
n alternate multiblock copolymers have been also synthesized and their morphological parameters are similar to those of AB diblock copolymers, such block copolymers show unique and interesting morphologies, different from those produced from AB diblock copolymers. Recently, novel characteristic and complicated morphological behavior has been observed in three-component ABC triblock terpolymers because many variables originated from the three components such as Flory parameters between A–B, B–C, and C–A interfaces and the unit numbers of A, B, and C segments must be considered [
8,
22,
23,
24,
25,
26,
27].
As a matter of fact, well-defined block polymers with a high degree of molecular and compositional homogeneity are very important to elucidate their basic properties, behavior, and morphologies. In order to synthesize such well-defined block polymers, the use of a living polymerization system is essential. Although various living polymerization systems via different mechanisms have been developed, particularly in the last 25 years, the living anionic polymerization of certain monomers such as styrene, 1,3-butadiene, isoprene, 2-vinylpyridine, and alkyl methacrylates are so far the best living polymerization systems from the view point of the following features: First, molecular weights can be precisely controlled in a wide range, from 10
3 to even 10
6 g/mol. Second, extremely narrow molecular weight distributions are attained,
Mw/
Mn values being 1.05 or even smaller. Third, the tolerance of various functional groups, much more than the numbers that one can consider, can be realized, although some of them are required to be protected during polymerization [
28,
29,
30,
31,
32,
33]. Finally, the resulting living anionic polymers have active chain-end anions that are highly reactive, but stable under appropriate conditions. These characteristics are ideally suited for the synthesis of well-defined block polymers. In fact, most of well-defined AB diblock copolymers, two-component tri- and alternate multiblock copolymers, and even ABC triblock terpolymers, have been so far synthesized by using this living anionic polymerization system where the corresponding monomers are sequentially added to the anionic initiators, known as “sequential polymerization”.
A further consideration for molecular design and precise synthesis of block polymers is the order of monomer addition in sequential polymerization. There is no limitation to the addition order in polymerization using monomers with similar reactivities, and any monomer pair and addition order are acceptable to synthesize almost all possible block polymers. In contrast, the number of block polymers to be synthesized is significantly limited in sequential polymerization using monomers with different reactivities, as the nucleophilicity of the living polymer chain-end anion does not match the electrophilicity of the other monomer, in certain cases. In fact, difficulty often arises in block polymer synthesis by sequential polymerization using monomers with different reactivities. This is recognized as a long-standing problem to be overcome.
In this article, we report on the successful development of specially designed linking methodologies, in conjunction with the living anionic polymerization system for block polymer synthesis in order to overcome the long-standing problem and realize the facile synthesis of synthetically difficult block polymers. Herein, we focus on multiblock polymers composed of three or more block segments, but not diblock copolymers.
2. Synthesis of Block Polymers by Sequential Polymerization
As mentioned in the introduction, well-defined block polymers are generally synthesized by living anionic polymerization, where two or more monomers are sequentially added to an anionic initiator, known as “sequential polymerization”. With the use of monomers with similar reactivities, the resulting living polymer chain-end anions also exhibit similar reactivities to each other and thereby crossover polymerization is possible among such monomers. As a result, almost all block polymers can be synthesized. For example, the following three monomers, styrene, 1,3-butadiene, and isoprene, are similar in reactivity. If styrene and 1,3-butadiene are used, both AB and BA diblock copolymers of polystyrene (PS)-
b-poly(1,3-butadiene) (PB) and PB-
b-PS are readily synthesized by the sequential addition of styrene and 1,3-butadiene, or 1,3-butadiene first, followed by styrene. The synthesis of triblock copolymers of ABA and BAB types, PS-
b-PB-
b-PS and PB-
b-PS-
b-PB, as well as (AB)
n alternate multiblock copolymers of (PS-
b-PB)
n are possible by the same sequential polymerization, where the two monomers are, in turn added, in sequential order based on the structure of the block polymer. With the use of three monomers, styrene, 1,3-butadiene, and isoprene, the sequential addition of these monomers in this order to
sec-BuLi results in the formation of an ABC triblock terpolymer of PS-
b-PB-
b-polyisoprene (PI). Two other triblock terpolymers of ACB and BAC types, PS-
b-PI-
b-PB and PB-
b-PS-
b-PI, are synthesized by changing the addition order of the three monomers. Among the three structurally different methacrylate monomers with similar reactivities, the above-mentioned block polymer synthesis is also possible without problem. In practice, ABC, ACB, and BAC triblock terpolymers were successfully synthesized from the following three methacrylate monomers:
tert-butyl methacrylate, 2-trimethylsilyloxyethyl methacrylate, and 2-(4-perfluorobutyl)ethyl methacrylate [
34,
35]. Treatment of poly(2-trimethylsilyloxyethyl methacrylate) with 0.1 N HCl quantitatively yielded poly(2-hydroxyethyl methacrylate) having a hydroxyl group in each monomer unit. Thus, interestingly, hydrophilic, hydrophobic, and oleophobic blocks are incorporated and arranged in different sequential order in these triblock terpolymers.
