3.1. Esterification by Anhydride Treatment
Peracetylation using acetic anhydrides is a simple and common approach to alter hydrophobicity of polyphenolics [
22]. In the current study, tannin was reacted with varying levels of acetic anhydride and 1-methylimidazole catalyst to determine any control of the anhydride reaction (
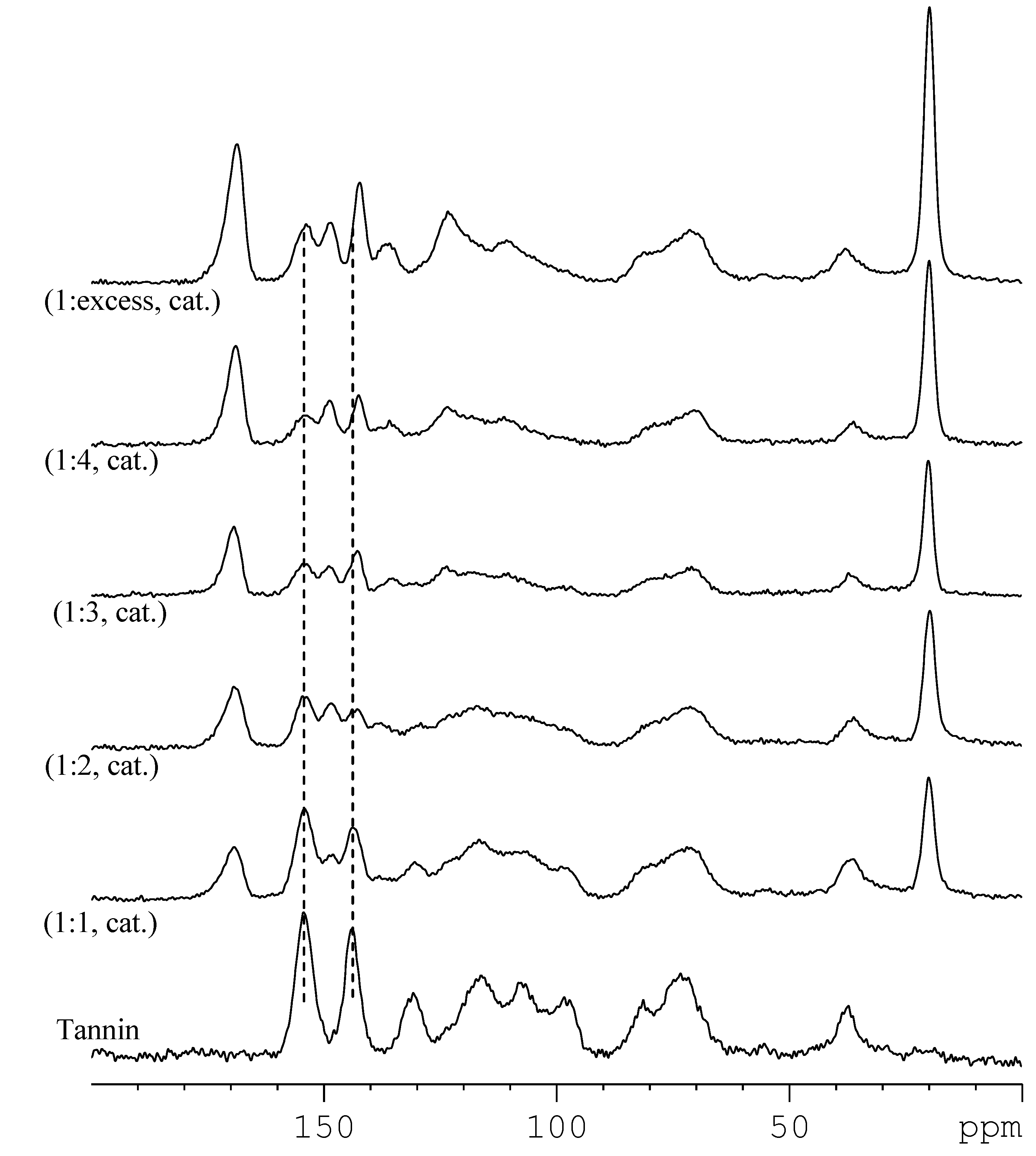
Table 1). Isolation and work up of various tannin acetates revealed products as pale brown powders with yields varying from modest to excellent. The limited solubility of some products necessitated characterization by solid state NMR which was also utilized to determine the average degree of substitution (DS). Generally, it was observed that with a greater acetic anhydride ratio there were corresponding increases in the carbonyl (169 ppm) and methyl (20 ppm) peaks of pendant acetate groups (
Figure 2). Upon esterification the tannin C-3’ and C-4’ (145 ppm) and C-5, 7, 9 (160 ppm) signals [
23] split and moved upfield (2–5 ppm). This was more pronounced for the A-ring signals (
Figure 1). While this may indicate substitution was occurring preferentially at the A-ring phenols (hydroxyl sites), only at high anhydride ratio was there evidence for these phenols being fully esterified. The degree of tannin esterification could be controlled by the level of anhydride addition (
Table 1). A maximum DS of 5.3 was achieved with excess acetic anhydride and was indicative of full esterification of all hydroxyls of the pine bark tannin flavanyl unit [
24]. Lastly, the degree of acetylation gave no relationship to the resulting tannin acetate solubility. The presence of both free phenol hydroxyls and acetate groups gives a relatively insoluble product compared to either full esterification (organic solvent soluble) or native tannin extract (water soluble). This suggests a minimum DS is required to permit a change in solubility (hydrophilic to hydrophobic) or greater ester chain length for hydrophobicity.
