3.1. Synthesis of Inorganic Nanoparticles
In this section, the microfluidic synthesis of metal and semiconductor nanoparticles as well as other colloidal materials will be briefly reviewed. When possible, the micro-scale synthesis will be compared to the conventional methods used for the same material. This section is not extensive. Several representative syntheses will be discussed for each material.
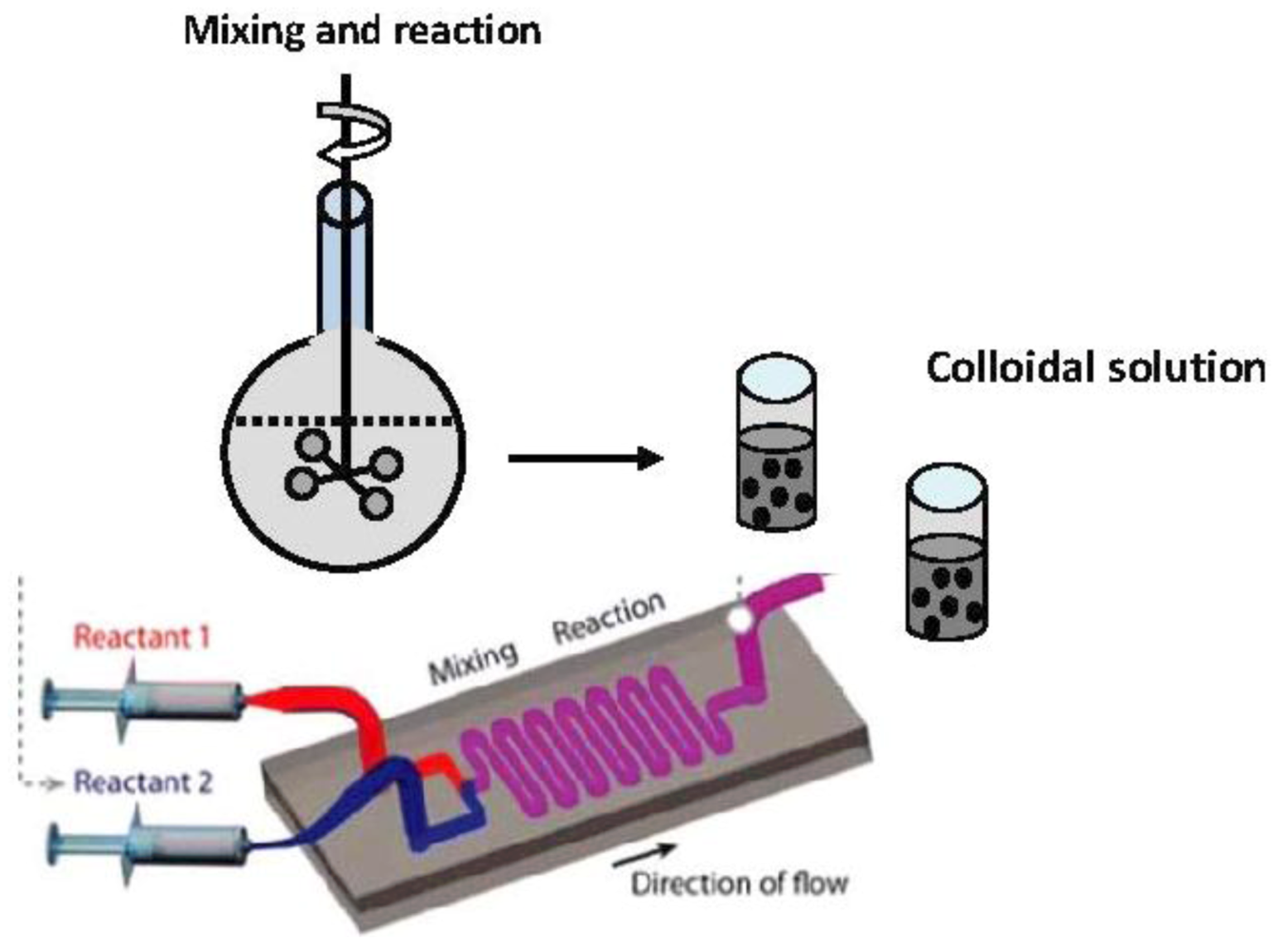
The most important advantage of the microfluidic devices for the synthesis of metal nanoparticles is the capability of controlling the temperature along the flow. This fact, together with an efficient mixing of reagents and the possibility to add reagents in any part of the channel, contribute to the generation of homogeneous nanoparticles in a well-controlled fashion. Three examples regarding the synthesis of gold nanoparticles in continuous flow systems are presented below in a chronological order to show the progress realized for a duration of less than a decade. Wagner and Köhler have used a simple microfluidic device (as shown in
Figure 8) to synthesize gold nanoparticles by using a mild reducing agent and poly(vinylpyrrolidone) as a capping agent [
20].
A mild reducing agent, ascorbic acid is used in this synthesis, in the presence of poly(vinylpyrrolidone) (PVP) as capping agent, and the size distribution of the gold nanoparticles is found considerably narrower than that obtained by conventional synthesis methods. The mixing is based on split and recombination of the laminar streams. Smaller gold nanoparticles (4–7 nm) were obtained when, instead the ascorbic acid, a much stronger reducing agent such as sodium borohydride was used [
21]. It seems that in this work, the size of nanoparticles was not influenced by the flow rate, or by the reagents ratios.
Figure 8.
Set-up of a microfluidic reactor used for the synthesis of gold nanoparticles [
20].
Figure 8.
Set-up of a microfluidic reactor used for the synthesis of gold nanoparticles [
20].
Thiol-passivated gold nanoparticles, generally called monolayer protected clusters, have been prepared for the first time, using a continuous flow microfluidic device. The authors (de Mello
et al.) have shown that nanoparticles generated by the device are smaller than those prepared by the batch method and, that their size distribution is significantly narrower [
22]. The schematic of the mixer is shown in
Figure 9.
The size histogram (
Figure 10) shows the narrow distribution of the thiol functionalized gold nanoparticles synthesized in the device using the mixer. The size distribution is compared to that of gold nanoparticles prepared through a bulk synthesis shown in
Figure 10c.
Figure 9.
(
a) Three dimensional schematic of a radial interdigitated mixer. Each mixer is fabricated in 3-layers. In the first two layers input flows are directed to two circular bus channels which, in turn, split the flow into several identical fluid laminae and deliver reagent streams towards a central mixing chamber. The final layer acts as a cap to enclose channels and as a guide for input and output capillaries. The output is from the centre of the uppermost layer; (
b) Photograph of the fabricated mixer. Microchannels are filled with dye solutions to show different shadings for the different channels [
22].
Figure 9.
(
a) Three dimensional schematic of a radial interdigitated mixer. Each mixer is fabricated in 3-layers. In the first two layers input flows are directed to two circular bus channels which, in turn, split the flow into several identical fluid laminae and deliver reagent streams towards a central mixing chamber. The final layer acts as a cap to enclose channels and as a guide for input and output capillaries. The output is from the centre of the uppermost layer; (
b) Photograph of the fabricated mixer. Microchannels are filled with dye solutions to show different shadings for the different channels [
22].
Figure 10.
Size histograms for particles made with a 2:1 thiol-gold ratio at (
a) 800 µL min
−1; (
b) 400 μL min
−1; (
c) the comparable bulk synthesis [
22].
Figure 10.
Size histograms for particles made with a 2:1 thiol-gold ratio at (
a) 800 µL min
−1; (
b) 400 μL min
−1; (
c) the comparable bulk synthesis [
22].
It can be seen that, while the bulk synthesis produced gold nanoparticles with non-uniform sizes, those produced in the microfluidic device provided with a mixer, show a narrow size distribution for both flow rates.
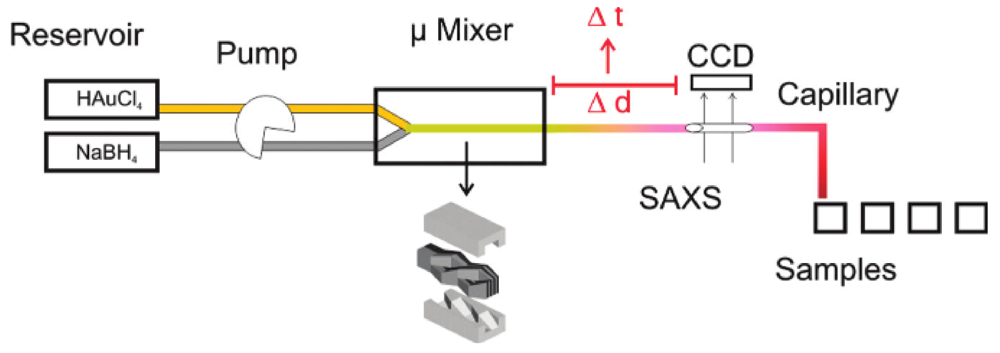
More recently, the nucleation and growth of gold nanoparticles prepared by reduction of gold ions by sodium borohydride was studied with small-angle X-ray scattering online, at a very high time resolution (100 ms) [
23]. The experimental setup used for the synthesis is shown in
Figure 11.
Figure 11.
Experimental setup for the synthesis of gold nanoparticles by using a static mixer coupled directly to SAXS-analysis in a flow cell [
23].
Figure 11.
Experimental setup for the synthesis of gold nanoparticles by using a static mixer coupled directly to SAXS-analysis in a flow cell [
23].
The ability to analyze online the nanoparticles is a considerable advantage. Coupling the microstructured mixer with small-angle X-ray scattering enabled online analysis of the formed nanoparticles, without requiring a synchrotron radiation facility. In general, by using this setup, the particle size is identical to sizes obtained in batch experiments. The main advantage of this setup is that kinetic information can be provided for rapid particle formation processes. In combination with XANES, the data show that initially, the gold precursor is converted into gold nuclei within less than 100 ms, followed by particle growth by coalescence as shown in
Figure 12.