As mentioned above, a significant limitation to block polymer synthesis is encountered by sequential polymerization using monomers with different reactivities [
1]. Let us consider block polymer synthesis using three monomers,
a,
b, and
c. The living anionic polymers derived from
a,
b, and
c are abbreviated as A
−, B
−, and C
−, respectively. The three monomers are different in reactivity and assumed to increase in reactivity from
a to
b to
c, that is,
a <
b <
c. In such a case, the living polymer chain-end anions should be reduced in anionic reactivity from A
− to B
− to C
−, that is, A
− > B
− > C
− as the living polymer chain-end anions are the conjugated bases of the corresponding monomers. When
a is polymerized with an appropriate anionic initiator, a living polymer, A
−, is produced. Since A
− is the most reactive living polymer among A
−, B
−, and C
−, the more reactive
b and
c are readily polymerized with A
− to give the two living diblock copolymers of AB
− and AC
−. The AB
− can further initiate the polymerization of the most reactive
c to result in a living triblock terpolymer of ABC
−, while the least reactive AC
− cannot polymerize the less reactive monomer
b having medium reactivity between
a and
c. Accordingly, an AC diblock copolymer is recovered in this case. Monomer
b is polymerized to produce a living polymer, B
−, and
a or
c is added to B
−. The B
−, having reactivity between A
− and C
−, cannot polymerize the least reactive monomer
a, but can polymerize the most reactive monomer
c to afford a living diblock copolymer of BC
−. When the least reactive monomer
a is added to the resulting BC
−, no more polymerization occurs. Finally,
c is polymerized to produce a living polymer, C
−. The C
− is the least reactive living polymer and is incapable of polymerizing either
a or
b, which are less reactive monomers than
c. Such reactivity relationships among monomers and their chain-end anions are summarized in
Table 1.
Table 1.
Reactivity relationships among monomers and their chain-end anions.
Table 1.
Reactivity relationships among monomers and their chain-end anions.
![Polymers 05 01012 i020]() |
Overall, three diblock copolymers of AB, AC, and BC, as well as one triblock terpolymer of ABC can be synthesized by sequential polymerization using three monomers with different reactivities. Unfortunately, diblock copolymers of BA, CA, and CB as well as two triblock terpolymers of ACB, and BAC cannot be synthesized due to the reactivity difference among living polymer chain-end anions and their monomers. However, BA, CA, and CB diblock copolymers are the same in block sequence as AB, AC, and BC diblock copolymers, although the sequential order is opposite in each case. Accordingly, there is no difference or limitation to diblock copolymer synthesis among sequential polymerizations using monomers with similar and different reactivities. On the other hand, a significant limitation was encountered in triblock terpolymer synthesis by sequential polymerization using monomers with different reactivities. Only synthesis of the ABC type among the three triblock terpolymers is possible; both ACB and BAC cannot be obtained by the same polymerization process, even with different addition orders.
What of the synthetic situation of triblock copolymers and alternate multiblock copolymers? With the use of three monomers, the synthesis of six triblock copolymers (ABA, BAB, ACA, CAC, BCB, and CBC) can be considered. None of the triblock copolymers shown above can be synthesized at all by sequential polymerization using three monomers with different reactivities, as is estimated from the reactivities of the monomers and chain-end anions shown in
Table 1. The living polymer B
− cannot polymerize the least reactive monomer
a and the least reactive C
− is not capable of polymerizing
a and
b. For the same reason, the synthesis of multiblock copolymers of (AB)
n, (AC)
n, and (BC)
n types is not possible. Moreover, other multiblock polymers, such as ABCA, ABCB, BACA, (ABC)
n, and so on, also cannot be synthesized.
Table 2 summarizes the synthetic possibilities of block polymers by sequential polymerization using monomers with similar and different reactivities. As can be seen, there is a serious problem in the synthesis of triblock polymers, multiblock polymers with more than four blocks, and alternate multiblock polymers. Almost all block polymers, except for the ABC triblock terpolymer, cannot be synthesized by sequential polymerization using monomers with different reactivities, while they can all be obtained by sequential polymerization using monomers with similar reactivities.
Table 2.
Synthetic possibilities of block polymers by sequential polymerization using monomers with similar and different reactivities.
Table 2.
Synthetic possibilities of block polymers by sequential polymerization using monomers with similar and different reactivities.
| Type of block polymers | Monomers |
|---|
| Similar reactivities | Different reactivities |
|---|
| a = b = c | a < b < c |
|---|
| diblock copolymer | AB, AC, BC | ○ | ○ |
| triblock copolymer | ABA, ACA, BCB | ○ | × |
| BAB, CAC, CBC |
| multiblock copolymer | (AB)n, (AC)n, (BC)n | ○ | × |
| triblock terpolymer | ABC, ACB, BAC | ○ | only ABC is possible |
| multiblock terpolymer | ABCA, ABCB, CABC | ○ | × |
| (ABC)n |
Nevertheless, several interesting triblock terpolymers and multiblock polymers with more than four blocks were synthesized by sequential polymerization using monomers with different reactivities. Stadler and Giebeler reported the first successful synthesis of an ABC triblock terpolymer, PS-
b-P2VP-
b-P
tBMA [
36]. The synthesis was achieved by the sequential addition of styrene, 2VP, and
tBMA to
sec-BuLi. 1,1-Diphenylethylene (DPE) was added to end-cap the chain-end anion of living P2VP prior to the polymerization of
tBMA with a living diblock copolymer of PS-
b-P2VP in order to prevent the undesirable addition of a chain-end anion to the carbonyl group of
tBMA.
Soon after, Müller and his coworkers synthesized a series of the same well-defined triblock terpolymers with different compositions in a similar manner (
Mn = 72–140 kg/mol and
Mw/
Mn = 1.02–1.04) [
37]. By using these polymers, they established a ternary phase diagram together with anticipated phase-separated structures. The results are very interesting. For example, by decreasing the P
tBMA volume fraction, the morphologies change from core-shell cylinders (a PS core surrounded by a P2VP shell in a P
tBMA matrix) via a core-shell gyroid in coexistence with a metastable perforated lamellar phase to a lamellar structure. In this sequence, the curvature of the PS/P2VP interface decreases systematically from a cylinder core to a gyroid core to lamellar phase. Further reduction of the P
tBMA block leads to yet another gyroid structure.
