Segregation of Benzoic Acid in Polymer Crystalline Cavities
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Techniques and Methods
3. Results and Discussion
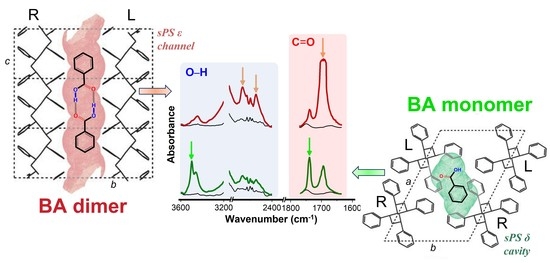
3.1. Co-Crystalline Phases sPS/BA
3.2. Co-Crystalline Phases PPO/BA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guerra, G.; Daniel, C.; Rizzo, P.; Tarallo, O. Advanced Materials Based on Polymer Cocrystalline Forms. J. Polym. Sci. B Polym. Phys. 2012, 50, 305–322. [Google Scholar] [CrossRef]
- Auriemma, F.; Daniel, C.; Golla, M.; Nagendra, B.; Rizzo, P.; Tarallo, O.; Guerra, G. Polymorphism of Poly(2,6-Dimethyl-1,4-Phenylene) Oxide (PPO): Co-Crystalline and Nanoporous-Crystalline Phases. Polymer 2022, 258, 125290. [Google Scholar] [CrossRef]
- Uda, Y.; Kaneko, F.; Kawaguchi, T. Selective Guest Uptake from Solvent Mixtures in the Clathrate Phase of Syndiotactic Polystyrene. Macromol. Rapid Commun. 2004, 25, 1900–1904. [Google Scholar] [CrossRef]
- Gowd, E.B.; Tashiro, K.; Ramesh, C. Structural Phase Transitions of Syndiotactic Polystyrene. Prog. Polym. Sci. 2009, 34, 280–315. [Google Scholar] [CrossRef]
- Itagaki, H.; Sano, T.; Okabe, T.; Sano, S.; Ebihara, H.; Tomono, F.; Dohra, H. Polymerization of Aniline in Tubular Cavities of the Crystalline Phase of Syndiotactic Polystyrene: Proposal of a Preparation Method of Sophisticated Polymer Composites. ACS Macro Lett. 2017, 6, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Nagendra, B.; Cozzolino, A.; Daniel, C.; Rizzo, P.; Guerra, G.; Auriemma, F.; De Rosa, C.; D’Alterio, M.C.; Tarallo, O.; Nuzzo, A. Two Nanoporous Crystalline Forms of Poly(2,6-Dimethyl-1,4-Phenylene)Oxide and Related Co-Crystalline Forms. Macromolecules 2019, 52, 9646–9656. [Google Scholar] [CrossRef]
- Alentiev, A.Y.; Levin, I.S.; Buzin, M.I.; Belov, N.A.; Nikiforov, R.Y.; Chirkov, S.V.; Blagodatskikh, I.V.; Kechekyan, A.S.; Kechekyan, P.A.; Bekeshev, V.G.; et al. Gas Transport Parameters, Density and Free Volume of Nanocrystalline Poly-2,6-Dimethylphenylene Oxide. Polymer 2021, 226, 123804. [Google Scholar] [CrossRef]
- Alentiev, A.Y.; Levin, I.S.; Belov, N.A.; Nikiforov, R.Y.; Chirkov, S.V.; Bezgin, D.A.; Ryzhikh, V.E.; Kostina, J.V.; Shantarovich, V.P.; Grunin, L.Y. Features of the Gas-Permeable Crystalline Phase of Poly-2,6-Dimethylphenylene Oxide. Polymers 2021, 14, 120. [Google Scholar] [CrossRef]
- Uda, Y.; Kaneko, F.; Tanigaki, N.; Kawaguchi, T. The First Example of a Polymer-Crystal–Organic-Dye Composite Material: The Clathrate Phase of Syndiotactic Polystyrene with Azulene. Adv. Mater. 2005, 17, 1846–1850. [Google Scholar] [CrossRef]
- De Girolamo Del Mauro, A.; Carotenuto, M.; Venditto, V.; Petraccone, V.; Scoponi, M. Gaetano Guerra Fluorescence of Syndiotactic Polystyrene/Trimethylbenzene Clathrate and Intercalate Co-Crystals. Chem. Mater. 2007, 19, 6041–6046. [Google Scholar] [CrossRef]
- Musto, P.; Rizzo, P.; Guerra, G. Host−Guest Interactions and Crystalline Structure Evolution in Clathrate Phases Formed by Syndiotactic Polystyrene and 1,2-Dichloroethane: A Two-Dimensional FTIR Spectroscopy Investigation. Macromolecules 2005, 38, 6079–6089. [Google Scholar] [CrossRef]
- Albunia, A.R.; Rizzo, P.; Coppola, M.; De Pascale, M.; Guerra, G. Azobenzene Isomerization in Polymer Co-Crystalline Phases. Polymer 2012, 53, 2727–2735. [Google Scholar] [CrossRef]
- Rizzo, P.; Gallo, C.; Cozzolino, A.; Coscia, N.; Micheletti, C.; Ventura, F.; Minei, P.; Pucci, A. Nanoporous-Crystalline and Amorphous Films of PPO Including off-on Vapochromic Fluorescent 7-Hydroxy Coumarin Guests. Polymer 2022, 249, 124833. [Google Scholar] [CrossRef]