Esterifying tannin with varying catalyst content and differing chain length anhydrides can give variable results (
Table 1). With acetylation, the use of only 1% w/w 1-methylimidazole catalyst proved equally effective as use with 10% catalyst. However, with higher chain length anhydrides, a 10% catalyst was required to maximize both DS and yield. With progressively higher chain length esters, isolated yields decrease, being less than anticipated for full esterification. NMR also suggests lower DS values were achieved with increasing anhydride chain length (
Figure 3). This was visually evident with the relative intensity of the ester carbonyl and methyl peaks decreasing across products isolated from acetic to hexanoic anhydride treatments. For example, excess anhydride produces a tannin hexanoate only partially esterified with a DS < 3 when compared with acetylation (
Figure 2,
Figure 3). This result indicates esterification
via anhydride treatment may become sterically inhibited at some phenol centers with greater chain length. Practically, it was also found using a longer chain anhydride requires a more involved isolation step to remove the corresponding acid by-product (
Figure 1) compared with acetic acid and acetylation. Overall, these results suggest anhydride treatments should be tailored to deliver only two long chain ester groups
via anhydride treatment with full esterification achieved by subsequent acetylation. This was demonstrated by preparing tannin hexanoate acetate (
Table 1). This mixed ester approach enabled efficiencies in ester product work up, isolated yield with the additional benefits of minimizing reagent use and improved solvent miscibility, as similarly achieved with tannin-fatty acid conjugates [
15].
Figure 2.
Comparison of solid state 13C NMR CPMAS spectra of various pine bark tannin acetate products formed with differing molar ratio of tannin:acetic anhydride and 1-methylimidazole catalyst (cat).
Figure 2.
Comparison of solid state 13C NMR CPMAS spectra of various pine bark tannin acetate products formed with differing molar ratio of tannin:acetic anhydride and 1-methylimidazole catalyst (cat).
In comparison to anhydride treatment, acyl chlorides are preferable for the synthesis of longer chain length esters (
Figure 1), due to the relative unavailability of such anhydrides, atom efficiency and ease of product work up [
19,
20]. As with anhydrides, control of the tannin:acyl chloride ratio can give defined DS values. Moreover, full esterification of the flavanyl unit phenols was achievable with long alkyl chain length acyl chlorides [
19] compared to anhydride use (
Table 1). This result, and the relatively high yields, suggests acyl chloride reactions with tannins are not subject to steric hindrance as observed with longer chain anhydride treatments (
Table 1). However, if a lower stearic acid chloride ratio is employed, the tannin stearate product tends to be only partially soluble [
15,
19] as found with acetylation (
Table 1,
Figure 2). As above, partial esterification and inherent insolubility may be overcome by acetylation of residual phenols, giving the respective mixed ester tannin stearate acetates, which are soluble in organic solvent [
15]. Transesterification is an alternative approach to synthesize tannins with high specificity for substitution of the tannin flavanyl B-ring [
20]. This method can be performed in several solvents, does not require anhydrous conditions and offers efficiencies in product workup. However, typically, transesterification achieves a DS < 1, being independent of the ester chain length and this, together with long reaction time employed, may not suit industrial application.
Figure 3.
Comparison of solid state 13C NMR spectra of isolated products from anhydride treatments with 10% 1-methylimidazole catalyst. (a) tannin acetate; (b) tannin propionate; (c) tannin butyrate and (d) tannin hexanoate.
Figure 3.
Comparison of solid state 13C NMR spectra of isolated products from anhydride treatments with 10% 1-methylimidazole catalyst. (a) tannin acetate; (b) tannin propionate; (c) tannin butyrate and (d) tannin hexanoate.