Hydrolysis of the P
tBMA block with HCl yielded a new ABC triblock terpolymer of PS-
b-P2VP-
b-poly(methacrylic acid), which exhibited pH-dependent solution properties. An intermolecular complexation of the P2VP and poly(methacrylic acid) blocks in micellar solution was observed [
36].
A variety of functional triblock terpolymers was also synthesized by the same research group. In such triblock terpolymers, they employed three different structural monomers, two of which are similar in reactivity, but different in reactivity from the third monomer. A triblock terpolymer of PS-
b-PB-
b-P
tBMA was an example of this type, which was synthesized by the sequential addition of styrene, 1,3-butadiene, and
tBMA to
sec-BuLi [
38]. Since styrene is similar in reactivity to 1,3-butadiene, another sequentially different triblock terpolymer of PB-
b-PS-
b-P
tBMA may be synthesized by changing the addition order of styrene and 1,3-butadiene. Furthermore, the following triblock terpolymers, belonging to the same category, were also synthesized by means of the same sequential polymerization technique with a careful monomer addition order: Poly(4-
tert-butoxystyrene)-
b-PB-
b-P
tBMA [
39], Poly[(5-
N,
N-dimethylamino) isoprene]-
b-PS-
b-P
tBMA [
40], PB-
b-P
tBMA-
b-Poly(2-dimethylaminoethyl methacrylate) [
41], and PS-
b-Poly(2-trimethhylsilyloxyethyl methacrylate)-
b-PMMA [
42].
The synthesis of multiblock polymers having more than four blocks by sequential polymerization was reported by Hadjichristidis and his coworkers, as illustrated in
Scheme 1. The first synthetic example was an ABCD tetrablock quaterpolymer of PS-
b-PI-
b-P2VP-
b-poly(ethylene oxide) (PEO), which was synthesized by the sequential addition of styrene, isoprene, 2VP, and EO to benzyl potassium as the initiator [
43]. The polymerizations of the first three monomers were carried out in THF at −78 °C for 1, 1, and 0.5 h, respectively, while the final polymerization of EO was performed in THF at 50 °C for five days. Moreover, a particular ABCDE pentablock quintopolymer, PS-
b-PI-
b-P2VP-
b-P
tBMA-
b-PEO, could also be synthesized in a similar manner by adding in turn the corresponding five monomers to benzyl potassium in THF. The polymerization conditions were −78 °C/1 h, −78 °C/1 h, −78 °C /0.5 h, −78 °C /0.5 h, and 50 °C/5 days. The resulting two polymers exhibited sharp monomodal SEC peaks, without any shoulder or tailing, and possessed predictable
Mn values of 223 and 92 kg/mol and narrow molecular weight distributions of
Mw/
Mn values of 1.08 and 1.04, respectively. Their compositions observed by
1H-NMR were consistent with those calculated from feed ratios. Thus, the resulting tetrablock quaterpolymer and pentablock quintopolymer were well-defined in structure and precisely controlled in chain length. Since the reactivity of each monomer increases in this order, styrene = isoprene < 2VP <
tBMA < EO, the choice of monomer addition order is reasonable. As the replacement of styrene by isoprene is acceptable, both PI-
b-PS-
b-P2VP-
b-PEO and PI-
b-PS-
b-P2VP-
b-P
tBMA-
b-PEO can be synthesized.
Scheme 1.
Synthesis of PS-b-PI-b-P2VP-b-PEO tetrablock quaterpolymer and PS-b-PI-b-P2VP-b-PtBMA-b-PEO pentablock quintopolymer.
Scheme 1.
Synthesis of PS-b-PI-b-P2VP-b-PEO tetrablock quaterpolymer and PS-b-PI-b-P2VP-b-PtBMA-b-PEO pentablock quintopolymer.
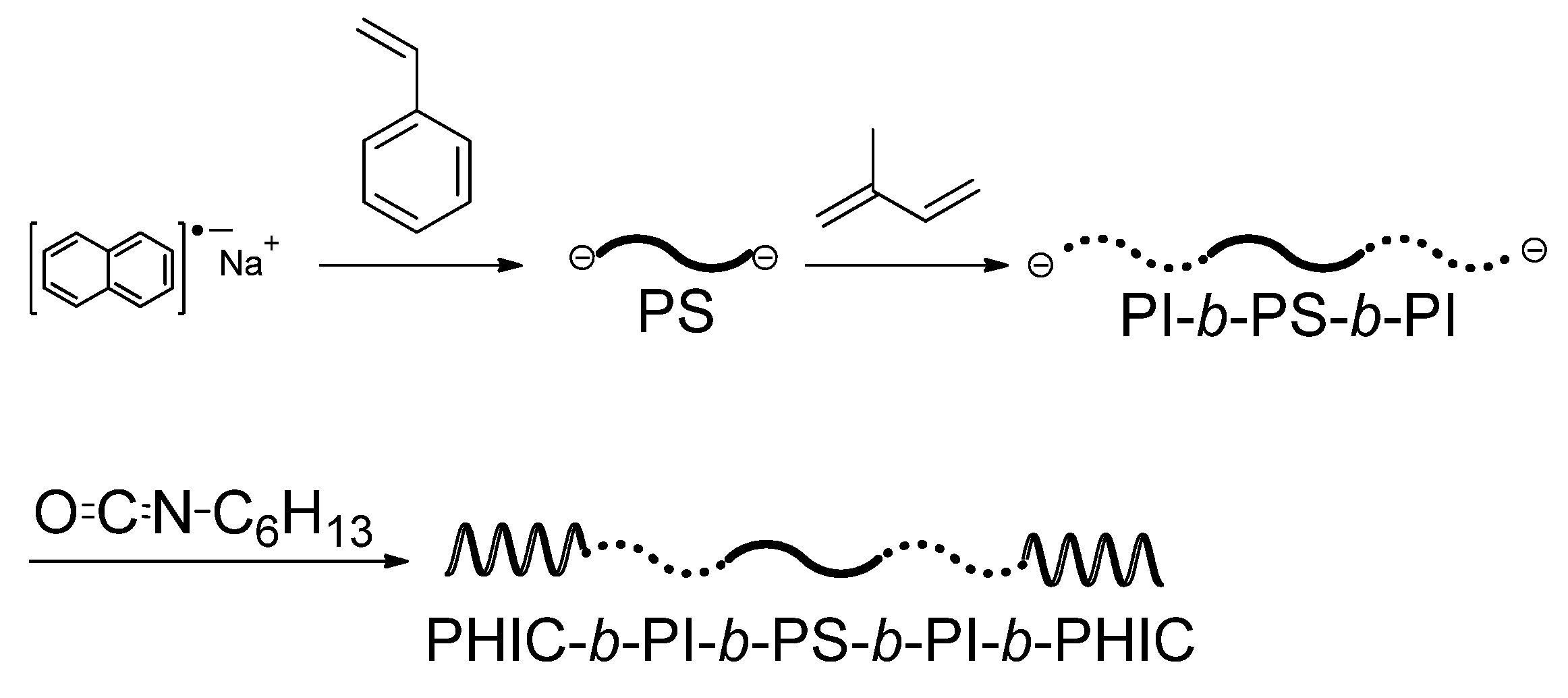
Lee and his coworkers were the first, successful, in achieving the living anionic polymerization of alkyl isocyanate monomers under somewhat special conditions at −98 °C with the use of organosodium initiators [
41,
42,
43,
44,
45,
46]. By using this living polymerization system, Hadjichristidis and his coworkers synthesized a symmetric CBABC pentablock terpolymer, poly(hexyl isocyanate) (PHIC)-
b-PI-
b-PS-
b-PI-
b-(PHIC) [