As in all the processes based on gold salts reduction, the final size of the particles is reached only after the precursor species are completely consumed.
Figure 12.
Mechanism of gold nanoparticles formation [
23].
Figure 12.
Mechanism of gold nanoparticles formation [
23].
Noble metal nanorods can be prepared from spherical metal seed crystals in a ‘growth solution’ containing the metal salt (HAuCl
4) in millimolar concentrations, a mild reducing agent (ascorbic acid) and a high concentration of cetyl-trimethyl-ammonium-bromide (CTAB), which forms rod-shaped micelles as a template for the anisotropic particle growth [
24]. The seed and growth solutions are injected with independent flow rates. For the preparation of silver rods, NaOH is added shortly after the mixing of seed and growth solutions. The flow is then directed through temperature-controlled tubing, allowing the crystals to grow at a fixed temperature. The extinction of the final product is measured by a flow-through spectrometer. The experimental results for gold and silver nanorod synthesis demonstrated the potential of the continuous flow setup for the synthesis of anisotropic particles as well. The continuous flow synthesis with online optical monitoring allows the reproducible and controlled synthesis of particles with specific shapes.
Thermal reduction of a few precursors of noble metals is possible by using the continuous flow approach shown in
Figure 13.
Figure 13.
Synthesis of silver nanoparticles in a tubular microreactor [
25].
Figure 13.
Synthesis of silver nanoparticles in a tubular microreactor [
25].
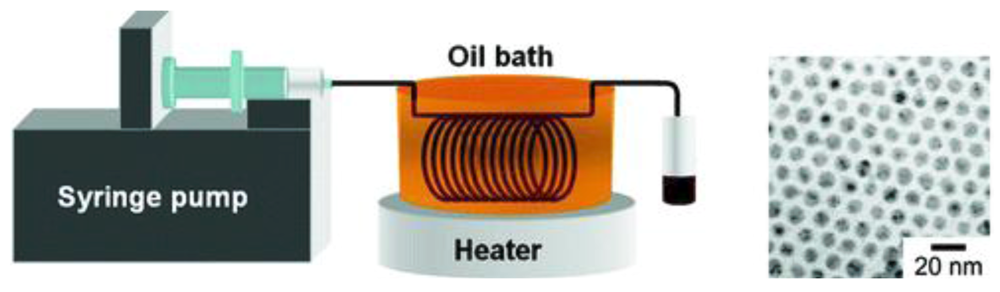
A continuous flow tubular micro-reactor is suitable for the synthesis of silver nanoparticles when a single-phase precursor such as silver pentafluoropropionate in isoamyl ether is thermally reduced at moderate temperatures. The produced silver nanoparticles have a narrow size distribution and, because of the nature of the procedure, there is no need for a mixer. Flow rates between 0.08 and 0.7 mL min−1 were tested and the TEM study showed that average diameters of silver nanoparticles prepared under these conditions were 8.6 +/− 0.9 and 8.6 +/− 1.0 nm for the molar ratios of TOA/silver pentafluoropropionate of 6 and 12, respectively.The experiments showed that low flow rates have to be used in order to obtain a narrow size distribution of silver nanoparticles. It is demonstrated that single-source precursors favour the formation of mono-disperse metal nanoparticles
Small copper nanoparticles with a narrow size distribution were produced in a microfluidic device by Kumar
et al. [
26], and Packirisamy
et al. [
27]. The most important property of microdevice-prepared copper nanoparticles is an improved stability to oxidation, very important for any application.
Cu nanoparticles with narrower size-distribution and a smaller size as shown in
Figure 14a could be synthesized by using the sulfobetaine-Cu(I) complex as the starting material, in comparison with Cu obtained by the conventional batch process by using CuCl
2. (
Figure 14b) UV-Vis spectroscopy, reaction calorimetry and XANES (X-ray absorption near edge structure) investigations showed that stable and water-soluble sulfobetaine-Cu(I) complex can be formed in a THF solution by using 3-(
N,
N-dimethyldodecylammonia) propanesulfonate as a stabilizer during the Cu nanocolloid formation from CuCl
2/THF solution. In this work, the important role of the starting material and stabilizer is emphasized.
Figure 14.
Comparison between the size distribution of copper nanoparticles obtained through the two methods: (
a) microreactor process and (
b) batch process [
26].
Figure 14.
Comparison between the size distribution of copper nanoparticles obtained through the two methods: (
a) microreactor process and (
b) batch process [
26].
In a recent work, copper nanoparticles have been synthesized in a microfluidic reactor and the spectra were compared to those corresponding to Cu nanoparticules prepared through the traditional batch method.
Cu2+ + BH4− + H2O → Cu0 + B(OH)3 + H2 → formation of Cu nanoparticles (1)
and their oxidation:
2Cu + ½O2 + H2O → Cu2O (2)
and
Cu2O + O2→ 2CuO (3)
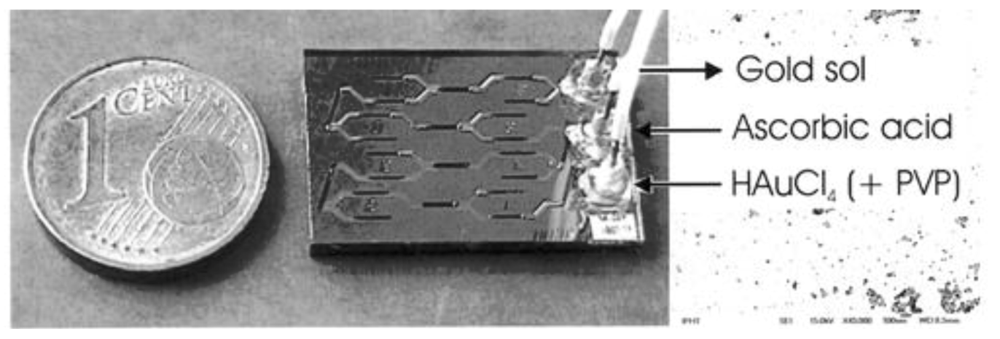
The schematic of the microfluidic reactor is shown in
Figure 15. The total volume of the microreactor is approximately 20 µL
3.
Copper nanoparticles were synthesized in the authors’ laboratory by using a poly(dimethylsiloxane) (PDMS) device.
Figure 15.
(
a) PDMS microreactor used for the synthesis of copper nanoparticles; (
b) Cu LSPR band corresponding to copper nanoparticles synthesized through the “flask method” microfluidic synthesis [
27].
Figure 15.
(
a) PDMS microreactor used for the synthesis of copper nanoparticles; (
b) Cu LSPR band corresponding to copper nanoparticles synthesized through the “flask method” microfluidic synthesis [
27].
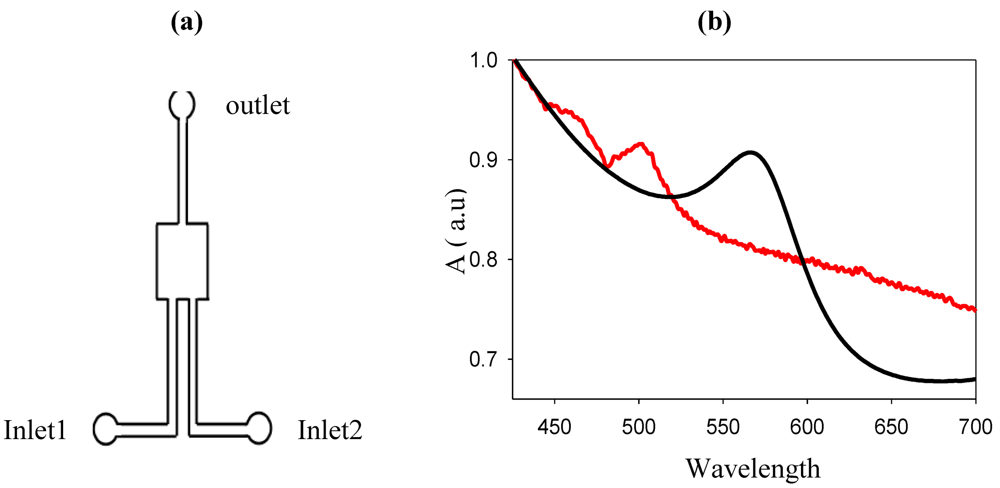
The copper sulfate solution (2.7 × 10
−3 M) is mixed with 4 mL of a sodium citrate solution (1%) outside the micro-reactor and then introduced into the micro-reactor, simultaneously with a sodium borohydride solution (0.02 M) through inlet 1 and 2, respectively. The spectra show the immediate formation of Cu nanoparticles. In the microfluidic environment, the Cu LSPR band is observed around 520 nm and corresponds to small (less than 5 nm) nanoparticles. In
Figure 16 the spectra of Cu synthesized inside and outside the micro-reactor are compared.
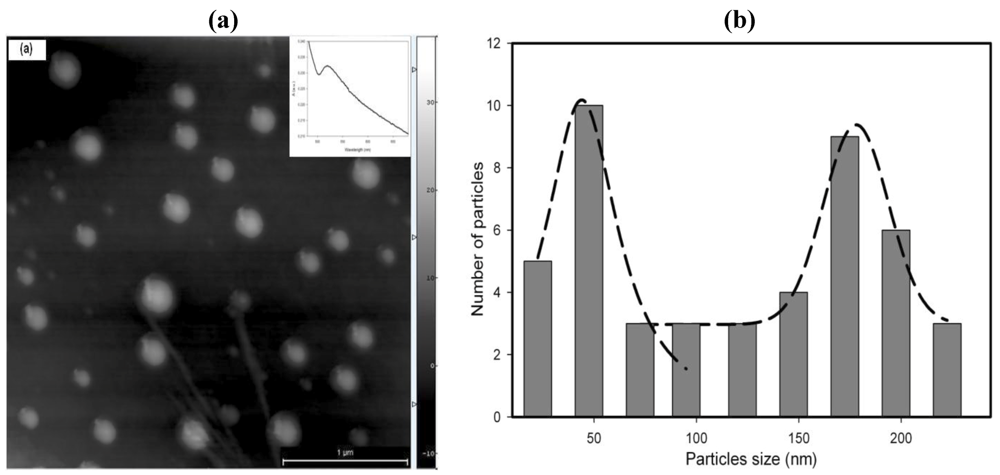
Figure 16.
