Synthesis and Properties of 1,8-Carbazole-Based Conjugated Copolymers
Abstract
:1. Introduction
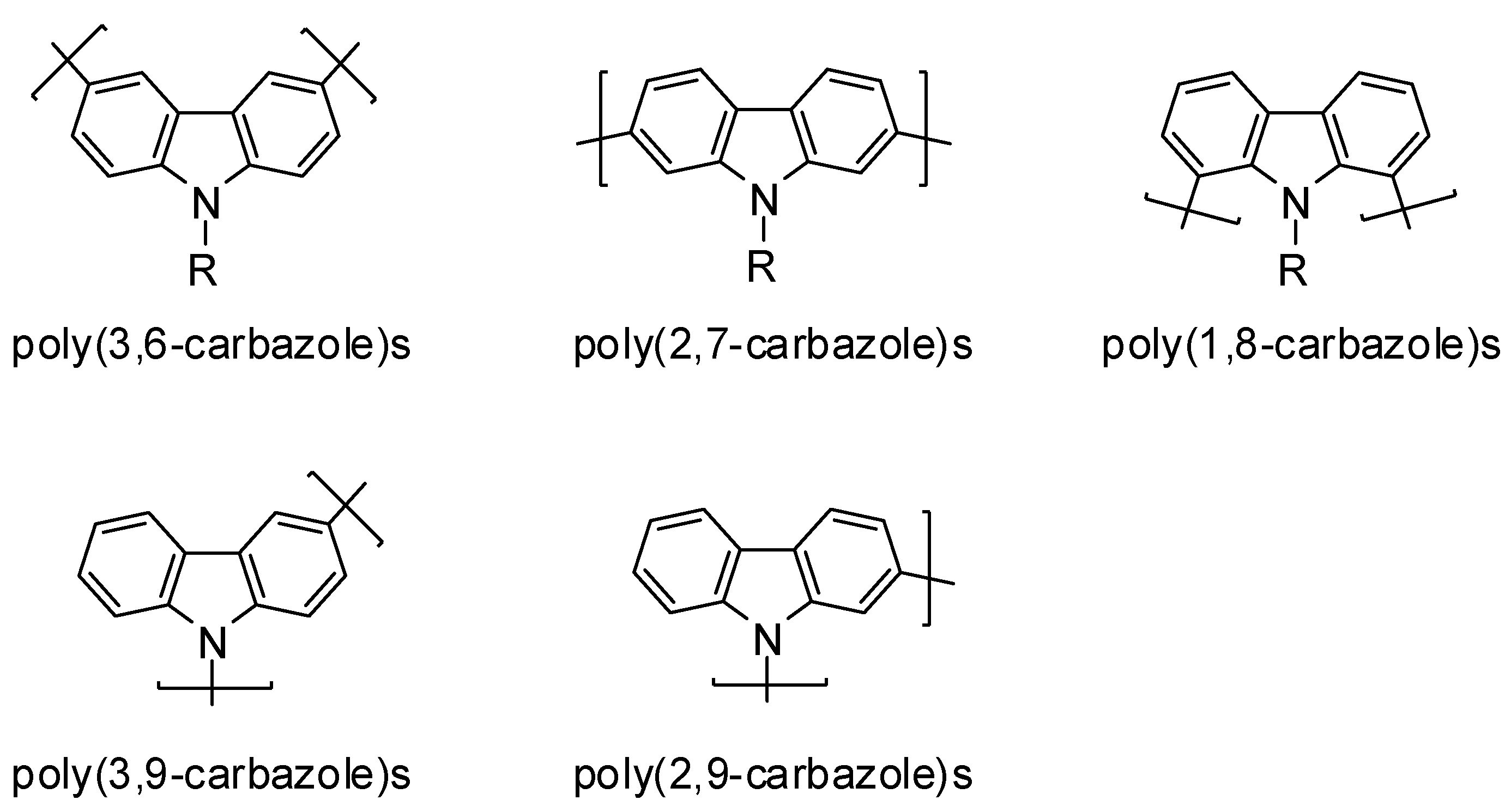
2. Results and Discussion
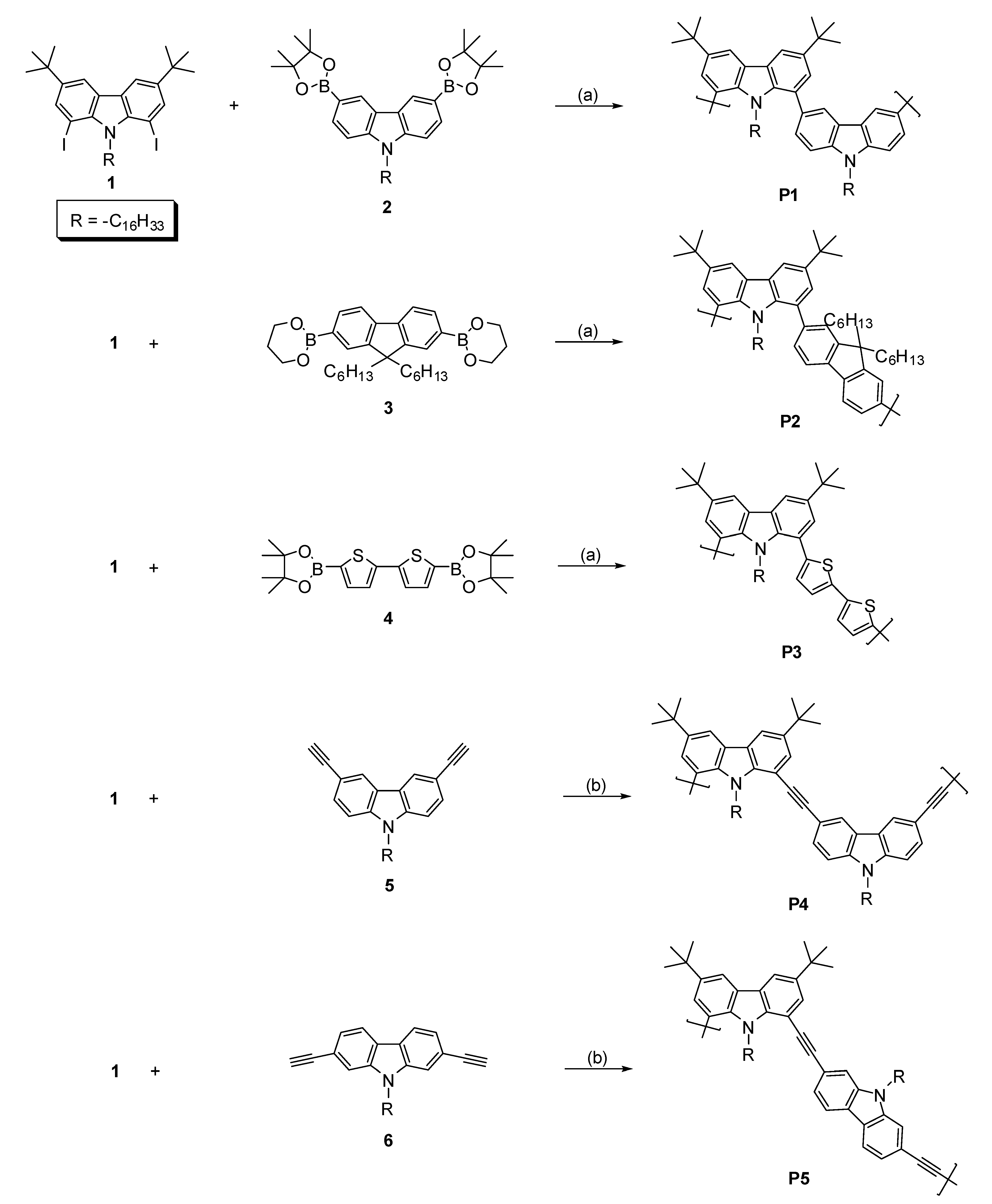
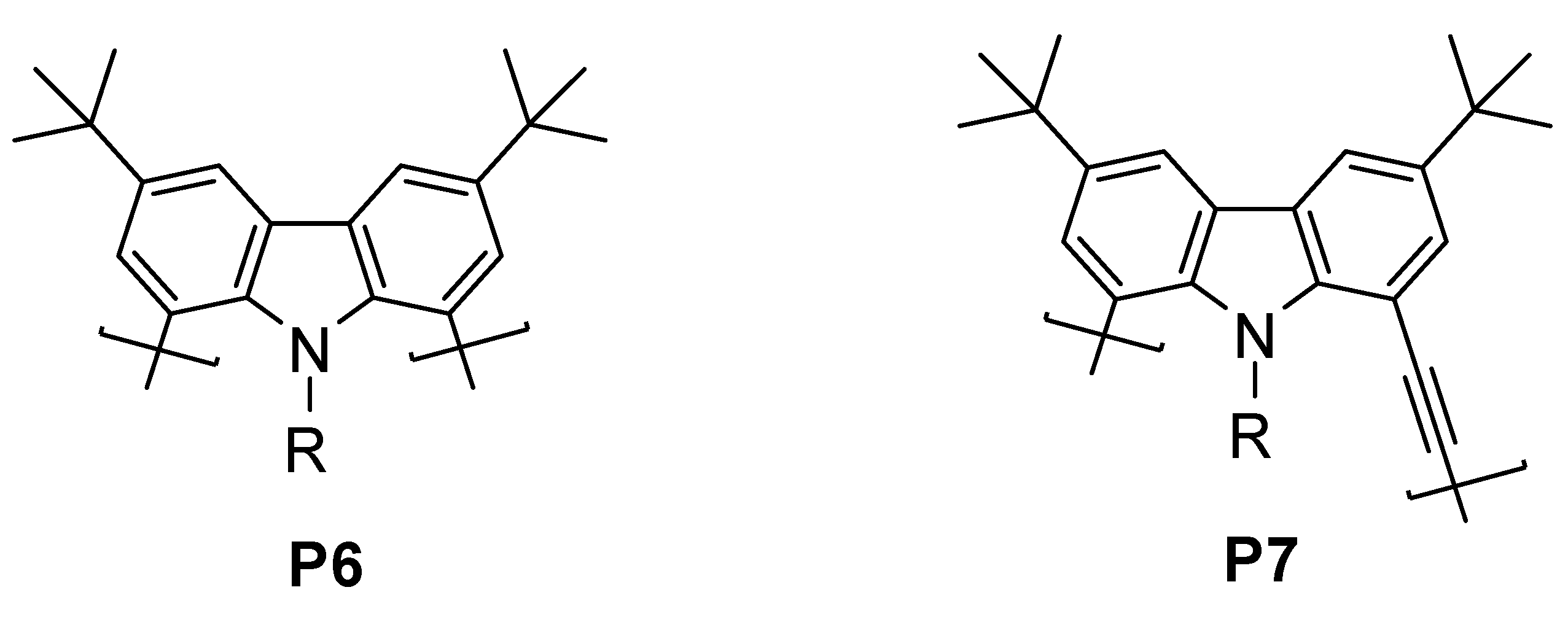
2.1. Polymer Synthesis
| Polymer | Yield (%) | Mw a | Mn a | Mw/Mn a | T5% (°C) b | Tg (°C) c |
|---|---|---|---|---|---|---|
| P1 | 28 | 1,400 | 1,300 | 1.1 | 259 | - |
| P2 | 61 | 1,700 | 1,400 | 1.2 | 276 | - |
| P3 | 15 | 2,000 | 1,800 | 1.1 | 276 | 63 |
| P4 | 99 | 6,900 | 4,300 | 1.6 | 340 | 77 |
| P5 | 100 | 13,000 | 7,200 | 1.8 | 380 | 80 |
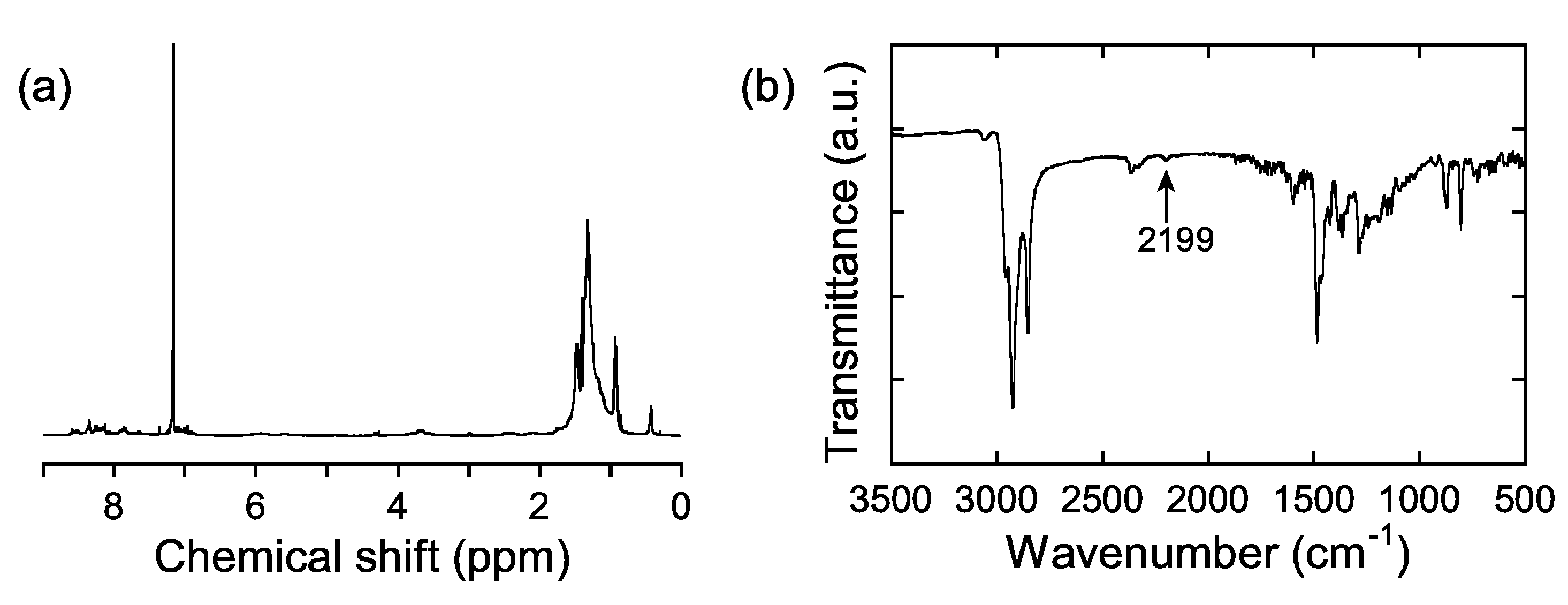
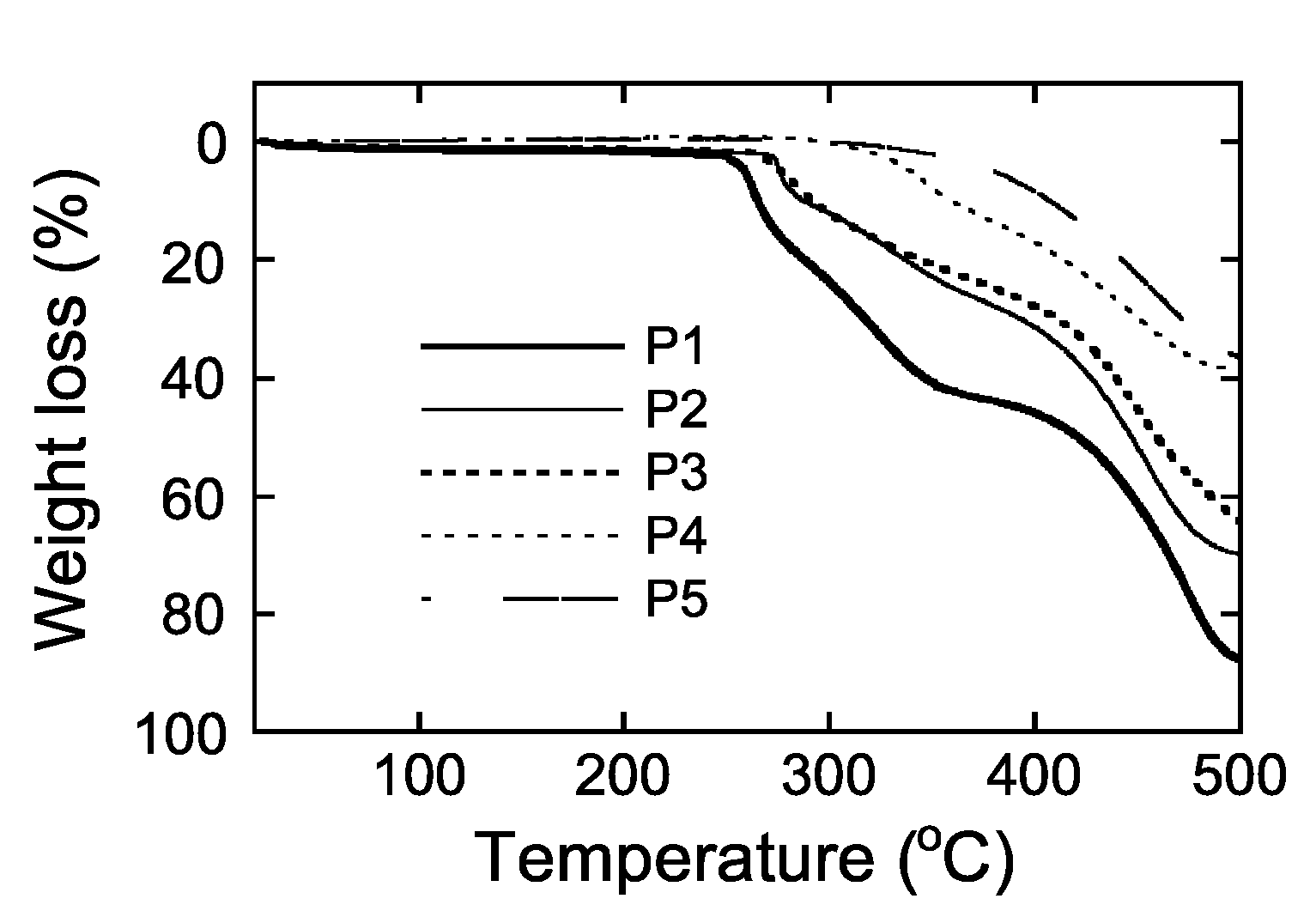
2.2. Thermal Properties
2.3. Electrochemistry
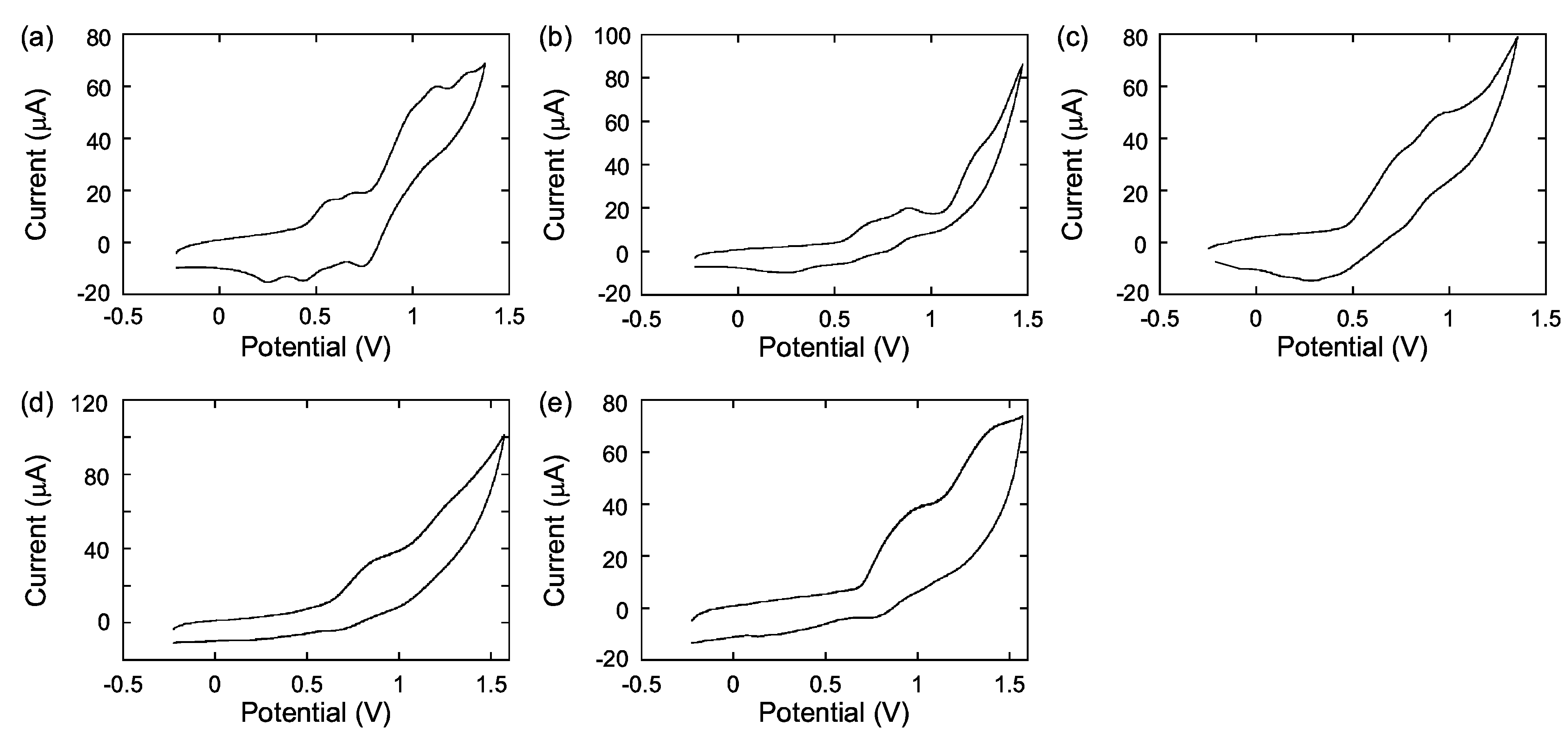
| in CH2Cl2 b | Film c | ||||||
|---|---|---|---|---|---|---|---|
| polymer | Eonset (V) | E○’ (V) d | Epa (V) d | Epc (V) d | Eonset (V) | Epa (V) | |
| P1 | 0.44 | 0.41 | 0.57 | 0.25 | 0.60 | 0.65 | |
| 0.58 | 0.70 | 0.45 | 0.83 | ||||
| 0.87 | 0.98 | 0.76 | 0.92 | ||||
| 1.12 | 1.31 | ||||||
| 1.28 | |||||||
| P2 | 0.55 | 0.48 | 0.67 | 0.29 | 0.66 | 0.71 | |
| 0.73 | 0.88 | 0.58 | 1.03 | ||||
| 1.22 | 1.27 | ||||||
| P3 | 0.48 | 0.51 | 0.73 | 0.30 | 0.75 | 1.11 | |