47]. Since the PHIC block is of special interest in that it adopts an extended rodlike helical conformation either in solution or in bulk, and possesses intriguing properties including optical activity and liquid crystalinity, the pentablock terpolymer containing PHIC segments is expected as a new and interesting functional material. The monomer reactivity increases from styrene and isoprene to HIC and, therefore, the order of monomer addition should be styrene, isoprene, followed by HIC in the sequential polymerization. In practice, the objective pentablock terpolymer could be synthesized by the sequential addition of styrene, isoprene, and HIC with sodium naphthalenide used as a difunctional initiator (see
Scheme 2). The addition of NaBPh
4 to the polymerization system is essential to prevent the undesirable trimerization by a back-biting from the living polymer chain-end anion. The analytical results of two pentablock terpolymers thus synthesized revealed a high degree of molecular and compositional homogeneity, although their molecular weight distributions were relatively broad (
Mn = 79.4–128 kg/mol,
Mw/
Mn = 1.25–1.32).
Scheme 2.
Synthesis of PHIC-b-PI-b-PS-b-PI-b-PHIC pentablock terpolymer.
Scheme 2.
Synthesis of PHIC-b-PI-b-PS-b-PI-b-PHIC pentablock terpolymer.
Although certain triblock terpolymers and multiblock polymers, having up to five blocks, could be successfully synthesized by this procedure, their syntheses are still significantly limited by sequential polymerization using monomers with different reactivities. With these limitations and thereby synthetic difficulties in mind, alternative approaches to overcome such problems will be introduced in the next two sections.
3. Synthesis of Block Polymers by a Linking Methodology Using a Heterofunctional Linking Agent, 2-(4-Chloromethylphenyl)ethyldimethylchlorosilane
Because of excellent thermal and oxidative stability, high chain flexibility, low glass transition temperature, low surface energy, and low solubility parameter, poly(dimethylsiloxane) (PDMS) is a very important and attractive material in a variety of industrial applications. Furthermore, PDMS is often used in lithographic applications due to its good oxygen reactive ion etching resistance and low absorption in deep UV regions. Consequently, well-defined block polymers containing PDMS segments are of special interest in offering high-performance specialty materials.
The living anionic polymerization of hexamethylcyclosiloxane (D
3) is undoubtedly the best procedure to obtain well-defined (PDMS)s, but it is not an easy matter to control the polymerization of D
3. The living anionic polymerization of D
3 has recently been much advanced by Hadjichristidis and his coworkers, with special care to well control the polymerization. Highly reactive living anionic polymers of styrene and isoprene initiate the polymerization of D
3 to afford well-defined AB diblock copolymers of PS-
b-PDMS and PI-
b-PDMS [
48]. It was also reported that an BAB triblock copolymer of PDMS-
b-poly(4-vinylpyridine) (P4VP)-
b-PDMS could be synthesized by sequential polymerization of 4VP and D
3 with difunctional sodium naphthalenide as the initiator [
49]. Unfortunately, no detailed characterization of molecular weight distribution was available in this report, but it was almost certain that living P4VP and possibly P2VP could polymerize D
3. On the other hand, less reactive living polymers of methyl methacrylate (MMA),
tBMA, and other methacrylate monomers cannot initiate the polymerization of D
3 because of their chain-end enolate anions with low nucleophilicities. Accordingly, a block copolymer, for example, P
tBMA-
b-PDMS cannot be synthesized by sequential polymerization of
tBMA, followed by the addition of D
3. More problematic is that a silanolate anion derived from D
3 is too low in nucleophilicity to initiate the polymerization of styrene, isoprene, 1,3-butadiene, 2VP, and even alkyl methacrylates. This strongly indicates that the use of D
3 in block polymer synthesis is especially limited to the preparation of a first-formed block in the sequence.