AFM image of Cu nanoparticles synthesized (
a) at room temperature; (
b) the corresponding size distribution. The spectrum of the stabilized Cu nanoparticles on a glass substrate is shown in the inset [
28].
Figure 16.
AFM image of Cu nanoparticles synthesized (
a) at room temperature; (
b) the corresponding size distribution. The spectrum of the stabilized Cu nanoparticles on a glass substrate is shown in the inset [
28].
Copper nanoparticles were synthesized through a “flask” process as well and their morphology is shown in
Figure 16.
The AFM image shows the presence of large particles with a broad size distribution.
Because of the anaerobic conditions, Cu nanoparticles are stable in the microfluidic environment.
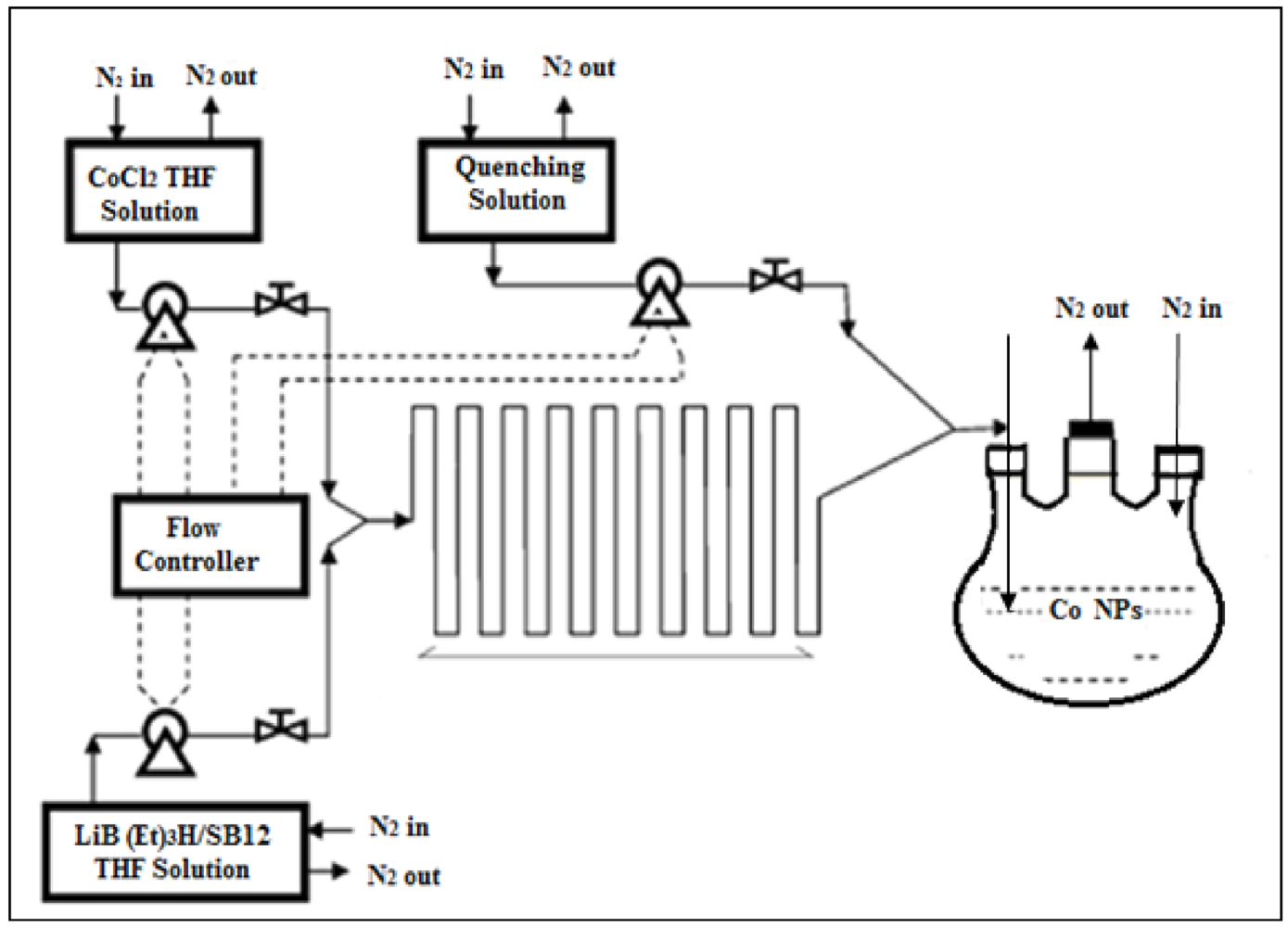
Another important application of microfluidic synthesis, the synthesis of nanoparticles with two or more crystalline phases, realized by Kumar
et al. is shown in the example below (
Figure 17) for cobalt nanoparticles [
29].
Figure 17.
Schematic of the phase-controlled synthesis of cobalt nanoparticles [
29].
Figure 17.
Schematic of the phase-controlled synthesis of cobalt nanoparticles [
29].
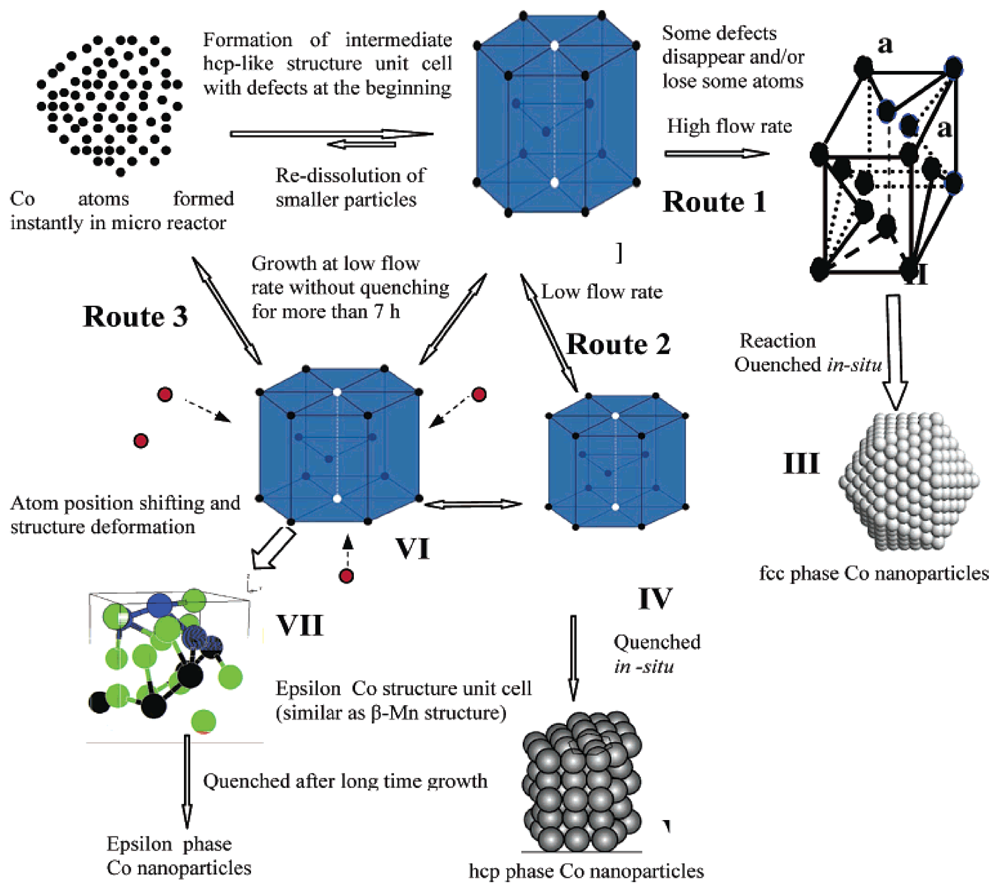
The different crystal structures are synthesized through manipulation of parameters such as flow rates, reaction times and quenching procedures. The formation route suggested by the authors is shown below (
Figure 18). They show that, by using high flow rates (high kinetic energy), the Co nanoparticles that are formed, have mainly a face-centered cubic structure, while at low flow rates, particles with hcp structures are favored. It is found that the flow rate has a marked effect only on the nucleation process as the growth of the particles, in this work, happens outside of the micro-reactor.
Semiconductor nanocrystals can also be synthesized through the continuous flow approach as shown in
Figure 19.
Alivisatos
et al. showed that the size of CdSe nanocrystals can be tuned by changing the parameters [
30].
Figure 18.
Proposed formation route for the different phases of Co nanoparticles [
29].
Figure 18.
Proposed formation route for the different phases of Co nanoparticles [
29].
Figure 19.
A chip-based microfluidic reactor for the synthesis of CdSe nanocrystals. Nanocrystals are sized through their continuous photoluminescence data [
30].
Figure 19.
A chip-based microfluidic reactor for the synthesis of CdSe nanocrystals. Nanocrystals are sized through their continuous photoluminescence data [
30].