3.2 Tannin Ester Properties
While tannin esterification may enhance hydrophobicity and miscibility in oils [
15], chemical modification may alter fundamental tannin properties including reduced antioxidant activity [
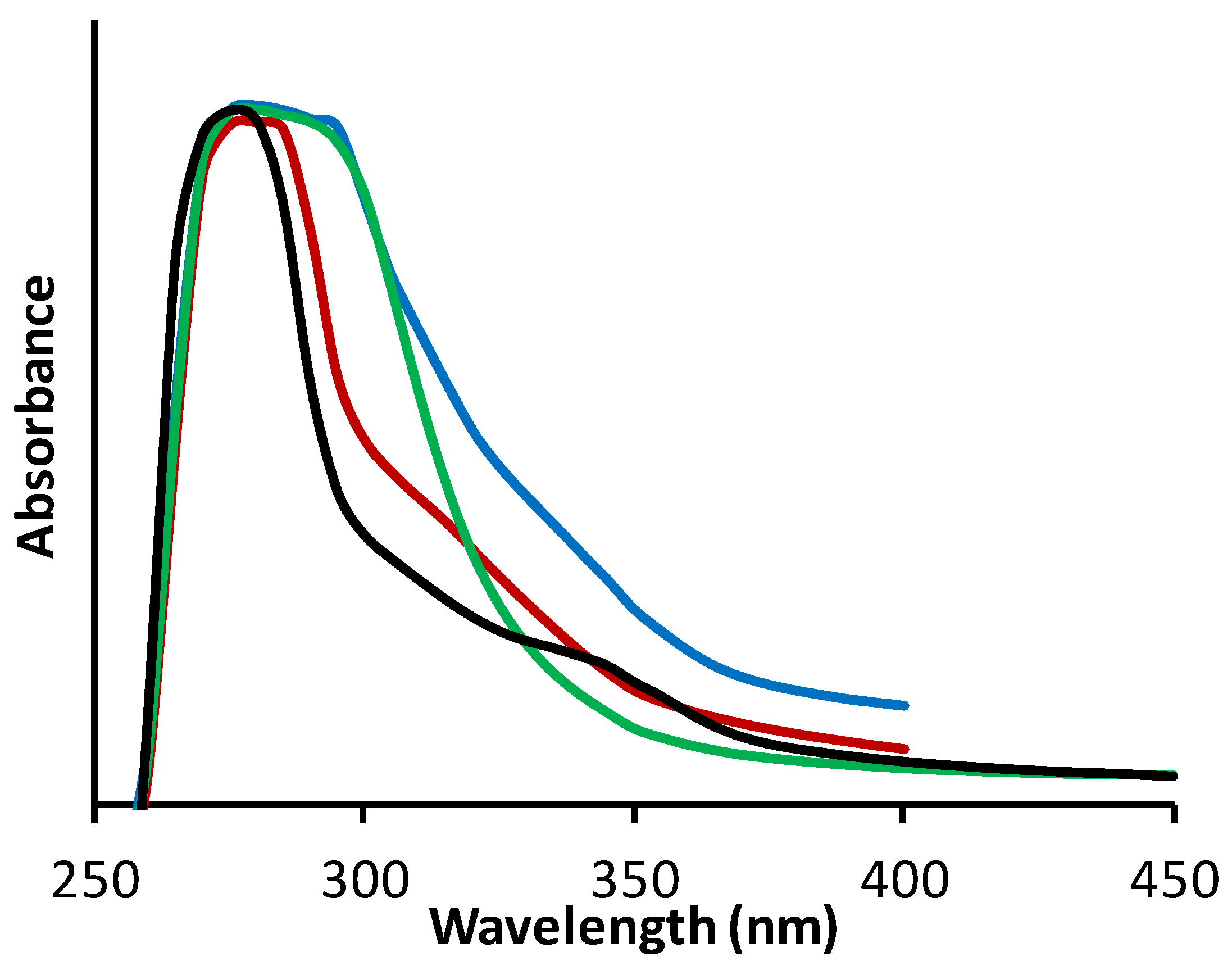
20], ultraviolet (UV) light absorption or those associated with tannin macromolecular behavior. For example, evaluation of various tannin esters (
Table 1) found a range of UV absorption profiles, with key differences observed in the UVB (285–315 nm) and UVA (315–385 nm) regions (
Figure 4). In general, unmodified pine bark tannin has the highest absorption over the UVA and UVB region which decreases upon esterification as evident for tannin acetate (DS = 5.3, TanAc). This decrease in UV absorption upon esterification correlated with the level of substitution. Tannin hexanoate (DS = 2, TanHex) retains good absorption in the UVB range similar to unmodified pine bark tannin, but this absorption diminishes in the UVA region. However, despite differing levels of substitution, the various tannin esters show potential as UVB absorbers.