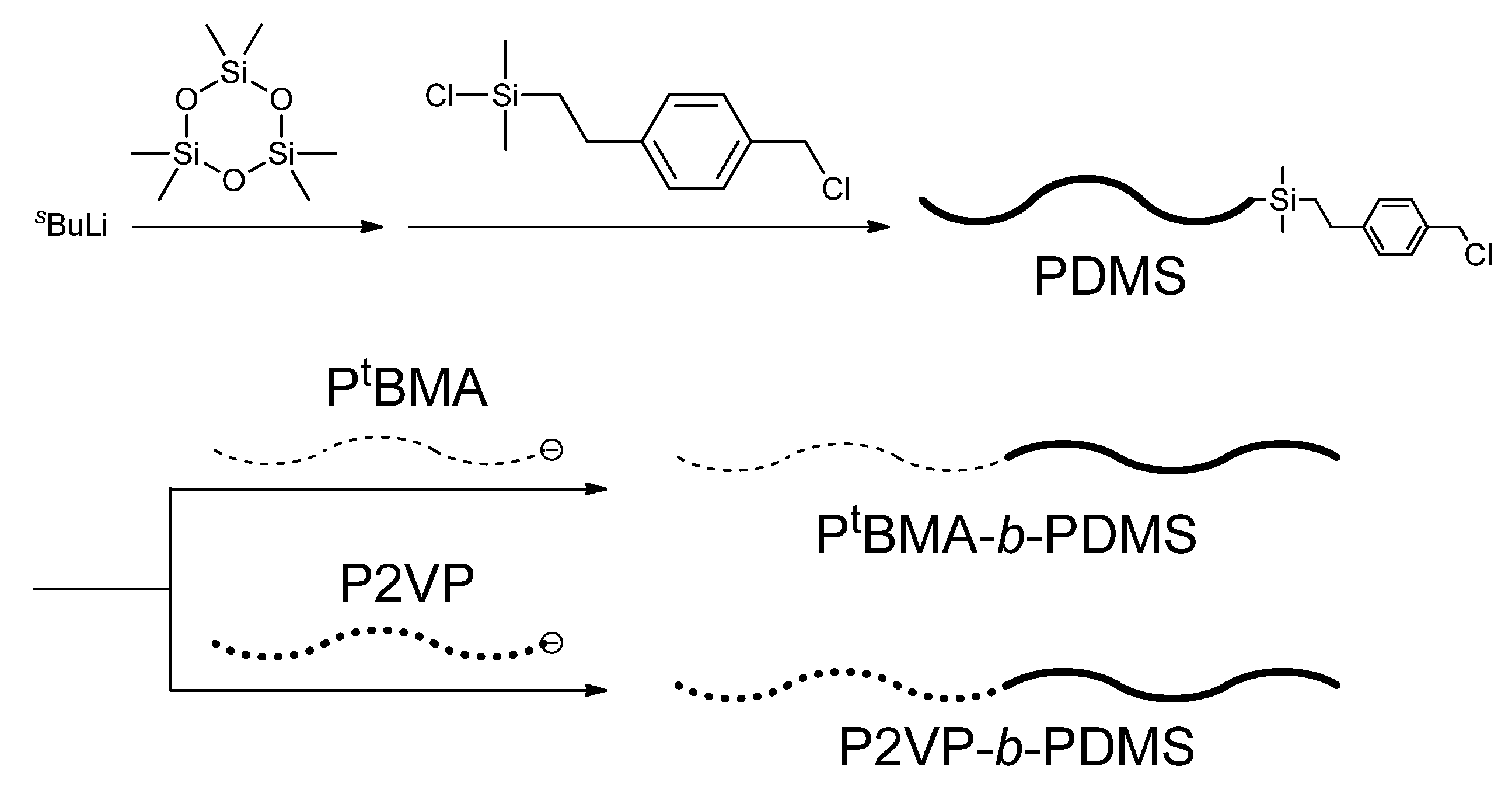
Hadjichristidis and his coworkers have developed an effective methodology for the synthesis of block polymers containing PDMS segments. As mentioned above, an AB diblock copolymer of PDMS-
b-P
tBMA could not be synthesized, as the living polymer of DMS was not nucleophilic enough to initiate the polymerization of
tBMA. In order to link the two polymer chains, they newly synthesized 2-(4-chloromethylphenyl)ethyldimethylchlorosilane (
1) as a heterofunctional linking agent on the basis of the greater reactivity of the silyl chloride than the benzyl chloride (BnCl) function toward the silanolate anion [
50]. The reaction sequence for the synthesis of the above diblock copolymer is given in
Scheme 3. The living PDMS was first prepared by the anionic polymerization of D
3 with
sec-BuLi and then reacted with a 1.1-fold excess of
1. The reaction of the silanolate anion with the silyl chloride function of
1 was observed by
1H-NMR analysis to be quite selective, while the BnCl function remained completely intact, resulting in an ω-chain-end-BnCl-functionalized PDMS. Living P
tBMA was prepared by the anionic polymerization of
tBMA with 1,1-diphenylhexyllithium in THF in the presence of LiCl at −78 °C and then reacted with the ω-chain-end-BnCl-functionalized PDMS. A two-fold excess of living P
tBMA toward the BnCl function was used and the reaction was carried out at −25 °C for three days. Unfortunately, only 30% conversion was obtained under such conditions. Quantitative conversion could be achieved by the addition of a catalytic amount of CsI to transform the chloride into a more reactive iodide. A well-defined PDMS-
b-P
tBMA (
Mn = 55.3 kg/mol,
Mw/
Mn = 1.03, PDMS (
w/
w, %) = 50%) was obtained in 100% yield. Thus, the linking of PDMS with P
tBMA, difficult to be linked by sequential polymerization, was successful.