| 0.86 | 0.94 | 0.78 | |||||
| P4 | 0.60 | 0.77 | 0.86 | 0.67 | 0.55 | 0.68 | |
| 1.15 | 1.25 | 1.05 | 0.91 | ||||
| P5 | 0.69 | 0.90 | 1.03 | 0.76 | 0.70 | 0.80 | |
| 1.41 | 0.97 | ||||||
2.4. UV-vis and Fluorescence Spectroscopies
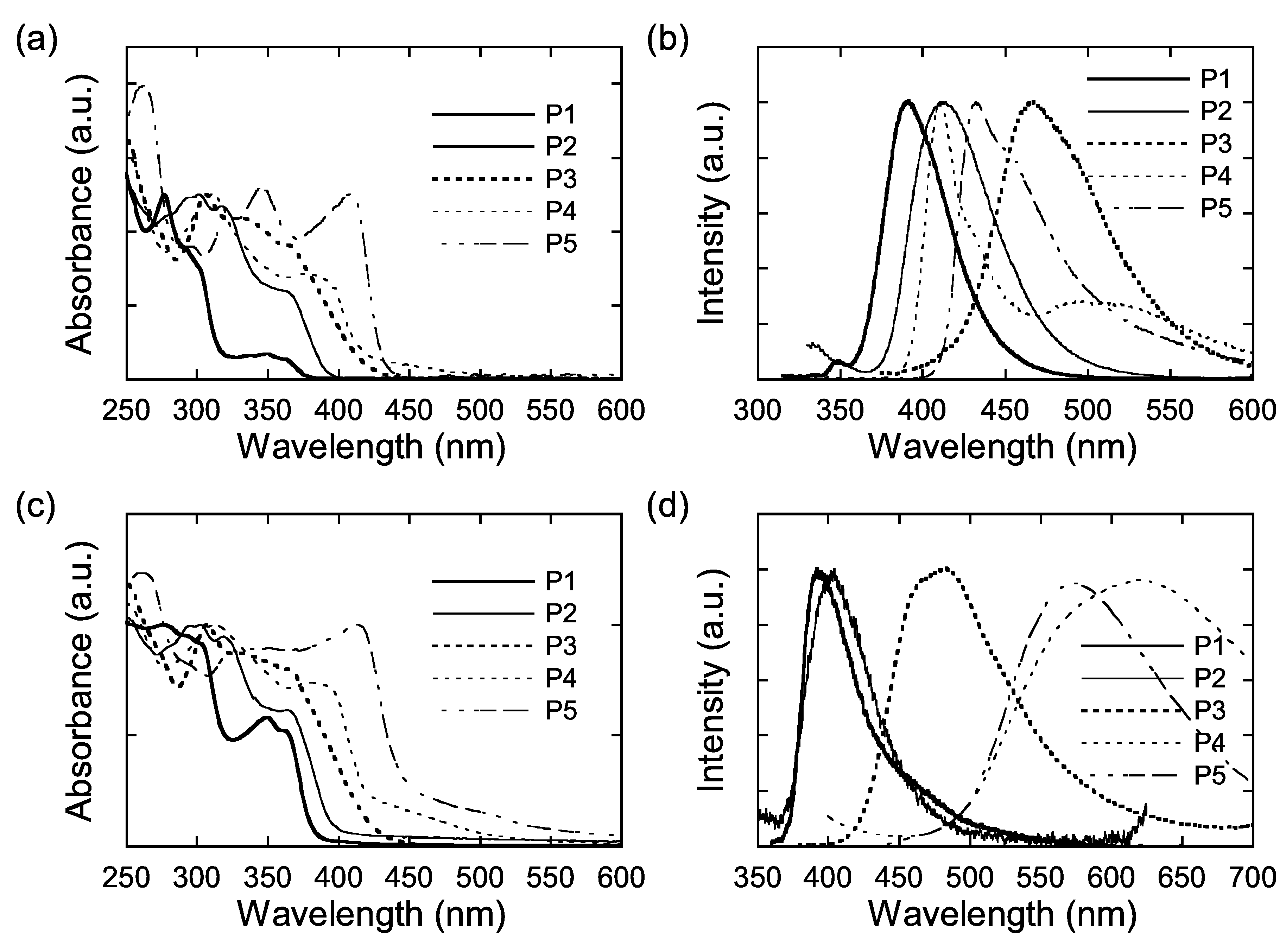
| in CH2Cl2 | Film | |||||
|---|---|---|---|---|---|---|
| polymer | λmax (nm) | λem (nm) [λex (nm)] | Stokes shift (cm−1) | Φf,sol a | λmax (nm) | λem (nm) [λex (nm)] |
| P1 | 362 | 392 [302] | 2114 | 0.250 | 362 | 392 [350] |
| P2 | 366 | 412 [317] | 3050 | 0.419 | 364 | 404 [319] |
| P3 | 366 | 467 [366] | 5909 | 0.125 | 366 | 486 [366] |
| P4 | 378 | 410, 497 [315] | 2065 | 0.352 | 380 | 616 [380] |
| P5 | 408 | 432, 457b [348] | 1362 | 0.496 | 414 | 569 [414] |

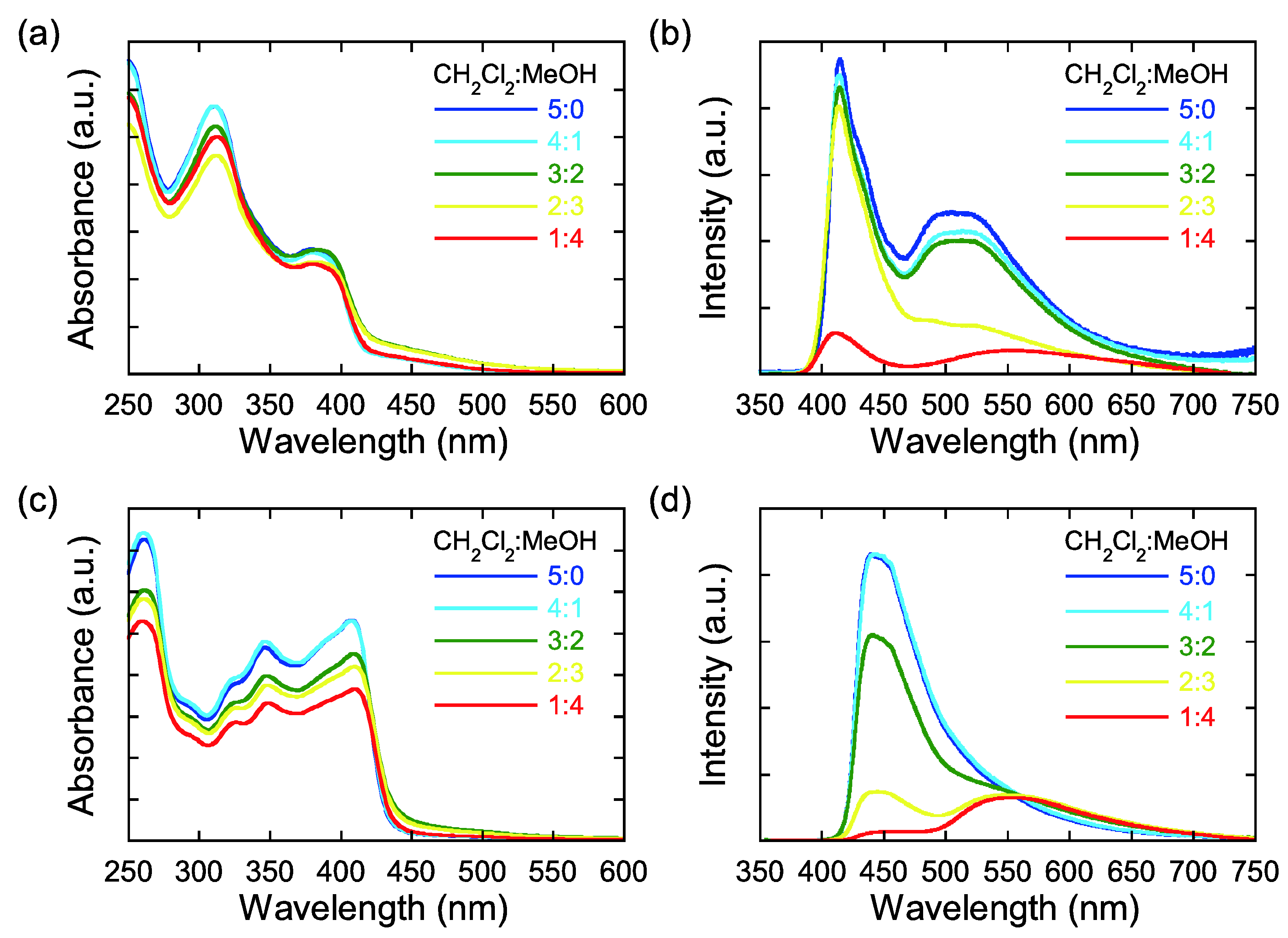
3. Experimental Section
3.1. Materials
3.2. Measurements
4. Conclusions
Acknowledgements
References and Notes
- Grazulevicius, J.V.; Strohriegl, P.; Pielichowski, J.; Pielichowski, K. Carbazole-containing polymers: Synthesis, properties and applications. Prog. Polym. Sci. 2003, 28, 1297–1353. [Google Scholar] [CrossRef]
- Wakim, S.; Aïch, B.-R.; Tao, Y.; Leclerc, M. Charge transport, photovoltaic, and thermoelectric properties of poly(2,7-carbazole) and poly(indolo[3,2-b]carbazole) derivatives. Polym. Rev. 2008, 48, 432–462. [Google Scholar] [CrossRef]
