Intelligent Polymeric Nanocarriers Responding to Physical or Biological Signals: A New Paradigm of Cytosolic Drug Delivery for Tumor Treatment
Abstract
:1. Introduction
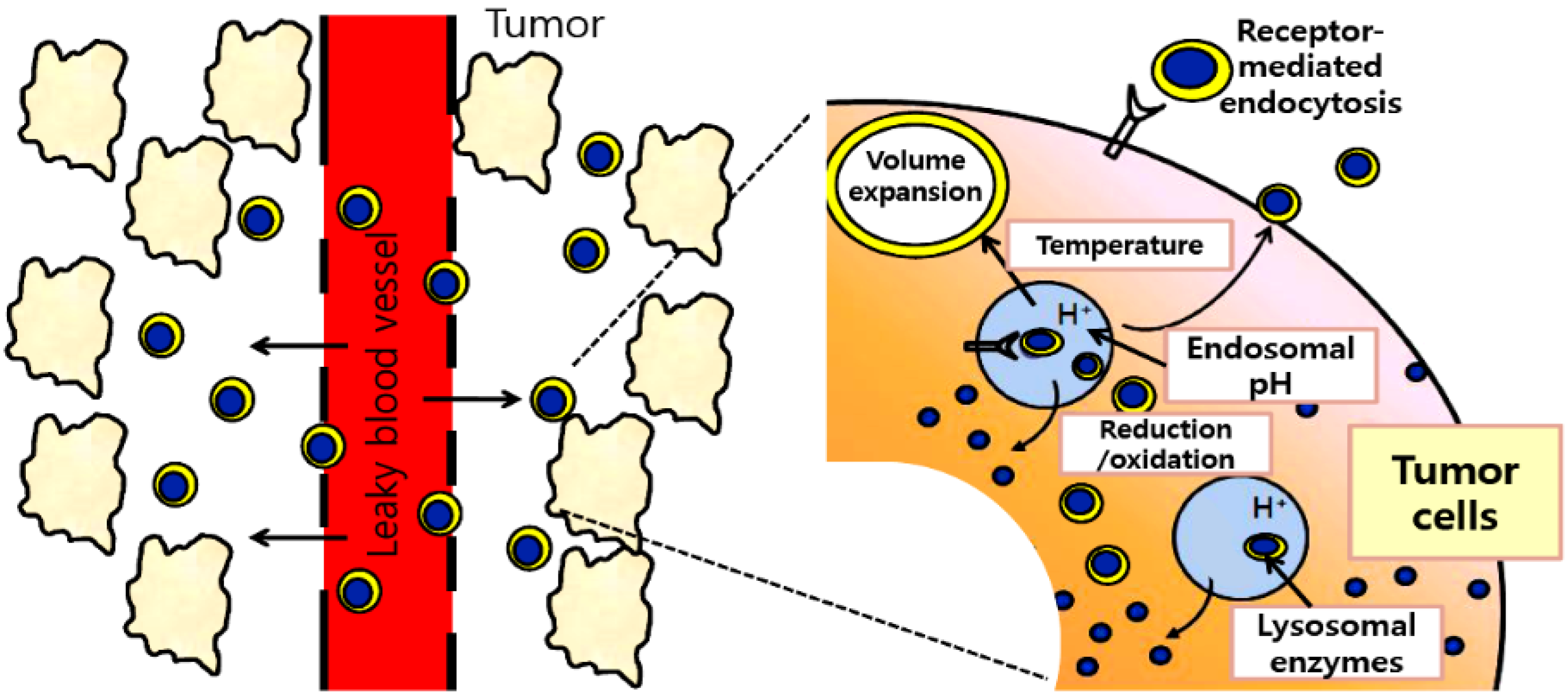
2. Stimuli-Responsive Delivery Systems
2.1. Acidic pH-Activating Systems
| Polymer | Description | Drug | Ref. |
|---|---|---|---|
| PolyHis-b-PEG | Protonation of the imidazole group in His block at lower pH (≤ pH 7.2), resulting drug release | DOX | [20] |
| Blended polymers of polyHis-b-PEG-folate /PLLA-b-PEG-folate | Micelles were destabilized in the pH range of 7.2-6.6, resulting drug release | DOX | [28,29] |
| Pullulan acetate and oligo-sulfadimethoxine (PA-g-OSDM) | Acid pH induced internal structural change, resulting drug release | DOX | [21] |
| Poly[(L-histidine)-co-(L-phenyl alanine)]-b-PEG (HF-b-PEG) | Drug release by the micelle destabilization at tumor extracellular pH | DOX | [46] |
| Poly(N-isopropylacrylamide) (PNIPAM) | A coil-to-globule transition at acidic pH was utilized to destabilize intracellular vehicle membrane | DOX | [47] |
| Folate-PEG-b-poly(aspartate hydrazone doxorubicin) [Folate-PEG-b- poly(Asp-Hyd-DOX)] | Hydrazone bond between drug and polymer was cleaved intracellularly at low pH (5.5), resulting in drug release | DOX | [48] |
| Poly(Ɛ-caprolactone-co-lactide)-b-PEG-b-poly(Ɛ-caprolactone-co-lactide) with sulfamethazine oligomer(OSM-PCLA-b-PEG-b-PCLA-OSM) | Rapid sol-to-gel transition with change in pH, resulting in triggering drug release | PTX | [49] |
| Poly(N-isopropylacrylamide-cobutylmethacrylate-co-acrylic acid) | A pH-dependent drug release behavior was observed in the pH range of pH 5.0-6.0 | Insulin | [50] |
| Enzyme-activated polymer | Enzymatic site | Drug | Ref. |
|---|---|---|---|
| N-(2-hydroxypropy1) methacrylamide-GFLG-mesochlorin e6 (HPMA-GFLG-Mce 6) | GFLG | Mce6 | [60] |
| N-(2-hydroxypropy1) methacrylamide-GFLG- Geldanamycin (HPMA-GFLG-GDM) | GFLG | GDM | [62] |
| Poly(ε-caprolactone)–b-poly(ethyl ethylene phosphate) | Disulfide bond | DOX | [84] |
| PEG-b-poly(amidoamine) dendrimer- succinic acid-PTX | Ester bond | PTX | [89] |
| Polyglycerol dendrimer- Ala-Phe-Lys-MTX | Ala-Phe-Lys | MTX | [90] |
| Trimeric pro-drug (DOX/CPT/etoposide) | Aldol, Retro-aldol | DOX, CPT, etoposide | [91] |
| Poly(γ-glutamic acid)-based nanoparticles (γ-PGA NPs) | Phenylalanine ethyl ester | OVA | [92] |
| N-(2-hydroxypropyl) methacrylamide with methacryloylglycylglycine 4-nitrophenyl ester (PHPMA) | Ester bond | CPT | [63] |
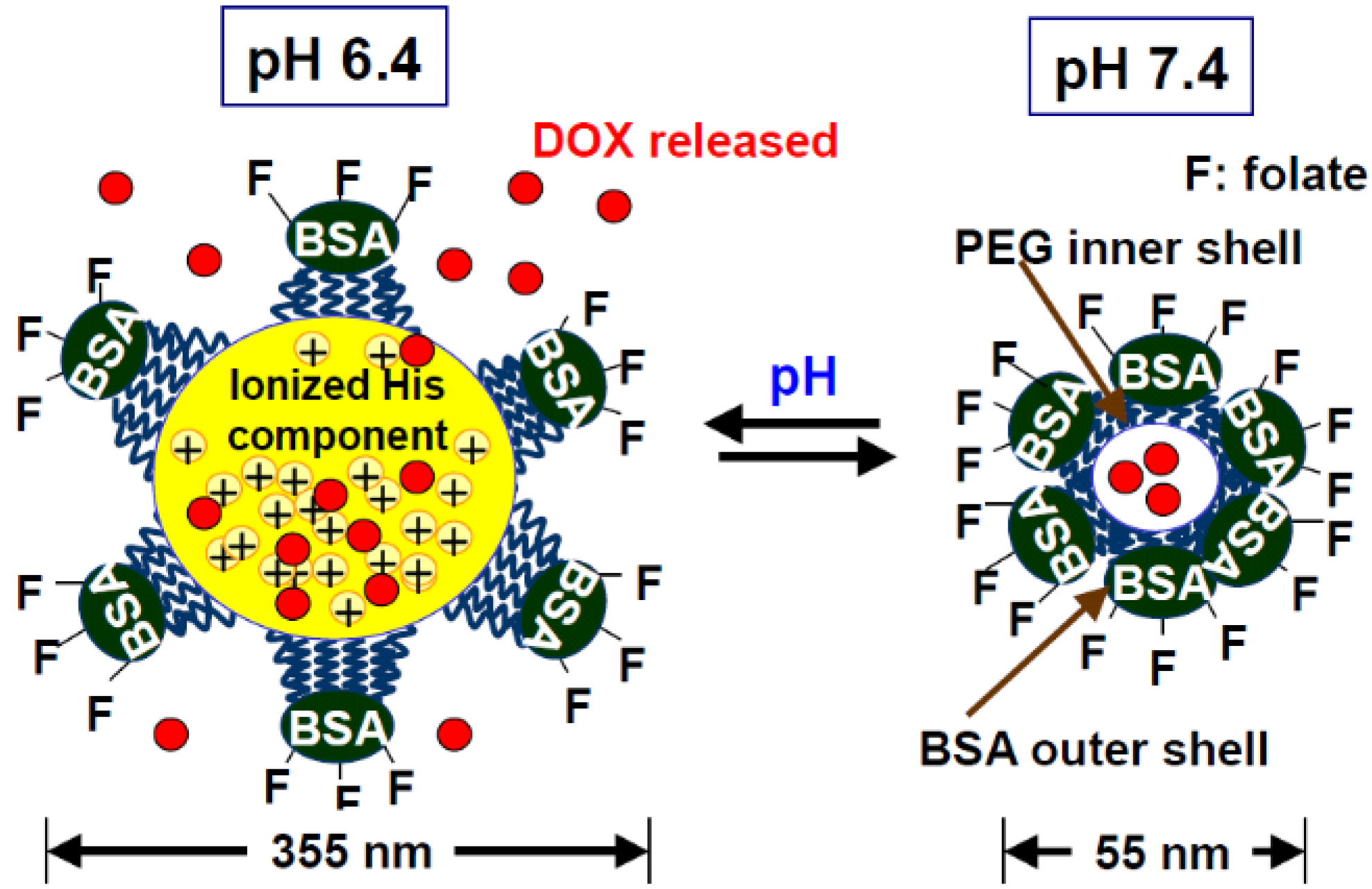
2.1.1. Synthetic anticancer drug delivery
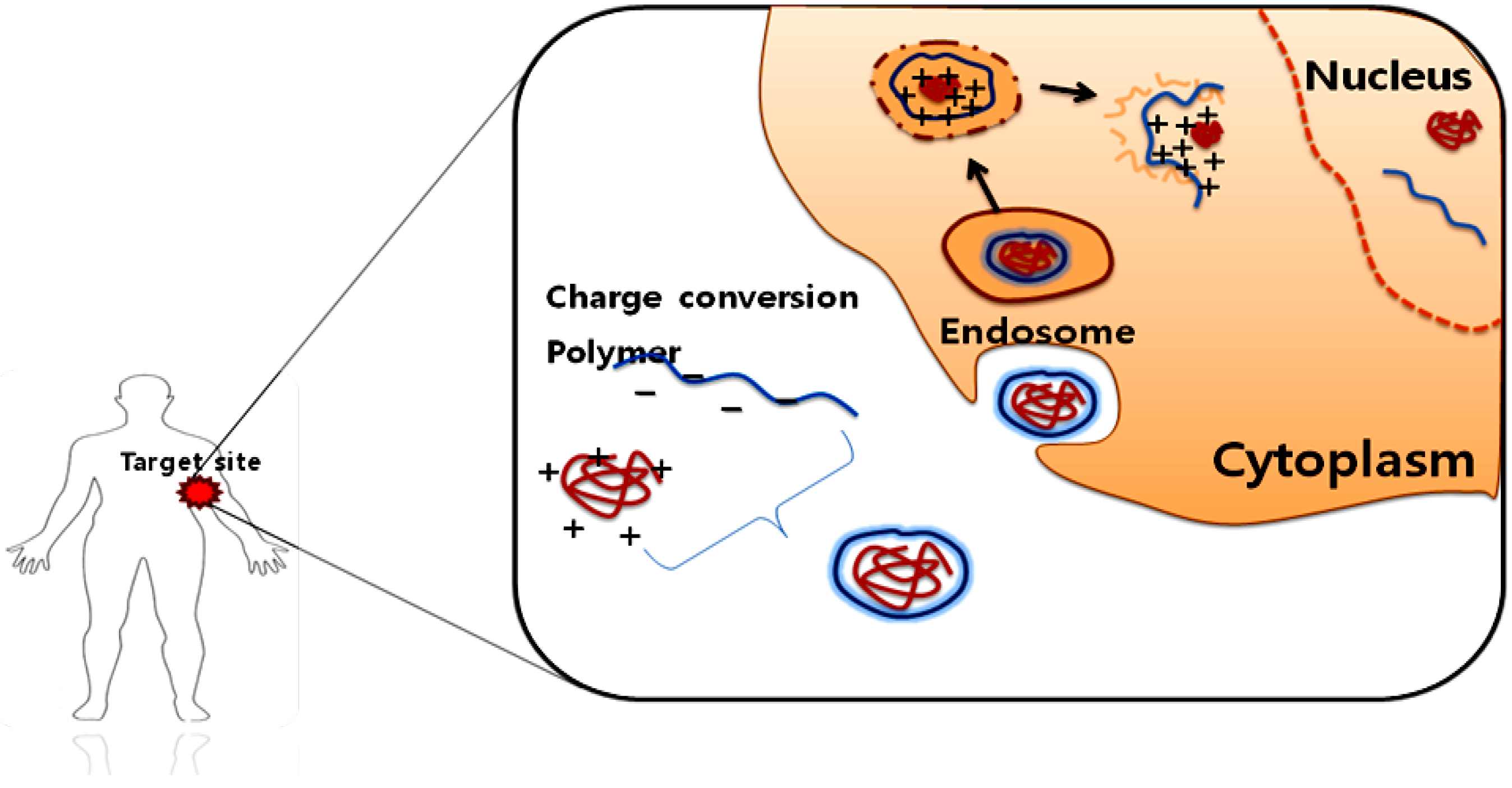
2.1.2. Protein drug delivery
2.2. Enzyme-Activating Systems
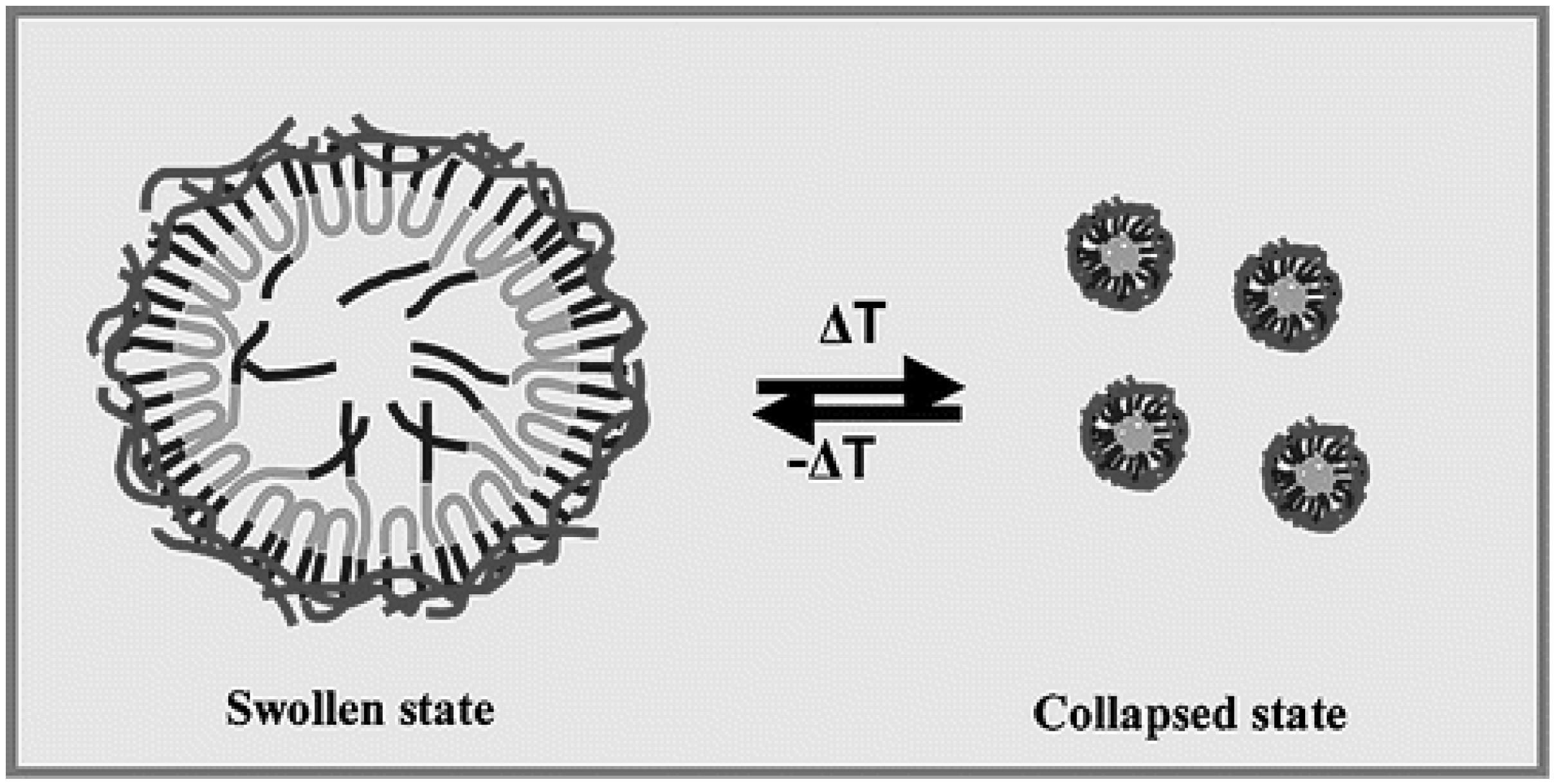
2.3. Temperature-Activating Systems
3. Conclusions
Acknowledgements
References
- Torchilin, V.P. Drug targeting. Eur. J. Pharm. Sci. 2000, 11, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Lammers, T.; Hennink, W.E.; Storm, G. Tumour-targeted nanomedicines: Principles and practice. Br. J. Cancer 2008, 99, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Chen, Z.G.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cao, W.; Jin, H.; Lovell, J.F.; Yang, M.; Ding, L.; Chen, J.; Corbin, I.; Luo, Q.; Zheng, G. Biomimetic nanocarrier for direct cytosolic drug delivery. Angew. Chem. Int. Ed. Engl. 2009, 48, 9171–9175. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Low, P.S. Folate-mediated delivery of macromolecular anticancer therapeutic agents. Adv. Drug Delivery Rev. 2002, 54, 675–693. [Google Scholar] [CrossRef]