Scheme 3.
Synthesis of PtBMA-b-PDMS and P2VP-b-PDMS diblock copolymers.
Scheme 3.
Synthesis of PtBMA-b-PDMS and P2VP-b-PDMS diblock copolymers.
Similarly, it was observed that the linking reaction of living P2VP quantitatively occurred with ω-chain-end-BnCl-functionalized PDMS in the presence of a catalytic amount of CsI even at −78 °C to afford a well-defined PDMS-
b-P2VP (
Mn = 20.2 kg/mol,
Mw/
Mn = 1.07, PDMS (
w/
w, %) = 20%) (see also
Scheme 3). Thus, the agent
1, was also effective to link P2VP with PDMS.
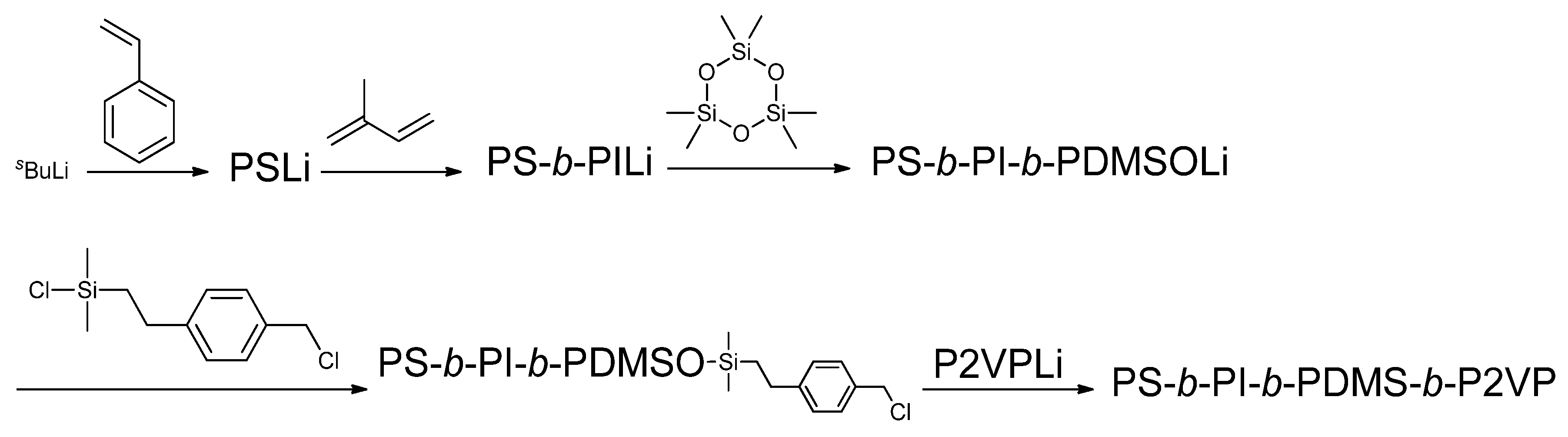
This methodology using
1 was extended to synthesize particular multiblock polymers, which cannot be synthesized by sequential polymerization. The first successful example is a tetrablock quaterpolymer of PS-
b-PI-
b-PDMS-
b-P2VP, as illustrated in
Scheme 4 [
51]. A living triblock terpolymer, PS-
b-PI-
b-PDMS, was first prepared by the sequential polymerization of styrene, isoprene, and then D
3 with sec-BuLi and subsequently reacted with
1 to give an ω-chain-end-BnCl-functionalized PS-
b-PI-
b-PDMS. Living P2VP was then reacted with the ω-chain-end-BnCl-functionalized PS-
b-PI-
b-PDMS in the presence of a catalytic amount of CsI at −78 °C for three days in order to complete the linking reaction. A well-defined and requisite structure of the resulting tetrablock quaterpolymer of PS-
b-PI-
b-PDMS-
b-P2VP, after isolation by fractional precipitation, was confirmed by SEC and
1H-NMR analyses. Typically, the tetrablock quaterpolymer possessed a predictable
Mn value of 98.8 kg/mol and a narrow molecular weight distribution, the
Mw/
Mn value being 1.03. The composition (
w/
w, %) of PS/PI/PDMS/P2VP was 30.5/25.5/25.3/21.1. Very interestingly, four phases of the microphase-separated structure in this tetrablock quaterpolymer were unequivocally observed. The PI, PDMS, and P2VP microphases formed triple coaxial cylinders with a hexagonal shape packed in a hexagonal arrary in the PS microphase, which formed the honeycomb-shaped matrix.
Scheme 4.
Synthesis of PS-b-PI-b-PDMS-b-P2VP tetrablock quaterpolymer.
Scheme 4.
Synthesis of PS-b-PI-b-PDMS-b-P2VP tetrablock quaterpolymer.