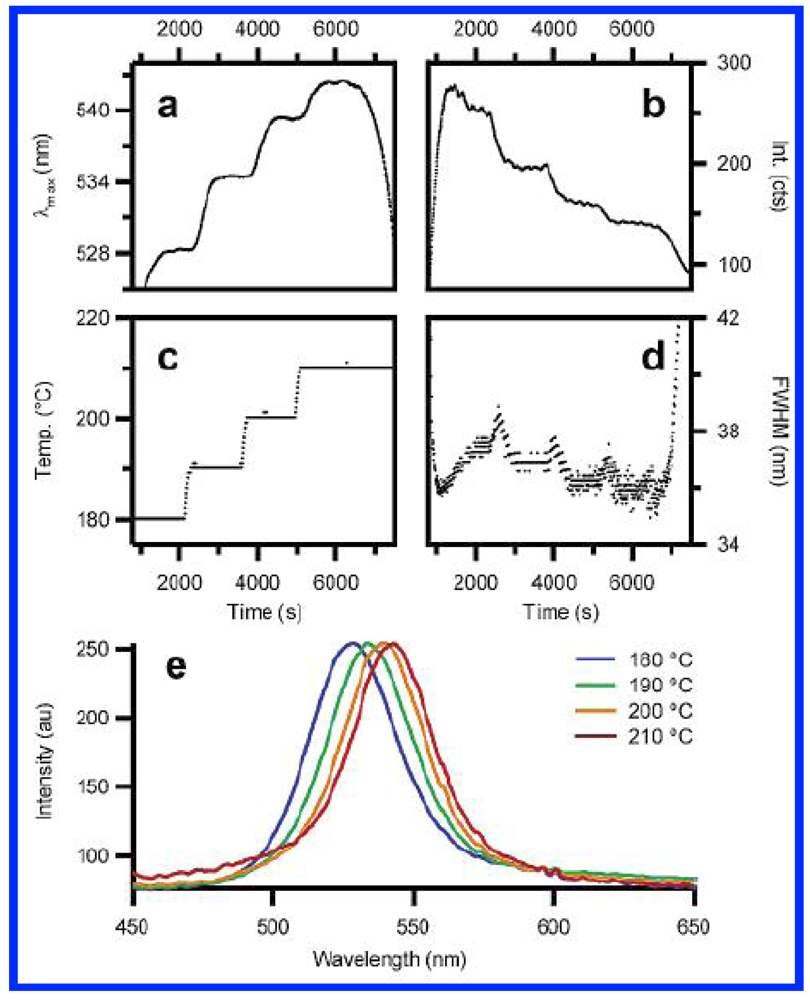
The tuning of the size of CdSe nanocrystals is done in this work by independently changing the temperature, the flow rate through heated microchannels, and the concentration of the precursor. The microfluidic reactor employed by the author works in a continuous flow regime as shown in
Figure 20. They make use of the major advantage of microfluidic reaction systems, that is, the ability to change rapidly the concentration and temperature on the scale of micrometers. The authors show that, while, similar data could have been obtained through flask synthesis, the experimental work would be very tedious.
The examples shown in this section have demonstrated the advantages of the devices based on continuous flow for the synthesis of high-quality Au, Ag, Cu, Co, nanoparticles. However, for obtaining nanoparticles with a narrow size distribution, the reactors should be integrated with mixers, pumps, and sometimes optical devices for characterizing the nanoparticles online.
The principal drawback of the continuous flow-based methods is the deposition of nanoparticles on the walls. As it has been shown in
Section 2.2, an alternative approach is the use of droplet microfluidics, where the confinement within small droplets prevents the contact of the nanoparticles with the walls, and hence, their deposition.
Figure 20.
Size control of CdSe nanocrystal synthesis via flow rate and concentration (Reproduced with permission from ref. [
30]).
Figure 20.
Size control of CdSe nanocrystal synthesis via flow rate and concentration (Reproduced with permission from ref. [
30]).
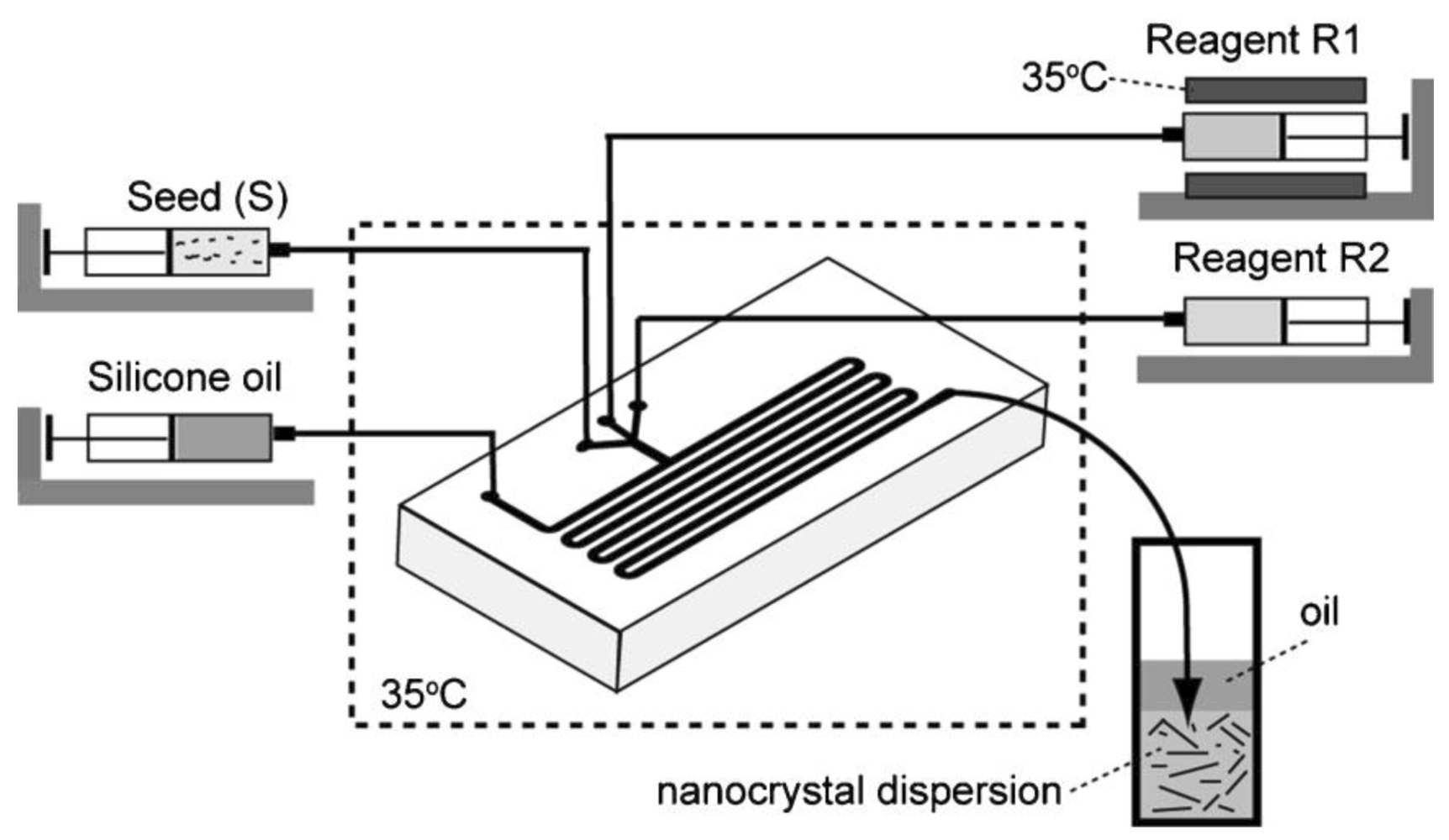
Gold nanorods of varying aspect ratios have been successfully prepared by implementing the T-junction droplet-based synthesis as shown in
Figure 21 [
31]. The authors adapted small-scale batch method [
32] to a flow version in a microfluidic environment (
Figure 19). The formation of droplets at the junction is determined by the microchannel inlet geometry, fluid viscosities, and interfacial tension, and the relative flow rates of the two immiscible fluids. Pico-liter droplets are formed containing both seeds and growth reagents and silicon oil is used as the continuous fluid. The most interesting results achieved in this study is the tunability of the shapes and thus of the UV-Visible absorption by changing parameters such as the concentration of reagents and their flow rates (
Figure 22). The authors (Duraiswamy and Khan) further intend to develop an integrated synthesis method that incorporates online synthesis of gold nanoparticle seeds, and extend the residence time of growing nanocrystals on-chip, so as to enable a fully continuous process. The microfluidic synthesis proves to be quite difficult in the case of using both a seed and a growth solution.
Figure 21.
Droplet-based synthesis of gold nanorods [
31].
Figure 21.
Droplet-based synthesis of gold nanorods [
31].
Figure 22.
UV/Vis absorbance spectra and the corresponding TEM images of rod-shaped particles of varying aspect ratios [
31].
Figure 22.
UV/Vis absorbance spectra and the corresponding TEM images of rod-shaped particles of varying aspect ratios [
31].
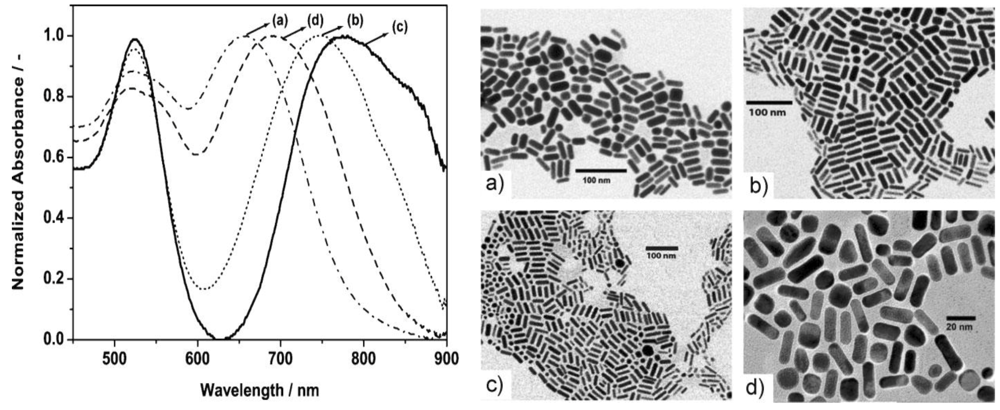
The size (aspect ratio) of the nanorods prepared through the droplet method appears to be uniform as shown in
Figure 23. It can be seen that, in addition to nanorods, a number of nanospheres are formed as well. However, this is a general occurrence, hardly avoidable in both batch methods and continuous synthesis as well.