- Sethuraman, V.A.; Bae, Y.H. TAT peptide-based micelle system for potential active targeting of anti-cancer agents to acidic solid tumors. J. Control. Release 2007, 118, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Jabr-Milane, L.S.; van Vlerken, L.E.; Yadav, S.; Amiji, M.M. Multi-functional nanocarriers to overcome tumor drug resistance. Cancer Treat. Rev. 2008, 34, 592–602. [Google Scholar] [CrossRef] [PubMed]
- van Vlerken, L.E.; Vyas, T.K.; Amiji, M.M. Poly(ethylene glycol)-modified nanocarriers for tumor-targeted and intracellular delivery. Pharm. Res. 2007, 24, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, N.; Kataoka, K. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol. Ther. 2006, 112, 630–648. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Sawa, T.; Konno, T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J. Control. Release 2001, 74, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Bharate, G.Y.; Daruwalla, J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur. J. Pharm. Biopharm. 2009, 71, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 64, 271–284. [Google Scholar] [CrossRef]
- Liu, H.; Moy, P.; Kim, S.; Xia, Y.; Rajasekaran, A.; Navarro, V.; Knudsen, B.; Bander, N.H. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res. 1997, 57, 3629–3634. [Google Scholar] [PubMed]
- Juweid, M.; Neumann, R.; Paik, C.; Perez-Bacete, M.J.; Sato, J.; van Osdol, W.; Weinstein, J.N. Micropharmacology of monoclonal antibodies in solid tumors: Direct experimental evidence for a binding site barrier. Cancer Res. 1992, 52, 5144–5153. [Google Scholar] [PubMed]
- Sudimack, J.; Lee, R.J. Targeted drug delivery via the folate receptor. Adv. Drug Delivery Rev. 2000, 41, 147–162. [Google Scholar] [CrossRef]
- Green, M.C.; Murray, J.L.; Hortobagyi, G.N. Monoclonal antibody therapy for solid tumors. Cancer Treat. Rev. 2000, 26, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Trowbridge, I.S.; Lopez, F. Monoclonal antibody to transferrin receptor blocks transferrin binding and inhibits human tumor cell growth in vitro. Proc. Natl. Acad. Sci. USA 1982, 79, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Shin, H.J.; Na, K.; Bae, Y.H. Poly(L-histidine)-PEG block copolymer micelles and pH-induced destabilization. J. Control. Release 2003, 90, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Lee, K.H.; Bae, Y.H. pH-sensitivity and pH-dependent interior structural change of self-assembled hydrogel nanoparticles of pullulan acetate/oligo-sulfonamide conjugate. J. Control. Release 2004, 97, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Tolcher, A.W.; Mita, M.; Meropol, N.J.; von Mehren, M.; Patnaik, A.; Padavic, K.; Hill, M.; Mays, T.; McCoy, T.; Fox, N.L.; Halpern, W.; Corey, A.; Cohen, R.B. Phase I pharmacokinetic and biologic correlative study of mapatumumab, a fully human monoclonal antibody with agonist activity to tumor necrosis factor-related apoptosis-inducing ligand receptor-1. J. Clin. Oncol. 2007, 25, 1390–1395. [Google Scholar] [CrossRef] [PubMed]