- Tao, X.-T.; Zhang, Y.-D.; Wada, T.; Sasabe, H.; Suzuki, H.; Watanabe, T.; Miyata, S. Hyperbranched polymers for electroluminescence applications. Adv. Mater. 1998, 10, 226–230. [Google Scholar]
- Zhang, Z.-B.; Fujiki, M.; Tang, H.-Z.; Motonaga, M.; Torimitsu, K. The first high molecular weight poly(n-alkyl-3,6-carbazole)s. Macromolecules 2002, 35, 1988–1990. [Google Scholar]
- Zhang, Z.-B.; Motonaga, M.; Fujiki, M.; McKenna, C.E. The first optically active polycarbazoles. Macromolecules 2003, 36, 6956–6958. [Google Scholar] [CrossRef]
- Liou, G.-S.; Hsiao, S.-H.; Chen, H.-W. Novel high-Tg poly(amine-imide)s bearing pendent N-phenylcarbazole units: synthesis and photophysical, electrochemical and electrochromic properties. J. Mater. Chem. 2006, 16, 1831–1842. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Sakaguchi, K.; Kasuga, H.; Kawakami, A.; Taka, H.; Kita, H.; Ogino, K. Synthesis of charge transporting block copolymers containing 2,7-dimethoxycarbazole units for light emitting device. Polymer 2010, 51, 616–622. [Google Scholar] [CrossRef]