Figure 4.
UV absorbance profiles of various tannin esters. Tannin (in water, pH7, blue), tannin acetate (in chloroform, red), tannin hexanoate (in dimethyl formamide, green) and tannin hexanoate acetate (in DMF, black).
Figure 4.
UV absorbance profiles of various tannin esters. Tannin (in water, pH7, blue), tannin acetate (in chloroform, red), tannin hexanoate (in dimethyl formamide, green) and tannin hexanoate acetate (in DMF, black).
Tannin esterification and ester chain length can alter the relative molecular mobility within the polyphenolic flavanyl unit and tannin oligomers. By employing solid state NMR and comparing native tannin with tannin propionate (TanPr, DS = 5.3), differing molecular motion characteristics were determined about the tannin flavanyl unit (
Figure 5). Spin-lattice relaxation (T
1ρH) values show the relative molecular motion of some carbons increase on adding propionate ester groups to the flavanyl unit. This increase was most significant at C-2,3, C-5,7,9 and C6’. This suggests the presence of propionate groups has increased the molecular mobility about these carbon centers. This characteristic, together with introduced hydrophobicity, has the potential to influence compatibility and polymer properties when integrated into a plastic.
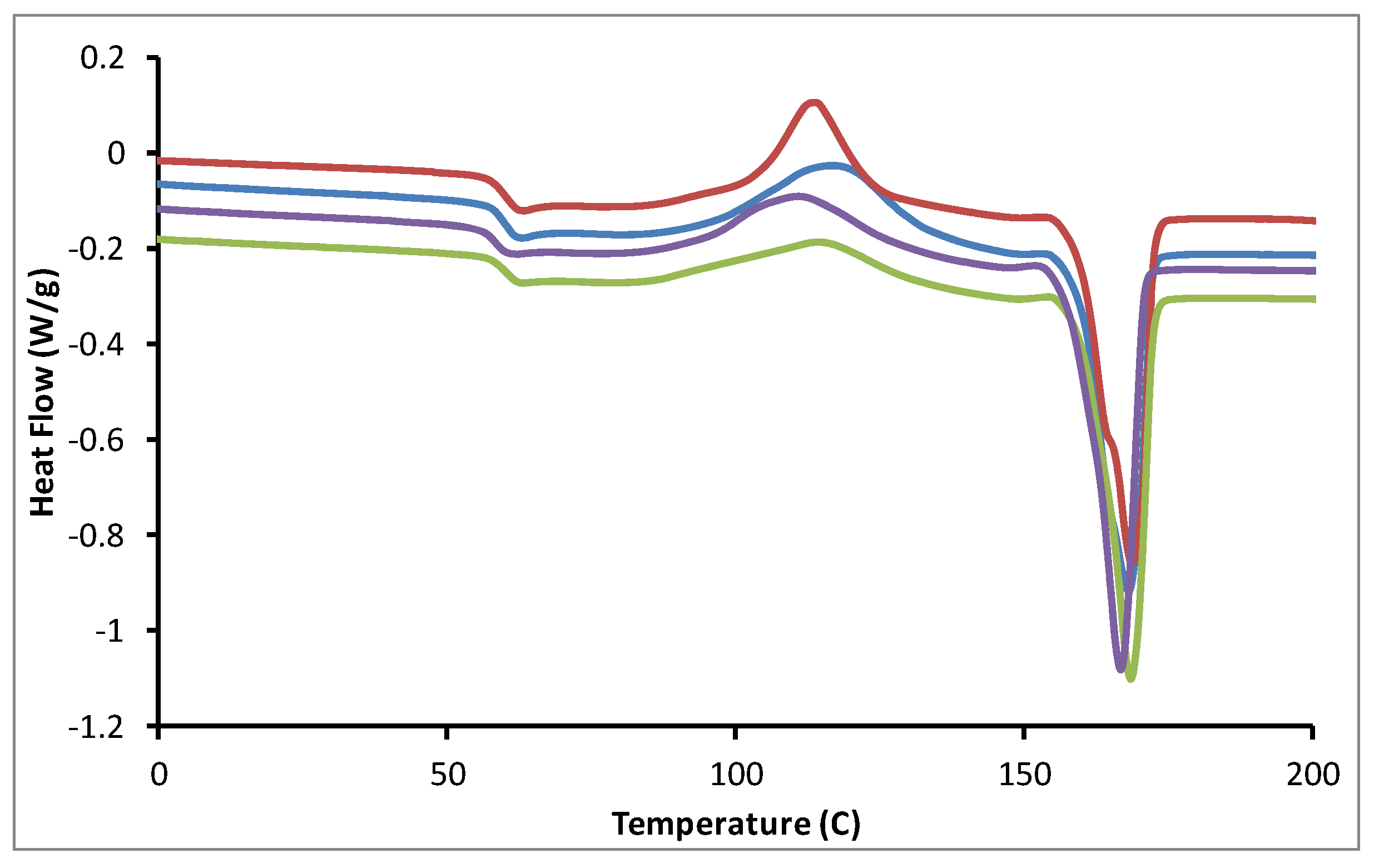
An evaluation of tannin ester properties by differential scanning calorimetry (DSC) was undertaken to assess the potential melt processing and polymer properties of modified tannins. DSC analysis revealed thermal behaviors were dependent on ester chain length (
Figure 6). Both tannin and tannin acetate do not have obvious melt features nor physically melt [
18]. However, modified tannins bearing longer chain esters show melt features, decreasing both in broadness and temperature with longer chain length. This broadness in the melt feature may be associated with the range of tannin oligomers present in the extract [
25] together with extent and randomness of esterification about the tannin flavanyl unit. In the case of ester chain lengths of 4 to 6 carbons, a board melt occurs between 80 °C and 160 °C. Such melt behavior is generally below typical plastic melt processing temperatures [