- Oosterwijk, E.; Bander, N.H.; Divgi, C.R.; Welt, S.; Wakka, J.C.; Finn, R.D.; Carswell, E.A.; Larson, S.M.; Warnaar, S.O.; Fleuren, G.J. Antibody localization in human renal cell carcinoma: A phase I study of monoclonal antibody G250. J. Clin. Oncol. 1993, 11, 738–750. [Google Scholar] [PubMed]
- Konda, S.D.; Aref, M.; Brechbiel, M.; Wiener, E.C. Development of a tumor-targeting MR contrast agent using the high-affinity folate receptor: Work in progress. Invest. Radiol. 2000, 35, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Cui, Y.; Levenson, R.M.; Chung, L.W.K.; Nie, S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 2004, 22, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.T.; Baik, H.J.; Lee, A.H.; Oh, Y.T.; Youn, Y.S.; Lee, E.S. The reversal of drug-resistance in tumors using a drug-carrying nanoparticular system. Int. J. Mol. Sci. 2009, 10, 3776–3792. [Google Scholar] [PubMed]
- Lee, E.S.; Shin, H.J.; Na, K.; Bae, Y.H. Poly(L-histidine)-PEG block copolymer micelles and pH-induced destabilization. J. Control. Release 2003, 90, 363–374. [Google Scholar] [CrossRef]
- Lee, E.S.; Na, K.; Bae, Y.H. Doxorubicin loaded pH-sensitive polymeric micelles for reversal of resistant MCF-7 tumor. J. Control. Release 2005, 103, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Na, K.; Bae, Y.H. Polymeric micelle for tumor pH and folate-mediated targeting. J. Control. Release 2003, 91, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.C.; Lee, M.; Bae, Y.H. Polymeric gene carriers. Crit. Rev. Eukaryot. Gene Expr. 2005, 15, 317–342. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Fukushima, S.; Harada, A.; Kataoka, K. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: Polymeric micelles that are responsive to intracellular pH change. Angew. Chem. Int. Ed. Engl. 2003, 42, 4640–4643. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Nishiyama, N.; Fukushima, S.; Koyama, H.; Yasuhiro, M.; Kataoka, K. Preparation and biological characterization of polymeric micelle drug carriers with intracellular pH-triggered drug release property: Tumor permeability, controlled subcellular drug distribution, and enhanced in vivo antitumor efficacy. Bioconjugate Chem. 2005, 16, 122–130. [Google Scholar] [CrossRef]
- Nishiyama, N.; Bae, Y.; Miyata, K.; Fukushima, S.; Kataoka, K. Smart polymeric micelles for gene and drug delivery. Drug Discov. Today Technol. 2005, 2, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Hruby, M.; Konak, C.; Ulbrich, K. Polymeric micellar pH-sensitive drug delivery system for doxorubicin. J. Control. Release 2005, 103, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Gao, Z.; Bae, Y.H. Recent progress in tumor pH targeting nanotechnology. J. Control. Release 2008, 132, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Gao, Z.; Kim, D.; Park, K.; Kwon, I.C.; Bae, Y.H. Super pH-sensitive multifunctional polymeric micelle for tumor pHe specific TAT exposure and multidrug resistance. J. Control. Release 2008, 129, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, A.; Akuta, T.; Okuyama, H.; Senda, T.; Yokoi, H.; Inokuchi, H.; Fujita, S.; Hayakawa, T.; Takeda, K.; Hasegawa, M.; Nakanishi, M. Protein transduction domain of HIV-1 Tat protein promotes efficient delivery of DNA into mammalian cells. J. Biol. Chem. 2001, 276, 26204–26210. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Kim, D.; Youn, Y.S.; Oh, K.T.; Bae, Y.H. A novel virus-mimetic nanogel vehicle. Angew. Chem. Int. Ed. Engl. 2008, 47, 2418–2421. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Fukushima, S.; Bae, Y.; Hiki, S.; Ishii, T.; Kataoka, K. A protein nanocarrier from charge-conversion polymer in response to endosomal pH. J. Am. Chem. Soc. 2007, 129, 5362–5363. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ishii, T.; Cabral, H.; Kim, H.J.; Seo, J.H.; Nishiyama, N.; Oshima, H.; Osada, K.; Kataoka, K. Charge-conversional polyionic complex micelles-efficient nanocarriers for protein delivery into cytoplasm. Angew. Chem. Int. Ed. Engl. 2009, 48, 5309–5312. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, J.; Lynn, D.M. Polyelectrolyte multilayers fabricated from 'charge-shifting' anionic polymers: A new approach to controlled film disruption and the release of cationic agents from surfaces. Soft Matter 2008, 4, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.R.; Oh, K.T.; Baik, H.J.; Youn, Y.S.; Lee, E.S. A charge-switched nano-sized polymeric carrier for protein delivery. Int. J. Pharm. 2010, 392, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.T.; Kim, D.; You, H.H.; Ahn, Y.S.; Lee, E.S. pH-sensitive properties of surface charge-switched multifunctional polymeric micelle. Int. J. Pharm. 2009, 376, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Kim, H.; Akashi, M. pH-dependent disruption of erythrocyte membrane by amphiphilic poly(amino acid) nanoparticles. J. Biomater. Sci., Polym. Ed. 2010, 21, 315–328. [Google Scholar] [CrossRef]
- Tada, S.; Chowdhury, E.H.; Cho, C.S.; Akaike, T. pH-sensitive carbonate apatite as an intracellular protein transporter. Biomaterials 2010, 31, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.M.; Bae, Y.H.; Jo, W.H. pH-induced micelle formation of poly(histidine-co-phenylalanine)-block-poly(ethylene glycol) in Aqueous Media. Macromol. Biosci. 2005, 5, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, M.H.; Garrec, D.L.; Sant, V.; Leroux, J.C.; Ranger, M. Preparation and characterization of water-soluble pH-sensitive nanocarriersfor drug delivery. Int. J. Pharm. 2004, 277, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Jang, W.D.; Nishiyama, N.; Fukushima, S.; Kataoka, K. Multifunctional polymeric micelles with folate-mediated cancer cell targeting and pH-triggered drug releasing properties for active intracellular drug delivery. Mol. Biosyst. 2005, 1, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.S.; Kim, J.H.; Kim, K.; Kim, Y.S.; Park, R.W.; Kim, I.S.; Kwon, I.C.; Lee, D.S. pH- and temperature- sensitive, injectable, biodegradable block copolymer hydrogels as carriers for paclitaxel. Int. J. Pharm. 2007, 331, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Bae, Y.H.; Kim, S.W. pH/temperature-sensitive polymers for macromolecular drug loading and release. J. Control. Release 1994, 28, 143–152. [Google Scholar] [CrossRef]
- Lee, R.J.; Wang, S.; Turk, M.J.; Low, P.S. The effects of pH and intraliposomal buffer strength on the rate of liposome content release and intracellular drug delivery. Biosci. Rep. 1998, 18, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Gerasimov, O.V.; Boomer, J.A.; Qualls, M.M.; Thompson, D.H. Cytosolic drug delivery using pH- and light- sensitive liposomes. Adv. Drug Delivery Rev. 1999, 38, 317–338. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, U.S. Liposomes in drug delivery: Progress and limitations. Int. J. Pharm. 1997, 154, 123–140. [Google Scholar] [CrossRef]