Figure 23.
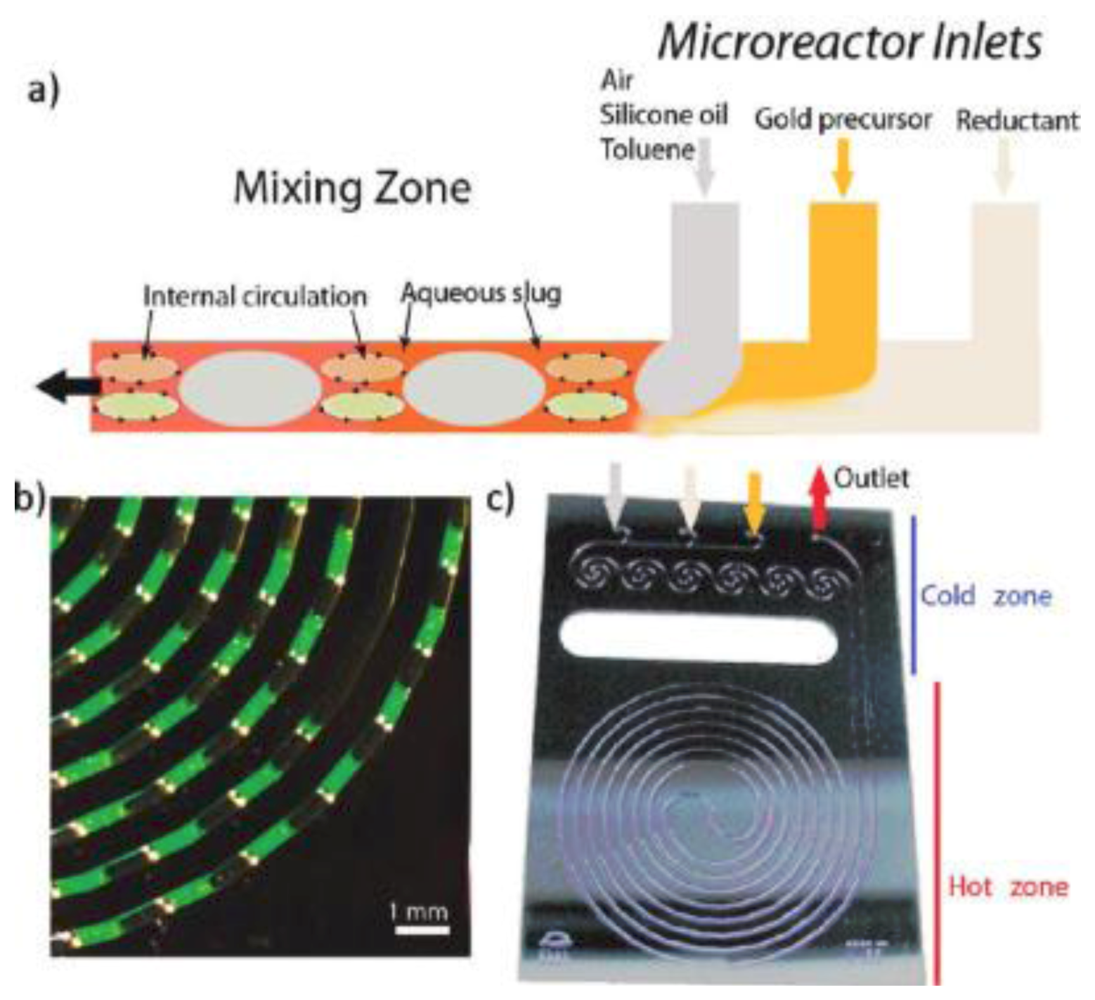
(
a) Schematic of the segmented flow generation in a microfluidic reactor; (
b) Detail of segmented slugs generated at a residence time of 10 s. The aqueous slug (continuous phase) contains fluorescein (green color); the disperse phase is toluene; (
c) Spiral silicon/Pyrex microfluidic reactor designed for gold nanocrystal synthesis (400 μm channel width and depth, 100 μL reaction zone volume) [
33].
Figure 23.
(
a) Schematic of the segmented flow generation in a microfluidic reactor; (
b) Detail of segmented slugs generated at a residence time of 10 s. The aqueous slug (continuous phase) contains fluorescein (green color); the disperse phase is toluene; (
c) Spiral silicon/Pyrex microfluidic reactor designed for gold nanocrystal synthesis (400 μm channel width and depth, 100 μL reaction zone volume) [
33].
The spectra exhibit two resonance maxima, at 520 nm that corresponds to transverse plasmon resonance (TPR) of anisotropic particles and the second peak, usually obtained between 550 nm to near-IR wavelengths (1200 nm), represents the longitudinal plasmon resonance (LPR) of anisotropic particles. The width of the band determines the polydispersity in the particles.
Because of the rapid mixing in the discrete slugs, flow segmentation has proved to generate more uniformly sized nanoparticles than the continuous flow approach [
33]. By using a silicon/Pyrex microreactor (
Figure 23), Jensen
et al. demonstrated that the narrow size distribution of gold nanoparticles is due to the homogeneous mixing in two-phase flow systems [
33].
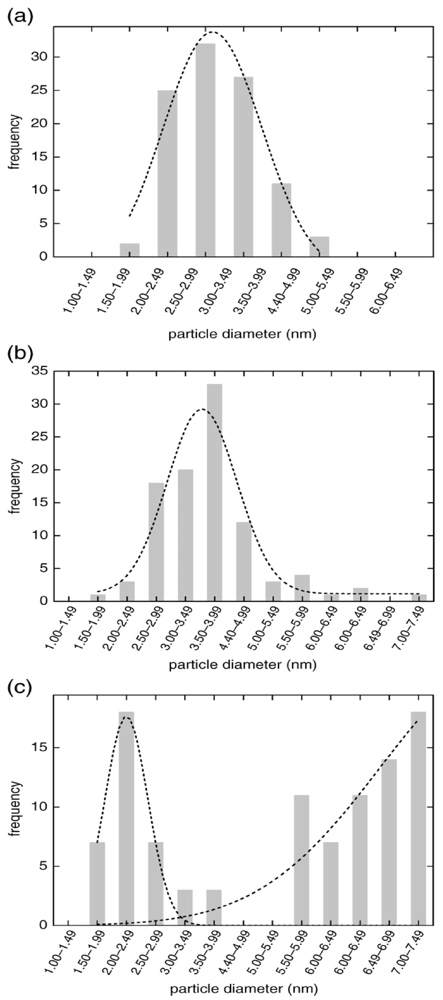
The system studied in this work is the rapid formation of gold nuclei with sodium borohydride, a very strong reducing agent. The particle size distribution diagram for three different reaction times shows that by using short times (10 s), a very narrow size distribution can be obtained when the two phases are carefully chosen.
It can be seen (
Figure 24d) that for a short reaction time (10 s) the particles are very small (less than 5 nm) and their size distribution is very narrow.
Figure 24.
TEM image of gold NPs synthesized in toluene-aqueous segmented flow: (a) [Au] = 1 mM; (b) Rt = 40 s; (c) Rt = 20 s; (d) Rt = 10 s. Particle size distribution diagram from AuNPs obtained in toluene-aqueous segemented flow at Rt = 40, 20, and 10 s, [Au] = 1 mM. Inset: gold NPs obtained in the toluene-aqueous phase, Rt = 10 s. Bottom: segmented slugs generated at Rt = 10 and 40 s (fluorescein was added to improve the optical resolution.
Figure 24.
TEM image of gold NPs synthesized in toluene-aqueous segmented flow: (a) [Au] = 1 mM; (b) Rt = 40 s; (c) Rt = 20 s; (d) Rt = 10 s. Particle size distribution diagram from AuNPs obtained in toluene-aqueous segemented flow at Rt = 40, 20, and 10 s, [Au] = 1 mM. Inset: gold NPs obtained in the toluene-aqueous phase, Rt = 10 s. Bottom: segmented slugs generated at Rt = 10 and 40 s (fluorescein was added to improve the optical resolution.
The examples shown in this section show some of the advantages of droplet-based methods. In spite of the complexity of the design and procedure, once the methodology well-established, the results in terms of size distribution are very gratifying.
3.2. Microfluidic-Assisted Synthesis of Polymeric Materials
Microfluidic synthesis of polymer particles provides the same advantages over the conventional methods as in the case of nanoparticles, that is, an improved control over their sizes, size distributions, morphologies as well as molecular weight distribution or average molecular weight of linear polymers [
34]. The batch methods, especially the emulsion and suspension polymerizations, totally lack of control of size and shape of particles and, composite materials cannot be prepared directly by using these methods. The convergence of particle technologies and microfluidics has enabled a considerable progress in the field of controlled synthesis of polymeric particles, especially important for applications such as painting formulation and drug delivery. However, before describing the use of microfluidic devices for polymer particle synthesis, the linear and branched polymers synthesis is briefly reviewed in this section.
Most of the work on the microfluidic synthesis of linear polymers was done by radical polymerization or copolymerization, initiated by thermal or photoinitiators [
35]. The authors (Wu
et al.) demonstrated the effect of the flow rate—that is the polymerization time—on the molecular mass of the polymer.