- Morin, J.-F.; Leclerc, M. Syntheses of conjugated polymers derived from N-Alkyl-2,7-carbazoles. Macromolecules 2001, 34, 4680–4682. [Google Scholar] [CrossRef]
- Dierschke, F.; Grimsdale, A.C.; Müllen, K. Efficient synthesis of 2,7-dibromocarbazoles as components for electroactive materials. Synthesis 2003, 16, 2470–2472. [Google Scholar]
- Blouin, N.; Leclerc, M. Poly(2,7-carbazole)s: Structure-property relationships. Acc. Chem. Res. 2008, 41, 1110–1119. [Google Scholar] [CrossRef]
- Boudreault, P.-L.; Blouin, N.; Leclerc, M. Poly(2,7-carbazole)s and related polymers. Adv. Polym. Sci. 2008, 212, 99–124. [Google Scholar]
- Leclerc, N.; Michaud, A.; Sirois, K.; Morin, J.-F.; Leclerc, M. Synthesis of 2,7-carbazolenevinylene-based copolymers and characterization of their photovoltaic properties. Adv. Funct. Mater. 2006, 16, 1694–1704. [Google Scholar] [CrossRef]
- Blouin, N.; Michaud, A.; Leclerc, M. A low-bandgap poly(2,7-carbazole) derivative for use in high-performance solar cells. Adv. Mater. 2007, 19, 2295–2300. [Google Scholar] [CrossRef]
- Blouin, N.; Michaud, A.; Gendron, D.; Wakim, S.; Blair, E.; Neagu-Plesu, R.; Belletête, M.; Durocher, G.; Tao, Y.; Leclerc, M. Toward a rational design of poly(2,7-carbazole) derivatives for solar cells. J. Am. Chem. Soc. 2008, 130, 732–742. [Google Scholar] [CrossRef]