A quite novel pentablock quintopolymer, PS-
b-PI-
b-PDMS-
b-P
tBMA-
b-P2VP, was also successfully synthesized by developing the same linking methodology shown in
Scheme 5 [
52]. The first step involved the preparation of an ω-chain-end-BnCl-functionalized triblock terpolymer of PS-
b-PI-
b-PDMS by the sequential polymerization of styrene, isoprene, and D
3 with
sec-BuLi, followed by reacting it with
1, in exactly the same manners as those described above. A living diblock copolymer was separately prepared by the polymerization where 2VP and
tBMA was sequentially polymerized with benzylpotassium and reacted with the ω-chain-end-BnCl-functionalized PS-
b-PI-
b-PDMS at −25 °C for three days. In this linking reaction, a two-fold excess of living diblock copolymer of P2VP-
b-P
tBMA was used for the BnCl function of the triblock terpolymer and a catalytic amount of (C
4H
9)
4NI was added to replace the chloride with the more reactive iodide to complete the reaction. Under such conditions, the linking reaction was observed to quantitatively take place to link the two block polymer chains. Thus, the target pentablock quintopolymer, PS-
b-PI-
b-PDMS-
b-P
tBMA-
b-P2VP, was successfully synthesized. A series of pentablock quintopolymers with different compositions could also be synthesized by changing the feed ratios. A high degree of molecular and compositional homogeneity was clearly exhibited in each of all block polymers, as listed in
Table 3.
Scheme 5.
Synthesis of PS-b-PI-b-PDMS-b-PtBMA-b-P2VP pentablock quintopolymer.
Scheme 5.
Synthesis of PS-b-PI-b-PDMS-b-PtBMA-b-P2VP pentablock quintopolymer.
The synthesis by this linking methodology is based on both the advanced living anionic polymerization of D3 and the selective linking reaction of the silanolate anion with the silyl chloride function of 1. With this methodology, a series of well-defined block polymers containing PDMS segments from diblock to even quite novel pentablock types have been successfully synthesized. Thus, the methodology using 1 herein developed becomes a very effective and versatile procedure for the synthesis of block polymers containing PDMS segments.
Table 3.
Molecular characteristics of poly(dimethylsiloxane) (PDMS) containing block polymers.
Table 3.
Molecular characteristics of poly(dimethylsiloxane) (PDMS) containing block polymers.
| Sample | Mn × 10−3 (g/mol) | Mw/Mn | Composition e (% w/w) |
|---|
| PDMS-b-PtBMA | 55.3 a | 1.02 c | 51/49 |
| PDMS-b-P2VP | 20.2 a | 1.07 c | 20/80 |
| PS-b-PI-b-PDMS-b-P2VP | 155.4 a | 1.04 d | 52/18/12/18 |
| PS-b-PI-b-PDMS-b-PtBMA-b-P2VP | 161.4 b | 1.05 b | 20/9/17/25/29 |
5. Future Outlook
Throughout this article, two linking methodologies in conjunction with living anionic polymerization system are introduced for the synthesis of various well-defined triblock polymers, multiblock polymers composed of more than four blocks, and alternate multiblock copolymers, which cannot be synthesized by sequential polymerization. The first linking methodology with a new heterofunctional linking agent, 1, was developed by Hadjichristidis and his coworkers. Since living PDMS is too low in nucleophilicity to initiate the polymerization of most vinyl monomers, which undergo living anionic polymerization, block polymer synthesis by sequential polymerization is very difficult or probably almost impossible. The first developed methodology based on the selective reaction of 1 with living PDMS and a further linking reaction with living anionic polymers or block polymers is very useful for the synthesis of multiblock polymers containing PDMS blocks. With this methodology, various well-defined PDMS-containing multiblock polymers even up to the pentablock quintopolymer, PS-b-PI-b-PDMS-b-PtBMA-b-P2VP, were successfully synthesized.
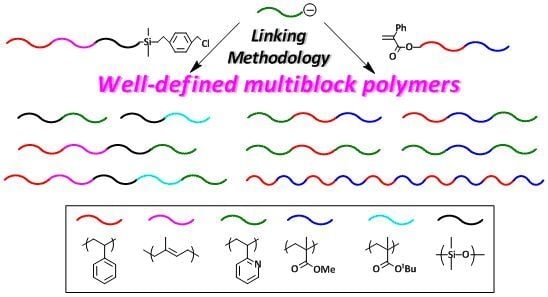
The second linking methodology using the PA reaction site was developed by Hirao and his coworkers in order to synthesize a variety of multiblock polymers composed of vinyl polymer blocks. As often mentioned, an ABC triblock terpolymer is the only synthetically possible triblock terpolymer by sequential polymerization using three monomers with different reactivities, (a) typically styrene, (b) 2VP, and (c) MMA. The success of the second methodology makes it possible to synthesize almost all block polymers. In fact, not only ACB (BCA) and BAC (CAB) triblock terpolymers, but also ABA, ACA, BAB, BCB, CAC, and CBC triblock copolymers as well as (AB)n and (AC)n (and possibly (BC)n) alternate multiblock copolymers were successfully synthesized. The synthesis of special triblock copolymers of BAB, CAC, and CBC having molecular asymmetry in both side blocks was also possible. Furthermore, a tetrablock terpolymer of ABCA was synthesized in a similar linking manner. Thus, multiblock polymers in any sequential order and a number of blocks as desired may possibly be synthesized by the linking reaction using the PA reaction site when using a, b, and c with different reactivities.