3]. In contrast, tannins bearing ester groups with 12 to 18 carbons melt below 50 °C [
19]. These melt behaviors are at physiological temperature and also suited for application in unsaturated resin systems [
15]. Overall, DSC analysis revealed tannins bearing ester chain lengths greater than propionate were likely suitable for processing into plastics.
Figure 5.
A comparison of the spin relaxation T
1ρH values for flavanyl unit carbons of tannin and tannin propionate using NMR spin locking experiments.
![Polymers 05 00344 i001]()
Tannin and
![Polymers 05 00344 i002]()
tannin propionate.
Figure 5.
A comparison of the spin relaxation T
1ρH values for flavanyl unit carbons of tannin and tannin propionate using NMR spin locking experiments.
![Polymers 05 00344 i001]()
Tannin and
![Polymers 05 00344 i002]()
tannin propionate.
Figure 6.
DSC thermograms of various tannin esters. Tannin acetate (orange), tannin propionate (blue), tannin hexanoate (DS = 2, red), tannin hexanoate acetate (black), tannin stearate (DS = 1, green) and tannin stearate (DS = 5, purple) with exo up.
Figure 6.
DSC thermograms of various tannin esters. Tannin acetate (orange), tannin propionate (blue), tannin hexanoate (DS = 2, red), tannin hexanoate acetate (black), tannin stearate (DS = 1, green) and tannin stearate (DS = 5, purple) with exo up.
3.3. Tannin Ester Modified Poly(Lactic Acid)
Based on the relative ease of anhydride treatments and their favorable properties, tannin esters with C2 to C6 chain lengths were compounded into poly(lactic acid) (PLA) at varying levels from 0.5% to 10% (w/w,
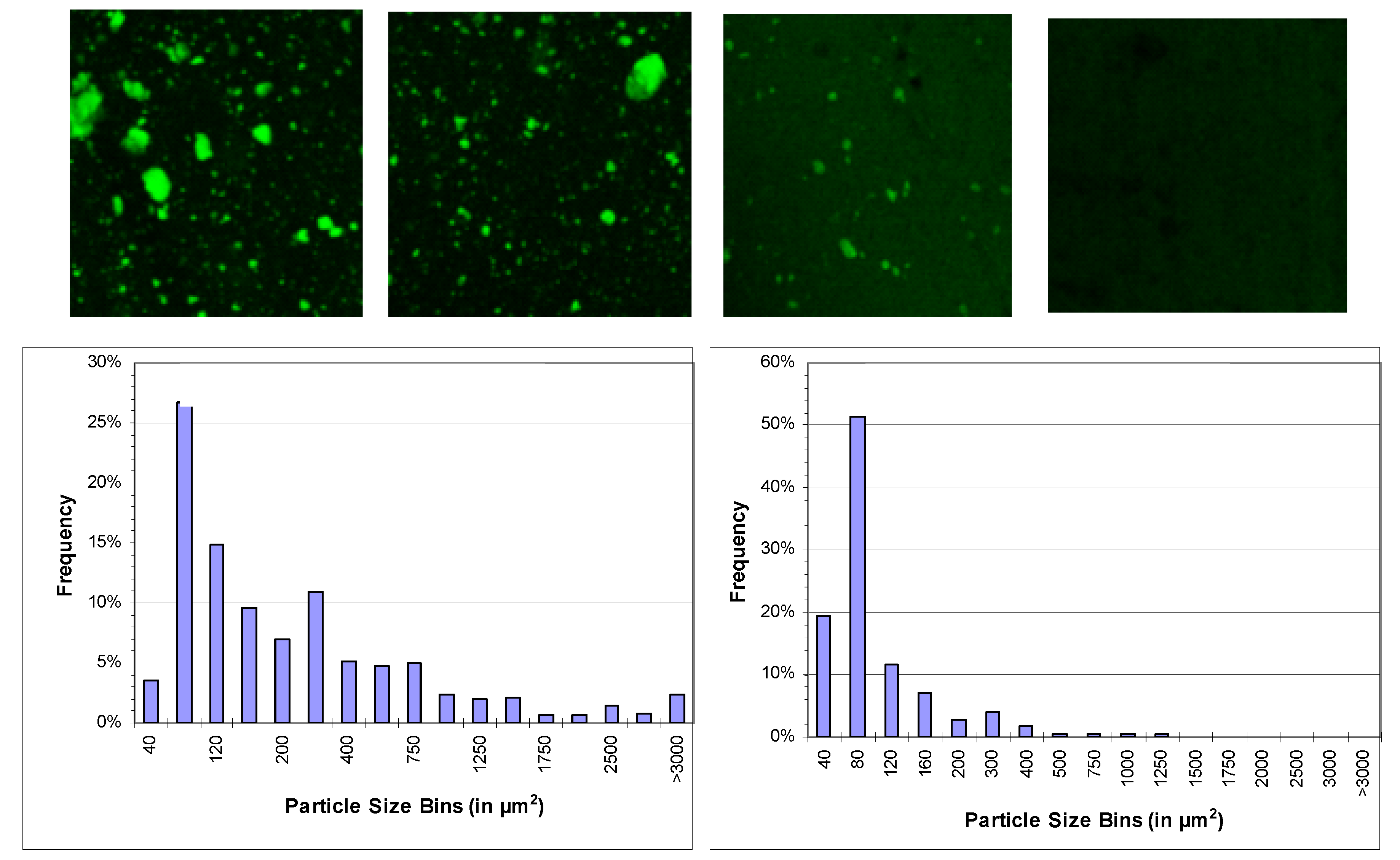
Table 1). Generally, the presence of these tannin additives color PLA, with this dependent on the ester and concentration in the plastic [supplementary material]. Analysis of the compounded plastic by fluorescence microscopy revealed the relative dispersion of each tannin ester (