- Zou, Y.; Gendron, D.; Badrou-Aïch, R.; Najari, A.; Tao, Y.; Leclerc, M. A high-mobility low-bandgap poly(2,7-carbazole) derivative for photovoltaic applications. Macromolecules 2009, 42, 2891–2894. [Google Scholar] [CrossRef]
- Park, S.H.; Roy, A.; Beaupré, S.; Cho, S.; Coates, N.; Moon, J.S.; Moses, D.; Leclerc, M.; Lee, K.; Heeger, A.J. Bulk heterojunction solar cells with internal quantum efficiency approaching 100%. Nat. Photonics 2009, 3, 297–303. [Google Scholar] [CrossRef]
- Wu, C.-W.; Lin, H.-C. Synthesis and characterization of kinked and hyperbranched carbazole/fluorene-based copolymers. Macromolecules 2006, 39, 7232–7340. [Google Scholar] [CrossRef]
- Tamura, K.; Shiotsuki, M.; Kobayashi, N.; Masuda, T.; Sanda, F. Synthesis of highly conjugated poly(3,9-carbazolyleneethynylenearylene)s emitting variously colored fluorescence. Polymer 2009, 50, 4479–4487. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Shiotsuki, M.; Kobayashi, N.; Masuda, T.; Sanda, F. Synthesis and properties of conjugated polymers containing 3,9- and 2,9-linked carbazole units in the main chain. J. Polym. Sci. A 2009, 47, 3509–3517. [Google Scholar] [CrossRef]