- Lee, K.D.; Oh, Y.K.; Portnoy, D.A.; Swanson, J.A. Delivery of macromolecules into cytosol using liposomes containing hemolysin from Listeria monocytogenes. J. Biol. Chem. 1996, 271, 7249–7252. [Google Scholar] [CrossRef] [PubMed]
- Samad, A.; Sultana, Y.; Aqil, M. Liposomal drug delivery systems: An update review. Curr. Drug Delivery 2007, 4, 297–305. [Google Scholar] [CrossRef]
- Obata, Y.; Tajima, S.; Takeoka, S. Evaluation of pH-responsive liposomes containing amino acid-based zwitterionic lipids for improving intracellular drug delivery in vitro and in vivo. J. Control. Release 2010, 142, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Leroux, J.; Roux, E.; Le Garrec, D.; Hong, K.; Drummond, D.C. N-isopropylacrylamide copolymers for the preparation of pH-sensitive liposomes and polymeric micelles. J. Control. Release 2001, 72, 71–84. [Google Scholar] [PubMed]
- Shi, G.; Guo, W.; Stephenson, S.M.; Lee, R.J. Efficient intracellular drug and gene delivery using folate receptor-targeted pH-sensitive liposomes composed of cationic/anionic lipid combinations. J. Control. Release 2002, 80, 309–319. [Google Scholar] [PubMed]
- Tachibana, R.; Harashima, H.; Shono, M.; Azumano, M.; Niwa, M.; Futaki, S.; Kiwada, H. Intracellular regulation of macromolecules using pH-sensitive liposomes and nuclear localization signal: Qualitative and quantitative evaluation of intracellular trafficking. Biochem. Biophys. Res. Commun. 1998, 251, 538–544. [Google Scholar] [PubMed]
- Tijerina, M.; Kopeckova, P.; Kopecek, J. Correlation of subcellular compartmentalization of HPMA copolymer-Mce6 conjugates with chemotherapeutic activity in human ovarian carcinoma cells. Pharm. Res. 2003, 20, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Kopecek, J.; Kopeckova, P.; Minko, T.; Lu, Z.R.; Peterson, C.M. Water soluble polymers in tumor targeted delivery. J. Control. Release 2001, 74, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Kasuya, Y.; Lu, Z.R.; Kopeckova, P.; Minko, T.; Tabibi, S.E.; Kopecek, J. Synthesis and characterization of HPMA copolymer–aminopropylgeldanamycin conjugates. J. Control. Release 2001, 74, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Zamai, M.; VandeVen, M.; Farao, M.; Gratton, E.; Ghiglieri, A.; Castelli, M.G.; Fontana, E.; D'Argy, R.; Fiorino, A.; Pesenti, E.; Suarato, A.; Caiolfa, V.R. Camptothecin poly[n-(2-hydroxypropyl) methacrylamide] copolymers in antitopoisomerase-I tumor therapy: Intratumor release and antitumor efficacy. Mol. Cancer. Ther. 2003, 2, 29–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasey, P.A.; Kaye, S.B.; Morrison, R.; Twelves, C.; Wilson, P.; Duncan, R.; Thomson, A.H.; Murray, L.S.; Hilditch, T.E.; Murray, T.; Burtles, S.; Fraier, D.; Frigerio, E.; Cassidy, J. Phase I clinical and pharmacokinetic study of PK1 [N-(2-Hydroxypropyl)methacrylamide copolymer doxorubicin]: First member of a new class of chemotherapeutic agents—drug-polymer conjugates. Clin. Cancer Res. 1999, 5, 83–94. [Google Scholar] [PubMed]