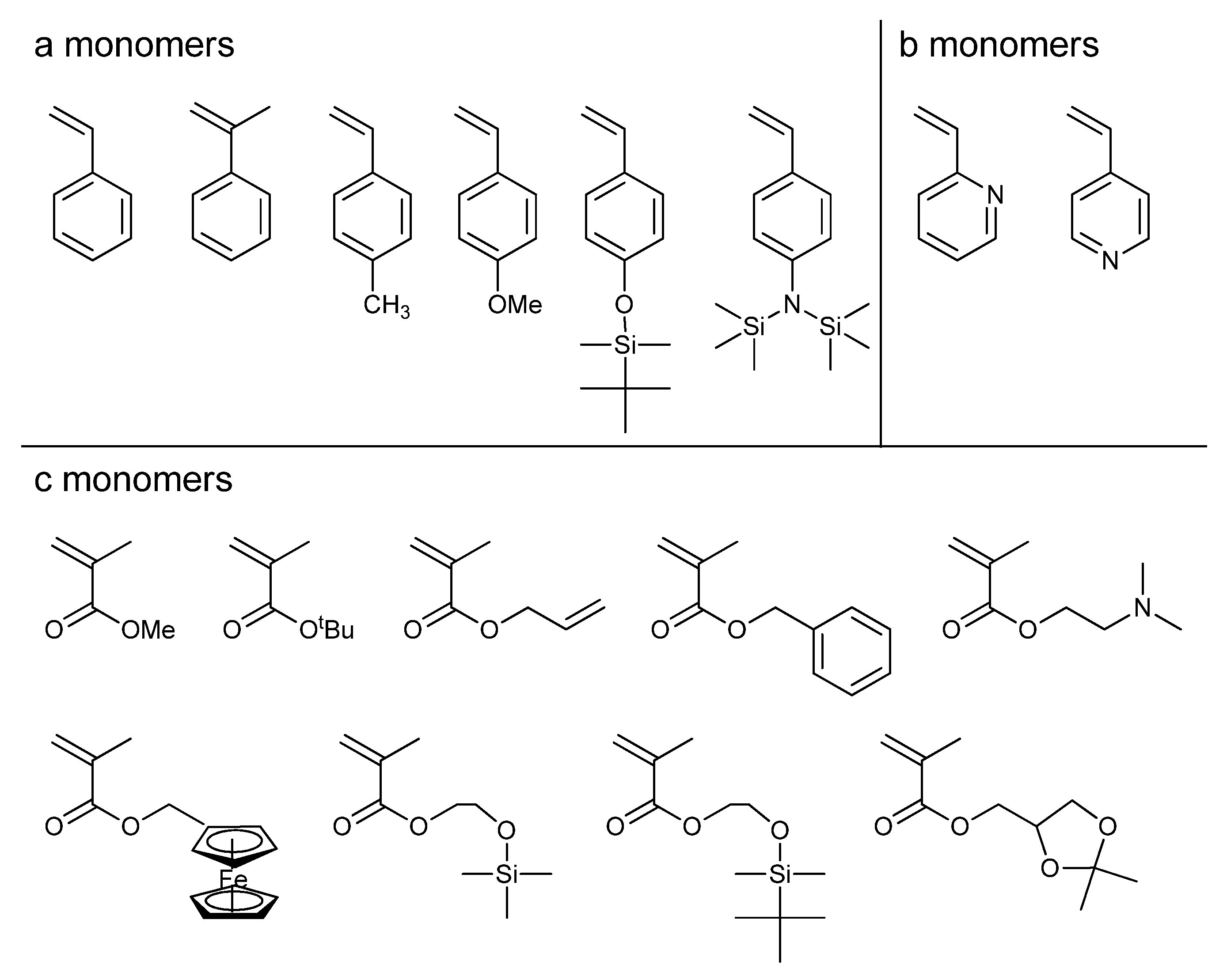
The numbers of synthetically possible block polymer further and greatly increased by using many other monomers listed in
Chart 1, in addition to styrene, 2VP, and MMA. In fact, various quite new triblock terpolymers represented as ABA′, ACA′, CAC′, and so on, were synthesized for the first time by the linking reaction using three structurally different monomers with two different reactivities. The PS-
b-P2VP-
b-PαMS and PMMA-
b-PS-
b-P
tBMA triblock terpolymers, for instance, are such typical examples. Moreover, a PαMS-
b-P4VP-
b-poly(4-methoxystyrene) triblock terpolymer of the A
1′B′A
2′ type could be synthesized. Further importantly, a wide variety of functional groups, such as hydroxyl, amino, formyl, and carboxyl functions, were introduced into the block polymers with the use of functional monomers, also listed in
Chart 1.
Thus, it should be mentioned that even now, a huge number of multiblock polymers with any sequential order and any block architecture can be synthesized by developing the linking methodologies introduced in this article. Since the first and second linking methodologies are based on the same concept, the combining of both methodologies may also allow access to a wider variety of multiblock polymers, which cannot be synthesized by any other methods.
It is known that phenyl vinyl sulfoxide [
67] and alkyl isocyanate monomers [
43,
44,
45,
46] undergo living anionic polymerization to result in interesting polymers, poly(4-phenyl vinyl sulfoxide), convertible to conductive rod-like poly(acetylene) and poly(alkyl isocyanate)s with helical conformation. These specialty functional polymers may also be used, at least to a certain extent, in the linking methodology to synthesize block polymers containing blocks with rod-like and helical conformations. One indicative example was introduced in the section 3. Furthermore, the living anionic polymerization of cyclic monomers [
68], such as epoxides, cyclic sulfides, lactones, lactides, and even NCA [
69], may be applied to the linking methodology, although a new reaction site capable of linking with their living anionic polymers is needed.
The linking methodologies introduced in this article are different from the recently developed methodologies based on Click reactions in terms of the direct use of living anionic polymers in the linking reaction. This is advantageous that one more chain-end modification for linking can be avoided and several possible reaction sites capable of linking can be used, taking into consideration about the use of highly reactive living anionic polymers. Moreover, to the best of our experience, the linking reaction can smoothly and efficiently take place among living anionic polymers and chain-end-(reaction site)-functionalized polymers with Mn values of several 10,000 g/mol orders. Although the direct comparison in linking efficiency seems difficult, most linking reactions by means of Click reactions so far reported are generally carried out among polymers having Mn values in the range of several 1000 and less than 10,000 g/mol.