- Sasano, T.; Sogawa, H.; Tamura, K.; Shiotsuki, M.; Masuda, T.; Sanda, F. Synthesis and properties of conjugated polymers containing 3,9-carbazolylene and silylenevinylene moieties in the main chain. J. Polym. Sci. A 2010, 48, 1815–1821. [Google Scholar] [CrossRef]
- Michinobu, T.; Kumazawa, H.; Shigehara, K. Nitrogen-linked aromatic poly(2,7-carbazole)s: Partially annulated poly(m-aniline)s. Chem. Lett. 2007, 36, 620–621,. [Google Scholar] [CrossRef]
- Michinobu, T.; Okoshi, K.; Osako, H.; Kumazawa, H.; Shigehara, K. Synthesis and optical properties of carbazole-containing donor-acceptor type conjugated polymers. J. Photopolym. Sci. Technol. 2007, 20, 255–256. [Google Scholar] [CrossRef]
- Michinobu, T.; Okoshi, K.; Osako, H.; Kumazawa, H.; Shigehara, K. Band-gap tuning of carbazole-containing donor-acceptor type conjugated polymers by acceptor moieties and π-spacer groups. Polymer 2008, 49, 192–199. [Google Scholar] [CrossRef]
- Michinobu, T.; Kumazawa, H.; Otsuki, E.; Usui, H.; Shigehara, K. Synthesis and properties of nitrogen-linked poly(2,7-carbazole)s as hole-transport material for organic light emitting diodes. J. Polym. Sci. A 2009, 47, 3880–3891. [Google Scholar] [CrossRef]