- Lammers, T.; Subr, V.; Peschke, P.; Kühnlein, R.; Hennink, W.E.; Ulbrich, K.; Kiessling, F.; Heilmann, M.; Debus, J.; Huber, P.E.; et al. Image-guided and passively tumour-targeted polymeric nanomedicines for radiochemotherapy. Br. J. Cancer 2008, 99, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Lammers, T.; Subr, V.; Ulbrich, K.; Peschke, P.; Huber, P.E.; Hennink, W.E.; Storm, G. Simultaneous delivery of doxorubicin and gemcitabine to tumors in vivo using prototypic polymeric drug carriers. Biomaterials 2009, 30, 3466–3475. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R. Designing polymer conjugates as lysosomotropic nanomedicines. Biochem. Soc. Trans. 2007, 35, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R. Development of HPMA copolymer-anticancer conjugates: Clinical experience and lessons learnt. Adv. Drug Deliv. Rev. 2009, 61, 1131–1148. [Google Scholar] [CrossRef] [PubMed]
- Etrych, T.; Chytil, P.; Jelinkova, M.; Rihova, B.; Ulbrich, K. Synthesis of HPMA copolymers containing doxorubicin bound via a hydrazone linkage. effect of spacer on drug release and in vitro cytotoxicity. Macromol. Biosci. 2002, 2, 43–52. [Google Scholar] [CrossRef]
- Christie, R.J.; Grainger, D.W. Design strategies to improve soluble macromolecular delivery constructs. Adv. Drug Delivery Rev. 2003, 55, 421–437. [Google Scholar] [CrossRef]
- Meers, P. Enzyme-activated targeting of liposomes. Adv. Drug Delivery Rev. 2001, 53, 265–272. [Google Scholar] [CrossRef]
- Hongrapipat, J.; Kopeckova, P.; Prakongpan, S.; Kopecek, J. Enhanced antitumor activity of combinations of free and HPMA copolymer-bound drugs. Int. J. Pharm. 2008, 53, 259–270. [Google Scholar] [CrossRef]
- Duncan, R.; Gac-Breton, S.; Keane, R.; Musila, R.; Sat, Y.N.; Satchi, R.; Searle, F. Polymer-drug conjugates, PDEPT and PELT: Basic principles for design and transfer from the laboratory to clinic. J. Control. Release 2001, 74, 135–146. [Google Scholar] [CrossRef]
- Hongrapipat, J.; Kopeckova, P.; Liu, J.; Prakongpan, S.; Kopecek, J. Combination chemotherapy and photodynamic therapy with Fab′ fragment targeted HPMA copolymer conjugates in human ovarian carcinoma cells. Mol. Pharm. 2008, 5, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Galande, A.K.; Hilderbrand, S.A.; Weissleder, R.; Tung, C.H. Enzyme-targeted fluorescent imaging probes on a multiple antigenic peptide core. J. Med. Chem. 2006, 49, 4715–4720. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Mok, H.; Lee, S.; Oh, Y.; Park, T.G. Target-specific intracellular delivery of siRNA using degradable hyaluronic acid nanogels. J. Control. Release 2007, 119, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Cerritelli, S.; Velluto, D.; Hubbell, J.A. PEG-SS-PPS:Reduction-sensitive disulfide block copolymer vesicles for intracellular drug delivery. Biomacromolecules 2007, 8, 1966–1972. [Google Scholar] [CrossRef]
- Dalkara, D.; Chandrashekhar, C.; Zuber, G. Intracellular protein delivery with a dimerizable amphiphile for improved complex stability and prolonged protein release in the cytoplasm of adherent cell lines. J. Control. Release 2006, 116, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Guo, B.; Li, X.; Cheng, R.; Meng, F.; Liu, H.; Zhong, Z. Shell-sheddable micelles based on dextran-SS-poly(ε-caprolactone) diblock copolymer for efficient intracellular release of doxorubicin. Biomacromolecules 2010, 11, 848–854. [Google Scholar] [CrossRef]