Figure 7). In the case of TanAc this was retained as relatively distinct domains within the plastic matrix indicative of poor miscibility or phase separation. These domains were present across the TanAc concentration range (0.5%–5%). In contrast, TanHex appeared fully dispersed within the plastic. In progressing from tannin acetate to hexanoate, it was evident the ester chain length determined how the tannin ester dispersed into the plastic. Tannin propionate and butyrate derivatives respectively show progressive dissolution and diminishing particle domains within the PLA compared to tannin acetate.
Figure 7.
Confocal microscopy images of plastic containing 5% loadings of tannin esters observing the inherent autofluorescence of tannin particles distributed in the plastic (top). TanAc (left), TanPr, TanBu and TanHex (right, with each image 500 × 500 μm). Representative particle size distributions of tannin esters in PLA. TanAc (bottom, left) and TanBu (bottom, right).
Figure 7.
Confocal microscopy images of plastic containing 5% loadings of tannin esters observing the inherent autofluorescence of tannin particles distributed in the plastic (top). TanAc (left), TanPr, TanBu and TanHex (right, with each image 500 × 500 μm). Representative particle size distributions of tannin esters in PLA. TanAc (bottom, left) and TanBu (bottom, right).
To evaluate any effect the tannin esters have on PLA thermal properties, both DSC and dynamic mechanical analysis (DMTA) were conducted on injection molded samples. Analysis revealed 10% TanHex and TanHexAc contents in PLA reduce the PLA glass transition (Tg) but does not impact the PLA melt (
Table 3 and
Figure 8). DMTA revealed both tannin esters significantly lowered the Tg onset compared to unmodified PLA (
ca. 50 °C). However, shorter chain length esters and lower quantities of tannin ester gave a reduced impact on PLA thermal properties [supplementary material]. The observed reduction in PLA Tg with tannin hexanoate while significant was less than that which can be achieved (≤ 38 °C) when blending PLA with poly(ethylene glycols) (PEGs) or citrates [
26,
27]. DSC analysis of the modified PLA samples was consistent with DMTA, showing a decrease in Tg of 6–7 °C upon addition of TanHex or TanHexAc and no change in the melt temperature (
ca. 168 °C). The onset temperature of crystallization within samples decreased with greater tannin ester content (
Figure 9, Supplementary Material).