- Michinobu, T.; Osako, H.; Shigehara, K. Alkyne-linked poly(1,8-carbazole)s: A novel class of conjugated carbazole polymers. Macromol. Rapid Commun. 2008, 29, 111–116. [Google Scholar] [CrossRef]
- Michinobu, T.; Osako, H.; Murata, K.; Shigehara, K. Blue, green, and red light emission of 1,8-carbazole-based conjugated polymers. Chem. Lett. 2010, 39, 168–169. [Google Scholar] [CrossRef]
- Michinobu, T.; Osako, H.; Shigehara, K. Synthesis of properties of conjugated poly(1,8-carbazole)s. Macromolecules 2009, 42, 8172–8180. [Google Scholar] [CrossRef]
- Morin, J.-F.; Leclerc, M.; Adès, D.; Siove, A. Polycarbazoles: 25 years of progress. Macromol. Rapid Commun. 2005, 26, 761–778. [Google Scholar] [CrossRef]
- Halkyard, C.E.; Rampey, M.E.; Kloppenburg, L.; Studer-Martinez, S.L.; Bunz, U.H.F. Evidence of aggregate formation for 2,5-dialkylpoly(p-phenyleneethynylenes) in solution and thin films. Macromolecules 1998, 31, 8655–8659. [Google Scholar] [CrossRef]
- Bouchard, J.; Belletête, M.; Durocher, G.; Leclerc, M. Solvatochromic properties of 2,7-carbazole-based conjugated polymers. Macromolecules 2003, 36, 4624–4630. [Google Scholar] [CrossRef]
- Liu, R.-R.; Lin, Z.-Q.; Shi, N.-E.; Zhao, J.-F.; Hou, X.-Y.; Qian, Y.; Xie, L.-H.; Huang, W. Synthesis and characterization of 1,8-carbazole-based π-conjugated copolymer with zigzagged conformation for stable deep-blue emission. Chem. Lett. 2010, 39, 522–523. [Google Scholar] [CrossRef]