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release 2008, 126, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Navath, R.S.; Wang, B.; Kannan, S.; Romero, R.; Kannan, R.M. Stimuli-responsive star poly(ethylene glycol) drug conjugates for improved intracellular delivery of the drug in neuroinflammation. J. Control. Release 2010, 142, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Oishi, M.; Hayama, T.; Akiyama, Y.; Takae, S.; Harada, A.; Yamasaki, Y.; Nagatsugi, F.; Sasaki, S.; Nagasaki, Y.; Kataoka, K. Supramolecular assemblies for the cytoplasmic delivery of antisense oligodeoxynucleotide: Polyion complex (PIC) micelles based on poly(ethylene glycol)-SS-oligodeoxynucleotide conjugate. Biomacromolecules 2005, 6, 2449–2454. [Google Scholar] [CrossRef] [PubMed]
- Tappel, A. Lysosomal and prostasomal hydrolytic enzymes and redox processes and initiation of prostate cancer. Med. Hypotheses 2005, 64, 1170–1172. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Wang, Y.; Li, Y.; Du, J.; Wang, J. Shell-detachable micelles based on disulfide-linked block copolymer as potential carrier for intracellular drug delivery. Bioconjugate Chem. 2009, 20, 1095–1099. [Google Scholar] [CrossRef]
- Saito, G.; Swanson, J.A.; Lee, K.D. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: Role and site of cellular reducing activities. Adv. Drug Delivery Rev. 2003, 55, 199–215. [Google Scholar] [CrossRef]
- Calderon, M.; Quadir, M.A.; Strumia, M.; Haag, R. Functional dendritic polymer architectures as stimuli-responsive nanocarriers. Biochimie 2010. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [PubMed]
- Hansen, J.M.; Go, Y.M.; Jones, D.P. Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Khandare, J.J.; Jayant, S.; Singh, A.; Chandna, P.; Wang, Y.; Vorsa, N.; Minko, T. Dendrimer versus linear conjugate: Influence of polymeric architecture on the delivery and anticancer effect of paclitaxel. Bioconjugate Chem. 2006, 17, 1464–1472. [Google Scholar] [CrossRef]
- Calderón, M.; Graeser, R.; Kratz, F.; Haag, R. Development of enzymatically cleavable prodrugs derived from dendritic polyglycerol. Bioorg. Med. Chem. Lett. 2009, 19, 3725–3728. [Google Scholar] [CrossRef] [PubMed]
- Haba, K.; Popkov, M.; Shamis, M.; Lerner, R.A.; Barbas, C.F., III; Shabat, D. Single-triggered trimeric prodrugs. Angew. Chem. Int. Ed. 2005, 44, 716–720. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Okada, N.; Oda, A.; Matsuo, K.; Matsuo, K.; Mukai, Y.; Yoshioka, Y.; Akagi, T.; Akashi, M.; Nakagawa, S. Development of amphiphilic gamma-PGA-nanoparticle based tumor vaccine: Potential of the nanoparticulate cytosolic protein delivery carrier. Biochem. Biophys. Res. Commun. 2008, 366, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Lee, S.H.; Park, T.G. Temperature-sensitive pluronic/poly(ethylenimine) nanocapsules for thermally triggered disruption of intracellular endosomal compartment. Biomacromolecules 2006, 7, 1864–1870. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Lee, J.H.; Choi, S.M.; Park, T.G. Thermally reversible Pluronic/Heparin nanocapsules exhibiting 1000-Fold volume transition. Langmuir 2006, 22, 1758–1762. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Choi, S.H.; Kim, S.H.; Park, T.G. Thermally sensitive cationic polymer nanocapsules for specific cytosolic delivery and efficient gene silencing of siRNA: Welling induced physical disruption of endosome by cold shock. J. Control. Release 2008, 125, 25–32. [Google Scholar] [PubMed]