- Rizzo, P.; Montefusco, T.; Guerra, G. Chiral Optical Films Based on Achiral Chromophore Guests. J. Am. Chem. Soc. 2011, 133, 9872–9877. [Google Scholar] [CrossRef] [PubMed]
- Albunia, A.R.; Rizzo, P.; Ianniello, G.; Rufolo, C.; Guerra, G. Syndiotactic Polystyrene Films with a Cocrystalline Phase Including Carvacrol Guest Molecules. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 657–665. [Google Scholar] [CrossRef]
- Rizzo, P.; Cozzolino, A.; Guerra, G. Chemical Stabilization of Hexanal Molecules by Inclusion as Guests of Nanoporous-Crystalline Syndiotactic Polystyrene Crystals. Macromolecules 2019, 52, 2255–2264. [Google Scholar] [CrossRef]
- Cozzolino, A.; Monaco, G.; Daniel, C.; Rizzo, P.; Guerra, G. Monomeric and Dimeric Carboxylic Acid in Crystalline Cavities and Channels of Delta and Epsilon Forms of Syndiotactic Polystyrene. Polymers 2021, 13, 3330. [Google Scholar] [CrossRef]
- Cozzolino, A.; Pappalardo, S.; Rizzo, P.; Guerra, G. Linear Hydrogen Bonded Aggregates of Carboxylic Acids in Crystalline Channels of Syndiotactic Polysty-Rene. Polymer 2022, 262, 125484. [Google Scholar] [CrossRef]
- Gadgoli, C.; Mishra, S.H. Antihepatotoxic Activity of P-Methoxy Benzoic Acid from Capparis Spinosa. J. Ethnopharmacol. 1999, 66, 187–192. [Google Scholar] [CrossRef]
- Park, E.-S.; Moon, W.-S.; Song, M.-J.; Kim, M.-N.; Chung, K.-H.; Yoon, J.-S. Antimicrobial Activity of Phenol and Benzoic Acid Derivatives. Int. Biodeterior. Biodegrad. 2001, 47, 209–214. [Google Scholar] [CrossRef]
- Amborabé, B.-E.; Fleurat-Lessard, P.; Chollet, J.-F.; Roblin, G. Antifungal Effects of Salicylic Acid and Other Benzoic Acid Derivatives towards Eutypa Lata: Structure–Activity Relationship. Plant Physiol. Biochem. 2002, 40, 1051–1060. [Google Scholar] [CrossRef]
- Benzoic Acid and Sodium Benzoate: IPCS, International Programm on Chemical Safety (IPCS); Wibbertmann, A.; International Programme on Chemical Safety (Eds.) Concise international chemical assessment document; World Health Organization: Geneva, Switzerland, 2000; ISBN 978-92-4-153026-2. [Google Scholar]
- Johnson, W.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Benzyl Alcohol, Benzoic Acid and Its Salts, and Benzyl Benzoate. Int. J. Toxicol. 2017, 36, 5S–30S. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.V.R.; Devi, K.P.; Buri, P. Influence of Molecular Size and Water Solubility of the Solute on Its Release from Swelling and Erosion Controlled Polymeric Matrices. J. Control. Release 1990, 12, 133–141. [Google Scholar] [CrossRef]
- Böttcher, H.; Jagota, C.; Trepte, J.; Kallies, K.-H.; Haufe, H. Sol–Gel Composite Films with Controlled Release of Biocides. J. Control. Release 1999, 60, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.S.; Deasy, P.B. Use of Commercial Porous Ceramic Particles for Sustained Drug Delivery. Int. J. Pharm. 2002, 246, 61–73. [Google Scholar] [CrossRef]
- López-Periago, A.; Argemí, A.; Andanson, J.M.; Fernández, V.; García-González, C.A.; Kazarian, S.G.; Saurina, J.; Domingo, C. Impregnation of a Biocompatible Polymer Aided by Supercritical CO2: Evaluation of Drug Stability and Drug–Matrix Interactions. J. Supercrit. Fluids 2009, 48, 56–63. [Google Scholar] [CrossRef]
- Sruthi, R.; Balagangadharan, K.; Selvamurugan, N. Polycaprolactone/Polyvinylpyrrolidone Coaxial Electrospun Fibers Containing Veratric Acid-Loaded Chitosan Nanoparticles for Bone Regeneration. Colloids Surf. B Biointerfaces 2020, 193, 111110. [Google Scholar] [CrossRef]