- Zhao, T.; Liu, Z.; Song, Y.; Xu., W.; Zhang, D.; Zhu, D. Novel diethynylcarbazole macrocycles: synthesis and optoelectronic properties. J. Org. Chem. 2006, 71, 7422–7432. [Google Scholar] [CrossRef]
- Iraqi, A.; Wataru, I. 3,6-Linked 9-alkyl-9H-carbazole main-chain polymers: Preparation and properties. J. Polym. Sci. A 2004, 42, 6041–6051. [Google Scholar] [CrossRef]
- Eaton, D.F. Reference materials for fluorescence measurement. Pure Appl. Chem. 1988, 60, 1107–1114. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Michinobu, T.; Osako, H.; Shigehara, K. Synthesis and Properties of 1,8-Carbazole-Based Conjugated Copolymers. Polymers 2010, 2, 159-173. https://doi.org/10.3390/polym2030159
Michinobu T, Osako H, Shigehara K. Synthesis and Properties of 1,8-Carbazole-Based Conjugated Copolymers. Polymers. 2010; 2(3):159-173. https://doi.org/10.3390/polym2030159
Chicago/Turabian StyleMichinobu, Tsuyoshi, Haruka Osako, and Kiyotaka Shigehara. 2010. "Synthesis and Properties of 1,8-Carbazole-Based Conjugated Copolymers" Polymers 2, no. 3: 159-173. https://doi.org/10.3390/polym2030159