Table 3.
Results of DSC analysis of selected PLA samples modified by tannin, TanHex and TanHexAc (see supplementary material).
Table 3.
Results of DSC analysis of selected PLA samples modified by tannin, TanHex and TanHexAc (see supplementary material).
| Additive | Cycle 1 | Cooling | Heating Cycle 2 |
|---|
| Tg (°C) | Crystallization (°C) | Tg (°C) | Melt (°C) |
|---|
| PLA | 58.5 | 155.7 | 64.3 | 167.7 |
| 1% TanHex | 58.0 | 155.9 | 63.8 | 168.0 |
| 5% TanHex | 56.1 | 145.0 | 63.6 | 167.3 |
| 10% TanHex | 54.7 | 139.3 | 57.3 | 167.2 |
| 1% TanHexAc | 59.6 | 155.9 | 63.7 | 168.3 |
| 5% TanHexAc | 56.1 | 144.9 | 59.6 | 167.1 |
| 10% TanHexAc | 53.8 | 139.1 | 57.8 | 166.7 |
| 1% Tannin | 58.6 | - | 63.5 | 168.2 |
| 5% Tannin | 58.3 | - | 64.4 | 168.2 |
| 10% Tannin | 57.6 | - | 64.5 | 167.9 |
Figure 8.
DMTA of PLA modified with 10% loadings of tannin hexanoate and tannin hexanoate acetate. PLA (red), TanHex (green) and TanHexAc (blue).
Figure 8.
DMTA of PLA modified with 10% loadings of tannin hexanoate and tannin hexanoate acetate. PLA (red), TanHex (green) and TanHexAc (blue).
A comparison of PLA flexural and tensile properties show the presence of the tannin esters can impact PLA mechanical properties contributing up to 15% decreases in PLA stiffness (
Table 4 and supplementary material). On addition of tannin acetate, similar modulus of elasticity (MOE) values as PLA (3894 MPa) were observed with up to 5% TanAc content. With longer ester chain length the MOE values decreased with greater tannin ester contents. For TanPr and TanBu, only 5% loadings achieved a significant decrease in PLA stiffness (<3800 MPa). However, both TanHex and TanHexAc exhibit decreases in MOE values across their entire loading range (0.5% to 10%). At 10% loading, decreases in MOE of up to 15% were achieved with TanHex (3480 MPa) and TanHexAc (~3400 MPa), respectively. Typically more significant decreases in PLA stiffness are observed with plasticizing additives such as PEGs or citrates [
27,
28]. In the case of adding unmodified tannin, this had a contrasting effect, stiffening PLA with increased MOE values at higher tannin contents (3915 MPa). This result indicates the native tannin likely acts as a filler, as observed with unmodified lignins in PLA [
6,
7]. The flexural strength decreased upon addition of all tannin esters compared with PLA (133 MPa). This was related to tannin modifier content for C2–C4 chain length esters, but appeared independent of loading for TanHex samples. Tensile testing revealed the presence of tannin modifiers in ≥1% loading decrease PLA tensile modulus values. For 5% TanHex, the tensile modulus was lowered by some 15%. Similarly, PLA tensile strength values were also reduced, with values dependent on tannin modifier loading.
Figure 9.
Representative DSC thermograms of PLA and PLA modified with 5% TanHex before and after artificial weathering exposure (exo up). PLA (red), PLA-exposure (green), TanHex (blue) and TanHex-exposure (purple).
Figure 9.
Representative DSC thermograms of PLA and PLA modified with 5% TanHex before and after artificial weathering exposure (exo up). PLA (red), PLA-exposure (green), TanHex (blue) and TanHex-exposure (purple).
Table 4.
Flexural testing results of modified PLA samples before and after artificial weathering.
Table 4.