- Lee, Y.; Park, S.Y.; Kim, C.; Park, T.G. Thermally triggered intracellular explosion of volume transition nanogels for necrotic cell death. J. Control. Release 2009, 135, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Kurisawa, M.; Yokoyama, M.; Okano, T. Gene expression control by temperature with thermo-responsive polymeric gene carriers. J. Control. Release 2000, 69, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Couffin-Hoarau, A.; Leroux, J. Report on the use of poly(organophosphazenes) for the design of stimuli-responsive vesicles. Biomacromolecules 2004, 5, 2082–2087. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, M.D.; Pennadam, S.S.; Ellis, J.; Yater, L.L.; Alexander, C.; G´orecki1, D.C. Enhanced gene expression through temperature profile-induced variations in molecular architecture of thermoresponsive polymer vectors. J. Gene Med. 2007, 9, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Wiradharma, N.; Gao, S.J.; Tong, Y.W.; Yang, Y.Y. Bio-functional micelles self-assembled from a folate-conjugated block copolymer for targeted intracellular delivery of anticancer drugs. Biomacromolecules 2007, 28, 1423–1433. [Google Scholar]
- Kabanov, A.V.; Batrakova, E.V.; Alakhov, V.Y. Pluronic® block copolymers as novel polymer therapeutics for drug and gene delivery. J. Control. Release 2002, 82, 189–212. [Google Scholar] [CrossRef] [PubMed]
- Schmaljohann, D. Thermo- and pH- responsive polymers in drug delivery. Adv. Drug Delivery Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, S.H.; Park, T.G. Temperature-sensitive pluronic/poly(ethylenimine) nanocapsules for thermally triggered disruption of intracellular endosomal compartment. Biomacromolecules 2006, 7, 1864–1870. [Google Scholar] [CrossRef] [PubMed]
- Chilkoti, A.; Dreher, M.R.; Meyer, D.E.; Raucher, D. Targeted drug delivery by thermally responsive polymers. Adv. Drug Delivery Rev. 2002, 54, 613–630. [Google Scholar] [CrossRef]
- Meyer, D.E.; Shin, B.C.; Kong, G.A.; Dewhirst, M.W.; Chilkoti, A. Drug targeting using thermally responsive polymers and local hyperthermia. J. Control. Release 2001, 74, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Sticca, R.P.; Dach, B.W. Rationale for hyperthermia with intraoperative intraperitoneal chemotherapy agents. Surg. Oncol. Clin. N. Am. 2003, 12, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Braun, R.D.; Dewhirst, M.W. Hyperthermia enables tumor-specific nanoparticle delivery: Effect of particle size. Cancer Res. 2000, 60, 4440–4445. [Google Scholar] [PubMed]
- Ponce, A.M.; Vujaskovic, Z.; Yuan, F.; Needham, D.; Dewhirst, M.W. Hyperthermia mediated liposomal drug delivery. Int. J. Hyperthermia 2006, 22, 205–213. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, B.R.; Baik, H.J.; Oh, N.M.; Lee, E.S. Intelligent Polymeric Nanocarriers Responding to Physical or Biological Signals: A New Paradigm of Cytosolic Drug Delivery for Tumor Treatment. Polymers 2010, 2, 86-101. https://doi.org/10.3390/polym2020086
Lee BR, Baik HJ, Oh NM, Lee ES. Intelligent Polymeric Nanocarriers Responding to Physical or Biological Signals: A New Paradigm of Cytosolic Drug Delivery for Tumor Treatment. Polymers. 2010; 2(2):86-101. https://doi.org/10.3390/polym2020086
Chicago/Turabian StyleLee, Bo Reum, Hye Jung Baik, Nam Muk Oh, and Eun Seong Lee. 2010. "Intelligent Polymeric Nanocarriers Responding to Physical or Biological Signals: A New Paradigm of Cytosolic Drug Delivery for Tumor Treatment" Polymers 2, no. 2: 86-101. https://doi.org/10.3390/polym2020086