Cationic and anionic polymerization were also performed in a microfluidic format [
36]. There are two different methods for producing polymer particles in microfluidic devices: single-phase (continuous flow) and multiphase (droplet) flow [
4,
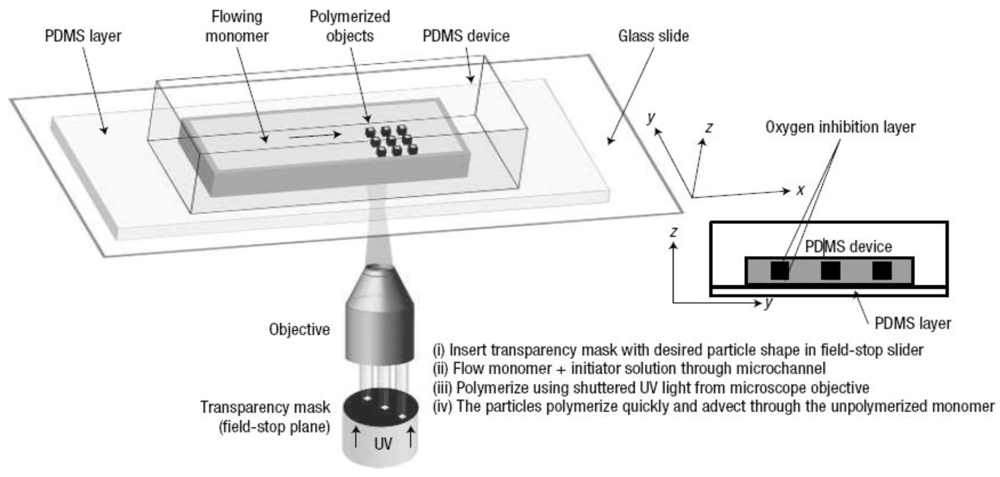
37]. The method of continuous flow (projection photolithography technique) developed by Dendukuri
et al. utilizes the objective of an optical microscope to UV irradiate through a mask, the solution flowing through the microchannel as shown in
Figure 25 [
38].
Figure 25.
Schematic representation of the continuous flow projection photolithography [
38].
Figure 25.
Schematic representation of the continuous flow projection photolithography [
38].
Through the mask, the desired particle shape can be “printed” into the monomer solution that will polymerize under the UV light, in the presence of a photo-initiator.
Monodispersed particles with diameters ranging from 20 to 1000 nm were generated by using a flow-focusing geometry by Whitesides
et al. The schematic of the flow-focusing geometry used for droplet formation is described by Kumacheva
et al. [
39].
In a recent work, size-tunable polymeric nanoparticles were synthesized by Langer
et al. in a novel 3D flow focusing in both the horizontal and the vertical dimensions [
40,
41].
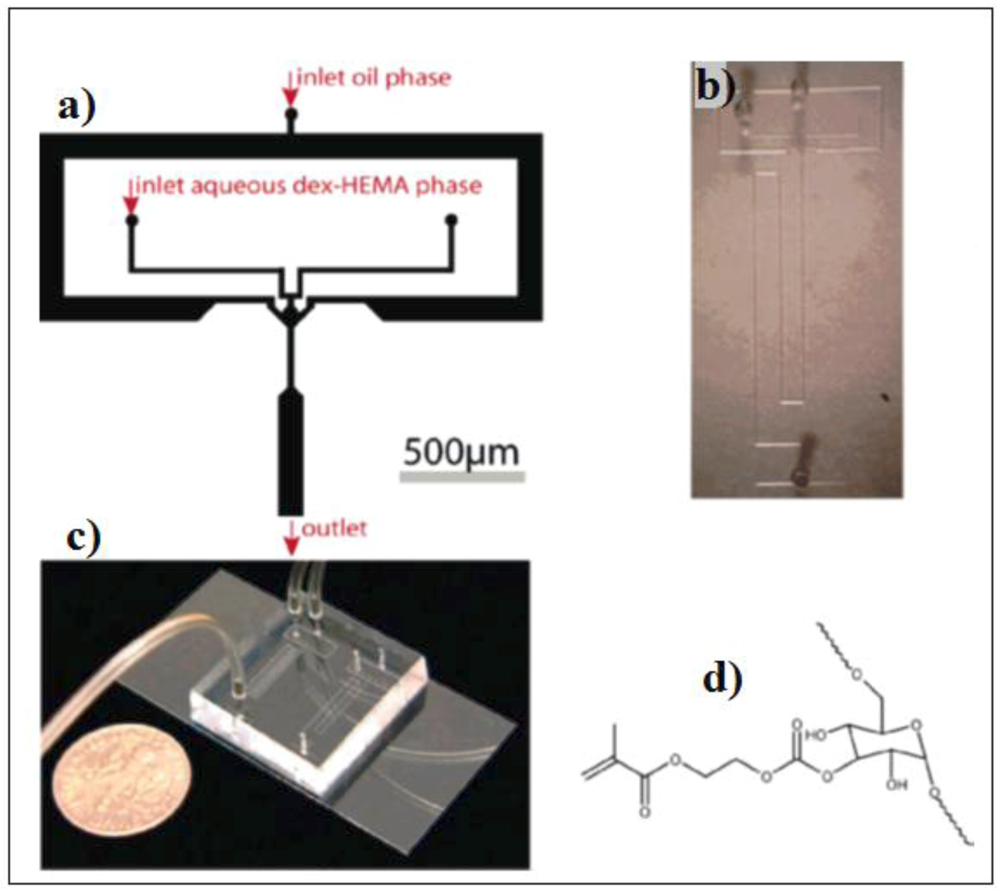
PLGA-PEG nanoparticles were synthesized by hydrodynamic flow focusing in a controlled nanoprecipitation process.
The figure below (
Figure 26) shows the microfluidic device as well as the TEM image of nanoparticles.
Figure 26.
Nanoprecipitation by hydrodynamic flow focusing. (
a) A microfluidic device for hydrodynamic flow focusing of polymeric nanoparticles in water, Scale bar 50 μm; (
b) TEM image of nanoparticles synthesized by nanoprecipitation of PLGA15K-PEG3.4K by hydrodynamic flow focusing showing spherical PLGA core-PEG corona structure of the nanoparticles [
41].
Figure 26.
Nanoprecipitation by hydrodynamic flow focusing. (
a) A microfluidic device for hydrodynamic flow focusing of polymeric nanoparticles in water, Scale bar 50 μm; (
b) TEM image of nanoparticles synthesized by nanoprecipitation of PLGA15K-PEG3.4K by hydrodynamic flow focusing showing spherical PLGA core-PEG corona structure of the nanoparticles [
41].
The authors showed that microfluidics may be used to tune the nanoparticle size and drug loading and release.
The polymer particles synthesized within the microfluidic device shown in the figure, are composed of poly(lactide-co-glycolide)-b-polyethyleneglycol (PLGA-PEG) block copolymers, a model biomaterial for drug delivery and, because they are biodegradable and biocompatible, are suitable for controlled drug release for therapeutic applications. The authors showed that the polydispersity of the small nanoparticles (30–230 nm), made with different concentrations of polymer precursor and molecular weights, has been found very low when 3D flow focusing was used. Flow focusing enables the rapid mixing of the polymer solutions with water. Drug loading and release of the resulting nanoparticles can be precisely controlled.
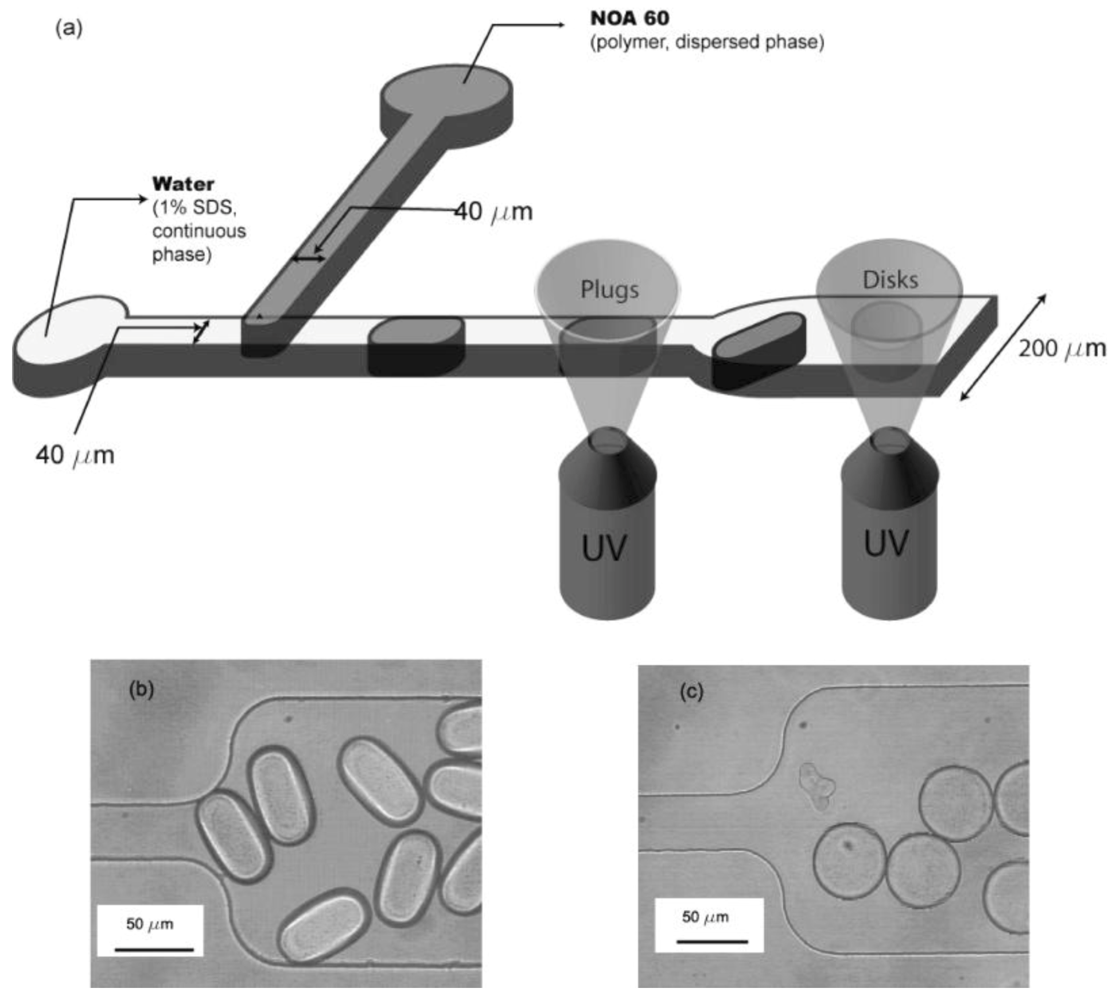
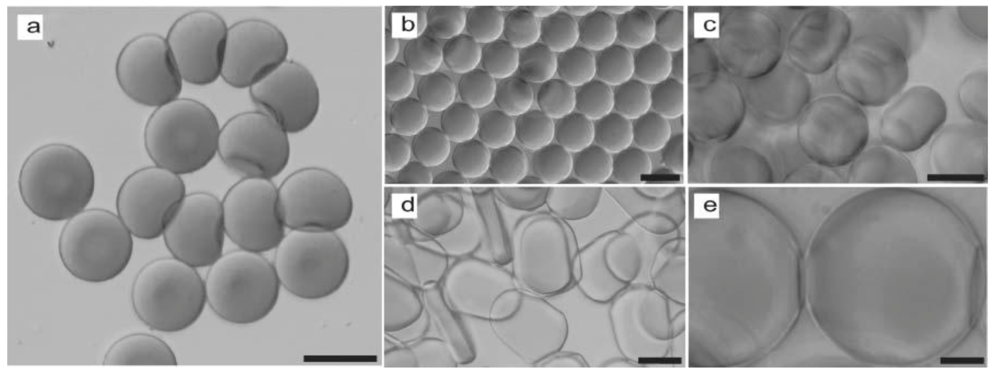
Non-spherical microparticles (plugs and disks) as shown in
Figure 27, were synthesized in a microfluidic device by Dendukuri
et al. [
42].