Flexural testing results of modified PLA samples before and after artificial weathering.
| Sample with tannin ester loading | Prior to artificial weathering | Post artificial weathering |
|---|
| Modulus | Strength | Modulus | Strength |
|---|
| (MPa) | StdDev | (MPa) | StdDev | (MPa) | StdDev | (MPa) | StdDev |
|---|
| PLA | 3894 | 33 | 113 | 1 | 3903 | 129 | 114 | 2 |
| 0.5% TanAc | 3902 | 25 | 111 | 1 | - | - | - | - |
| 1% TanAc | 3826 | 38 | 111 | 1 | 3922 | 22 | 110 | 1 |
| 5% TanAc | 3868 | 62 | 104 | 2 | 3929 | 61 | 107 | 1 |
| 0.5% TanPr | 3904 | 16 | 105 | 1 | - | - | - | - |
| 1% TanPr | 3872 | 41 | 107 | 1 | 3896 | 33 | 112 | 0 |
| 5% TanPr | 3787 | 21 | 104 | 1 | 3835 | 127 | 105 | 2 |
| 0.5% TanBu | 3879 | 39 | 108 | 2 | - | - | - | - |
| 1% TanBu | 3885 | 44 | 108 | 2 | 3903 | 77 | 111 | 2 |
| 5% TanBu | 3770 | 34 | 105 | 2 | 3855 | 161 | 106 | 1 |
| 0.5% TanHex | 3846 | 14 | 105 | 1 | - | - | - | - |
| 1% TanHex | 3749 | 27 | 104 | 0 | 3753 | 171 | 106 | 2 |
| 5% TanHex | 3605 | 19 | 95 | 1 | 3532 | 121 | 96 | 1 |
| 10% TanHex | 3480 | 19 | 85 | 0 | 3160 | 263 | 80 | 4 |
| 1% TanHexAc | 3771 | 25 | 104 | 1 | 3785 | 88 | 107 | 2 |
| 5% TanHexAc | 3654 | 16 | 95 | 1 | 3614 | 86 | 97 | 1 |
| 10% TanHexAc | - | - | - | - | 3429 | 223 | 69 | 16 |
| 1% Tannin | 3805 | 34 | 104 | 1 | 3807 | 121 | 109 | 1 |
| 5% Tannin | 3915 | 23 | 99 | 1 | 3895 | 110 | 101 | 4 |
| 10% Tannin | - | - | - | - | 3996 | 74 | 92 | 2 |
3.4. Artificial Weathering
Although PLA is unsuited to exterior application [
29], the PLA samples were subjected to accelerated weathering as a means to artificially age the plastic and evaluate any retention of PLA properties due to the presence of tannin esters. This test included both water condensation and UV irradiation (
Table 2) with samples expected to exhibit some degradation due to PLA hydrolysis and UV-promoted oxidation [
7,
29]. Results reveal that after 500 h of artificial weathering, the PLA flexural modulus increased, but this was not statistically significant due to high variability between test specimens (
Table 4). This variability marred all test specimens after aging. Flexural modulus values for aged TanAc, TanPr and TanBu modified PLA samples were similar to original values, but this did not differ to PLA at the 95% confidence level. For the TanHex and TanHexAc samples, a 5% loading led to retention of flexural properties after exposure. This result suggests both TanHex and TanHexAc may confer a protection role which may be as a UV absorber within PLA. Evaluation by DSC analysis revealed aged samples lose crystallinity, but retain similar Tg and melt temperatures (
Figure 9). Results also show any changes in sample weights after accelerated weathering were negligible.
The results from the colorimetric analysis of tannin ester-modified PLA after accelerated weathering are shown in
Table 5. This revealed some variability in color stability with samples typically lightening in color. Unmodified PLA showed the smallest change in ∆E, a likely consequence of this polymer being transparent with little scope for significant color change. Of the modified PLA samples, those modified with TanAc had the highest variability and may be linked to the dispersal of TanAc (
Figure 7). TanHex offered the best color stability of the samples which was probably due to the even dispersion of TanHex throughout the PLA matrix (
Figure 7).
Table 5.
Results of colorimetric analysis after artificial weathering exposure.
Table 5.
Results of colorimetric analysis after artificial weathering exposure.
| Sample | ∆L | ∆a | ∆b | ∆E |
|---|
| PLA | 0.26 | 0.34 | 1.43 | 1.49 |
| TanAc 1% | 4.60 | 7.51 | 2.14 | 9.06 |
| TanAc 5% | 12.43 | 1.36 | 1.48 | 12.59 |
| TanPr 1% | 6.75 | 0.57 | 6.08 | 9.10 |
| TanPr 5% | 9.58 | 0.19 | 0.31 | 9.59 |
| TanBu 1% | 4.89 | 3.75 | 4.14 | 7.42 |
| TanBu 5% | 9.32 | 0.61 | 0.65 | 9.36 |
| TanHex 1% | 5.08 | 0.58 | 3.56 | 6.23 |
| TanHex 5% | 5.42 | 0.89 | 3.69 | 6.62 |


 Tannin and
Tannin and  tannin propionate.
tannin propionate.
 Tannin and
Tannin and  tannin propionate.
tannin propionate.