By using appropriate channel geometries, curable polymer droplets formed at a T-junction, are constrained to adopt nonspherical shapes and are subsequently, irradiated with UV light to photopolymerize them and preserve the shapes. Two microchannels with different heights are used to form the droplets having the two shapes, 38 µm for the plugs and 16 µm for the disks.
As seen in
Figure 28, the non-spherical polymer particles (larger than 10 µm), fabricated through this method, are monodisperse plugs and disks. By varying the flow rates of the two phases, the dimensions of the two nonspherical microparticles can be tuned.
Figure 27.
Microchannel geometry used to create plugs and disks: (
a) schematic of channel with plug and disk creation zones marked; (
b) polymerized plugs in the 200 µm section of the channel, 38 µm height; (
c) polymerized disks in the 200 µm section of the channel, 16 µm height [
42].
Figure 27.
Microchannel geometry used to create plugs and disks: (
a) schematic of channel with plug and disk creation zones marked; (
b) polymerized plugs in the 200 µm section of the channel, 38 µm height; (
c) polymerized disks in the 200 µm section of the channel, 16 µm height [
42].
Figure 28.
SEM images of non-spherical colloids formed under varying conditions (
a) plug formed at
Qd = 0.05
íL/min and
Ca = 1.6 × 10
−3; (
b) disk formed at
Qd = 0.05 µL/min and
Ca = 4.8 × 10
−3; (
c) collection of plugs formed at
Qd = 0.05 µL/min and
Ca = 1.6 × 10
−3; (
d) collection of disks formed at
Qd = 0.05 µL/min and
Ca = 9.6 × 10
−3 [
43].
Figure 28.
SEM images of non-spherical colloids formed under varying conditions (
a) plug formed at
Qd = 0.05
íL/min and
Ca = 1.6 × 10
−3; (
b) disk formed at
Qd = 0.05 µL/min and
Ca = 4.8 × 10
−3; (
c) collection of plugs formed at
Qd = 0.05 µL/min and
Ca = 1.6 × 10
−3; (
d) collection of disks formed at
Qd = 0.05 µL/min and
Ca = 9.6 × 10
−3 [
43].
3.3. Polymer Microparticles/Inorganic Nanoparticle Composite Materials (Multiscale Materials)
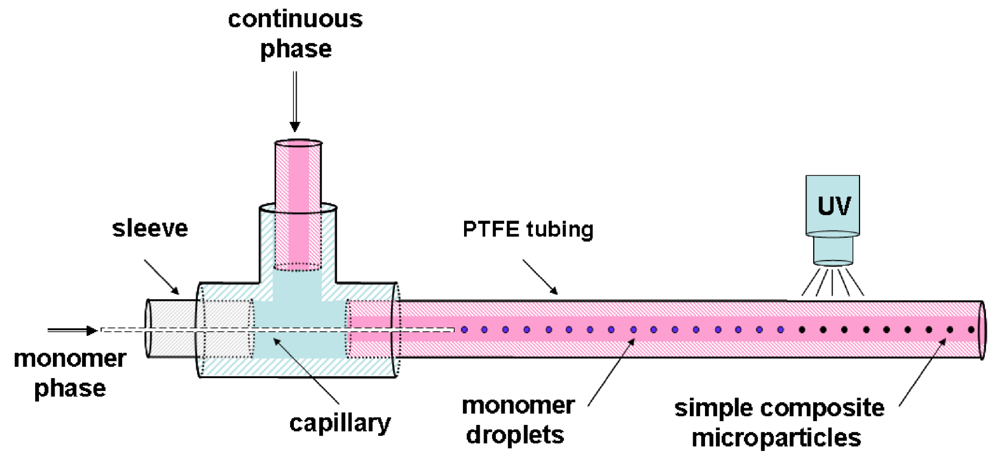
Recently, a new microfluidic route allowing the incorporation of inorganic nanoparticles in polymer microparticles was reported by Köhler
et al. [
43].
Simple composite microparticles have been fabricated in the capillary-based system shown in
Figure 29.
Figure 29.
Schematic drawing of the capillary-based system for the production of simple composite [
43].
Figure 29.
Schematic drawing of the capillary-based system for the production of simple composite [
43].
In this work, Au and ZnO nanoparticles are synthesized previously in a microfluidic device and, in a second step, integrated into the polymer. The monomer phase was a mixture of an acrylate-based monomer, a photoinitiator and the gold nanoparticle (or ZnO) solution in toluene.
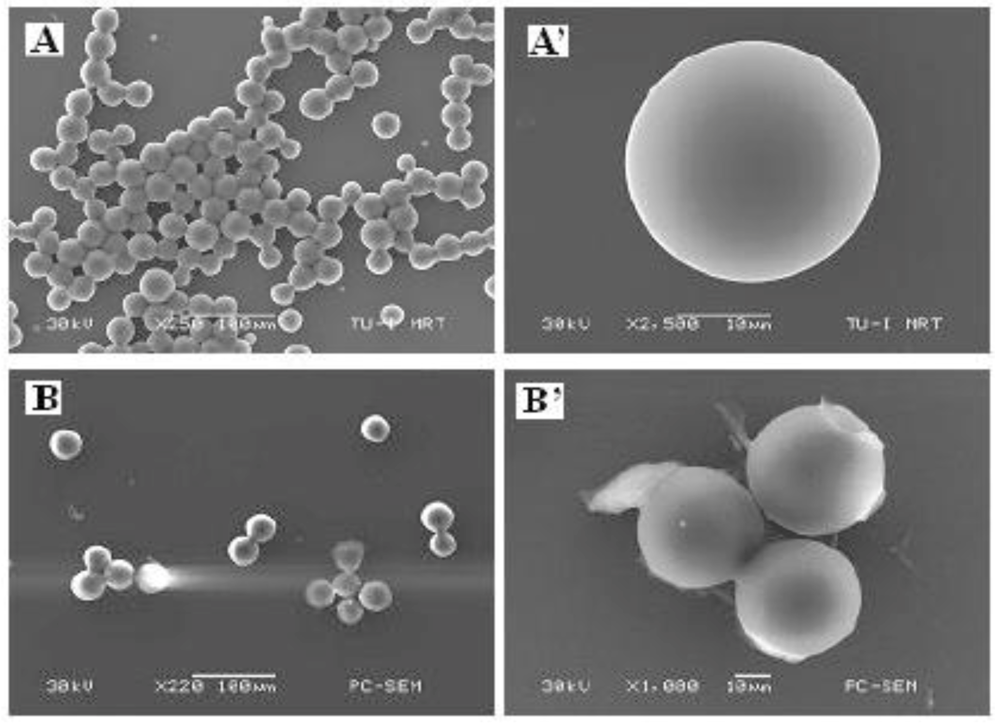
The droplets are hardened through thermal- or UV-induced polymerization. The polymer particles are in the size range of a few to hundreds of micrometers but the size distribution was found very narrow as shown in
Figure 30.
Figure 30.
Micrographs of nano-Au/Poly(TPGDA) microparticles (A,A’) and nano-ZnO/Poly(TPGDA) microparticles (B,B’) at two different magnifications [
43].
Figure 30.
Micrographs of nano-Au/Poly(TPGDA) microparticles (A,A’) and nano-ZnO/Poly(TPGDA) microparticles (B,B’) at two different magnifications [
43].
Au nanoparticles with a size around 13 nm are found to be dispersed randomly in the polymer matrix. The micrograph shows that ZnO nanoparticles are concentrated in the central part of the polymer bead.
The synthesis of composite materials demonstrated a good control over the size and size distribution.
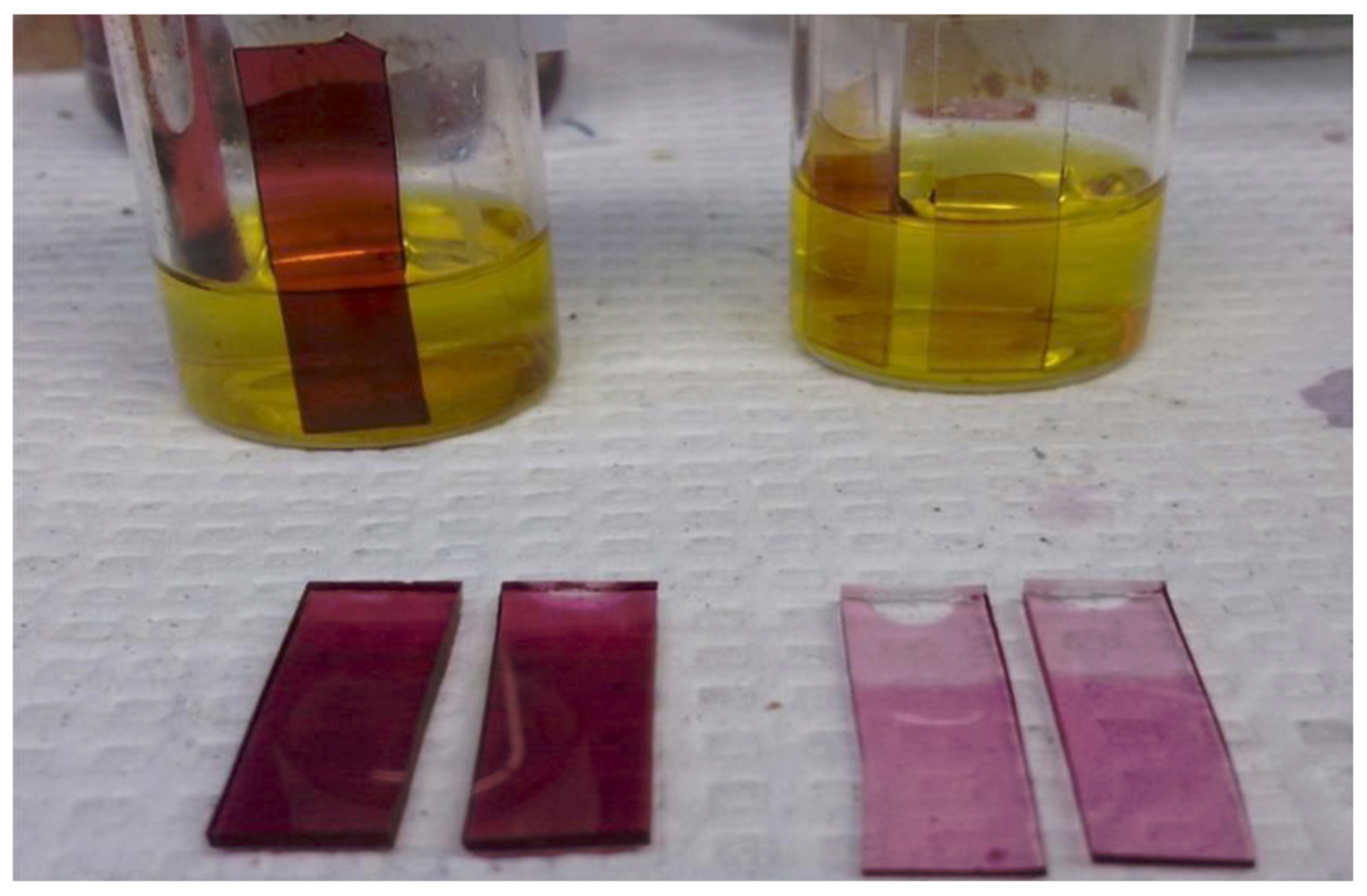
In the authors’ laboratory, gold-PDMS nanocomposite was fabricated both at the macro scale (
Figure 31) and in the channel, by introducing a gold chloride solution directly in the channel of a PDMS microfluidic device as shown in
Figure 32.
Figure 31.
Fabrication of Au-PDMS at the macro-scale.
Figure 31.
Fabrication of Au-PDMS at the macro-scale.
Figure 32.
Fabrication of Au—PDMS nanocomposite through the reduction of Au ions inside the microfluidic channel. PDMS microfluidic device. (
a) Dependence of the absorbance of Gold LSPR band on the time of reaction; and (
b) SEM image of Au nanoparticles formed in the channel [
44].
Figure 32.
Fabrication of Au—PDMS nanocomposite through the reduction of Au ions inside the microfluidic channel. PDMS microfluidic device. (
a) Dependence of the absorbance of Gold LSPR band on the time of reaction; and (
b) SEM image of Au nanoparticles formed in the channel [
44].
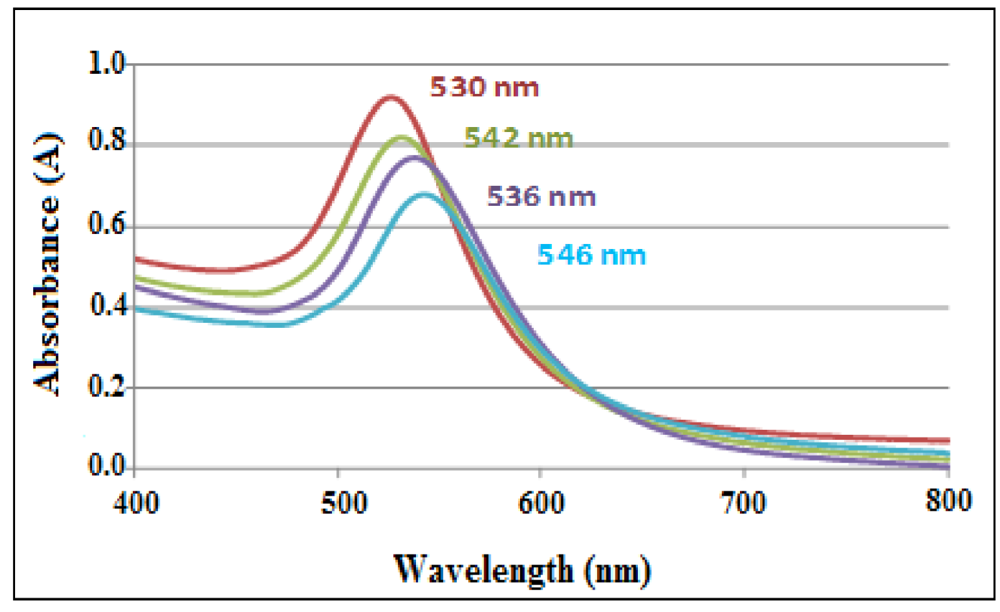
By immersing a piece of PDMS in a gold chloride solution, the gold ions are reduced by the curing agent in the polymer and the gold nanoparticles formed by reduction are embedded in the surface layers of the polymer. The figure shows that the absorbance of the Au Localized Surface Plasmon Resonance band (LSPR) increases when the solution is left in the channel for a longer time (
Figure 33).
Figure 33.
Plasmon band of Au in the nanocomposite corresponding to different stages of the immunoassay.
Figure 33.
Plasmon band of Au in the nanocomposite corresponding to different stages of the immunoassay.
Sensing experiments carried out with a Au-PDMS platform, demonstrated the sensitivity of the gold-PDMS nanocomposite to antigen-antibody interactions carried out in an immunoassay format.
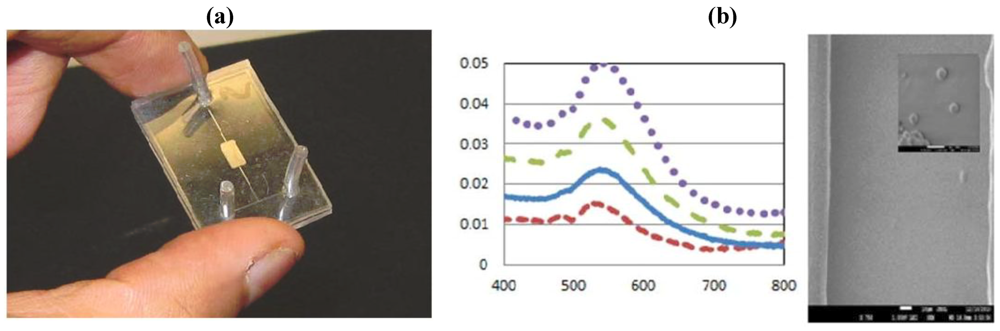
The device shown in
Figure 34 was used for biosensing of a hormone polypeptide molecule by using an immunosensing format. Gold nanoparticles were functionalized by pumping a linker molecule solution through the channel and, subsequently, adsorbing the antibody and the antigen onto the immobilized gold nanoparticles. By using the shift of the AuLSPR band, the antigen can be quantified.
Figure 34.
(
a) SEM image of gold nanoparticles embedded into the channel and (
b) photograph of the device [
45].
Figure 34.
(
a) SEM image of gold nanoparticles embedded into the channel and (
b) photograph of the device [
45].
However, the outlook is promising; the direct use of microfluidics for biosensing is still in an incipient stage. For the time being, only the continuous flow microfluidics is used for biosensing, droplet-based methods are not yet developed for this application. For the development of analytical microfluidic chip, new functionalization methods have to be developed.
Multi-functional polycaprolactone size-controlled microcapsules entrapping an anticancer drug, together with fluorescent and supermagnetic nanoparticles, were produced by microfluidic emulsification by Chang
et al. [
46]. This work demonstrates a proof-of-concept approach for fabrication of multifunctional materials. The drug delivery system enabled magnetic targeting through the super paramagnetic nanoparticles, fluorescence imaging through QDs and drug controlled release properties.
Microfluidics offers new strategies for fabricating polymer particles. With traditional methods it is difficult to prepare monodisperse spherical or non-spherical polymer microparticles. To be used in commercial applications, the microfluidic production of polymer particles will have to be scaled up. In addition, efforts will have to be made to synthesize nanometer-sized polymer particles as well.