- Biswal, A.K.; Thodikayil, A.T.; Saha, S. PH-Sensitive Acetalated Dextran/PLGA-Based Double-Layered Microparticles and Their Application in Food Preservation. ACS Appl. Bio. Mater. 2021, 4, 2429–2441. [Google Scholar] [CrossRef]
- Golla, M.; Nagendra, B.; Daniel, C.; Rizzo, P.; Guerra, G. Axial Orientation of Co-Crystalline Phases of Poly(2,6-Dimethyl-1,4-Phenylene)Oxide Films. Polymers 2020, 12, 2394. [Google Scholar] [CrossRef]
- Petraccone, V.; Auriemma, F.; Poggetto, F.D.; De Rosa, C.; Guerra, G.; Corradini, P. On the Structure of the Mesomorphic Form of Syndiotactic Polystyrene. Makromol. Chem. 1993, 194, 1335–1345. [Google Scholar] [CrossRef]
- Monaco, G.; Aquino, F.; Zanasi, R.; Herrebout, W.; Bultinck, P.; Massa, A. Model-Averaging of Ab Initio Spectra for the Absolute Configuration Assignment via Vibrational Circular Dichroism. Phys. Chem. Chem. Phys. 2017, 19, 28028–28036. [Google Scholar] [CrossRef] [PubMed]
- Austin, A.; Petersson, G.A.; Frisch, M.J.; Dobek, F.J.; Scalmani, G.; Throssell, K. A Density Functional with Spherical Atom Dispersion Terms. J. Chem. Theory Comput. 2012, 8, 4989–5007. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R. Gaussian 16 Rev. A.03.; Wallingford CT (2016) GaussView 5.0.; Gaussian, Inc.: Wallingford, UK, 2016. [Google Scholar]
- Boczar, M.; Szczeponek, K.; Wójcik, M.J.; Paluszkiewicz, C. Theoretical Modeling of Infrared Spectra of Benzoic Acid and Its Deuterated Derivative. J. Mol. Struct. 2004, 700, 39–48. [Google Scholar] [CrossRef]
- Linstrom, P. NIST Chemistry WebBook, NIST Standard Reference Database 69. J. Phys. Chem. Ref. Data Monogr. 1997, 9, 1–1951. [Google Scholar]
- Stepanian, S.G.; Reva, I.D.; Radchenko, E.D.; Sheina, G.G. Infrared Spectra of Benzoic Acid Monomers and Dimers in Argon Matrix. Vib. Spectrosc. 1996, 11, 123–133. [Google Scholar] [CrossRef]
- Chatani, Y.; Shimane, Y.; Inagaki, T.; Ijitsu, T.; Yukinari, T.; Shikuma, H. Structural Study on Syndiotactic Polystyrene: 2. Crystal Structure of Molecular Compound with Toluene. Polymer 1993, 34, 1620–1624. [Google Scholar] [CrossRef]






| Solid BA [35] | Solid BA [36] s = Strong m = Medium w = Weak vw = Very Weak | BA in Mesomorphic or in CC ε sPS | BA in CC α PPO |
|---|---|---|---|
| 552 | 552 vw | na | 547 // |
| 668 | 664 vw | 664 // | 664 // |
| 685 | 682 vw | 685 ⊥ | 685 ⊥ |
| 708 | 707 s | 712 ⊥ | 712 ⊥ |
| - - | 800 vw | 797 // | 797 // |
| 806 | 807 vw | 808 nd | 808 nd |
| 936 | 932 w | 936 nd | 936 nd |
| 1026 | 1023 vw | na | na |
| 1073 | 1070 vw | na | 1070 |
| 1129 | 1125 vw | 1125 nd | 1125 |
| 1181 | 1184 vw | na | na |
| 1294 | 1290 m | 1289 // | na |
| 1327 | 1323 m | 1318 nd | na |
| 1426 | 1420 w | 1417 // | na |
| 1454 | 1450 w | na | na |
| 1497 | 1495 vw | na | na |
| 1584 | 1580 vw | na | na |
| 1688 | 1685 s | 1695 // | 1695 nd |
| - - | 2560 vw | 2548 // | 2548 // |
| - - | 2603 vw | 2600 // | 2600 // |
| - - | 2670 vw | 2672 // | 2672 // |
| 3012 | 3005 vw | na | na |
| 3073 | 3078 vw | na | na |
| BA Isolated in Argon | BA Vapor s = Strong m = Medium w = Weak vw = Very Weak | BA in CC δ sPS |
|---|---|---|
| 565 | 575 vw | na |
| 628 | 632 vw | 632 ⊥ |
| 711 | 715 w | 714 // |
| - - | 817 vw | 808 nd |
| 937 | - - | 936 nd |
| 1027 | 1010 vw | na |
| 1066 | - - | na |
| 1086 | 1084 m | 1091 ⊥ |
| 1169 | - - | 1173 ⊥ |
| 1185 | 1184 m | 1189 ⊥ |
| 1275 | 1290 vw | 1277 |
| 1347 | 1370 vw | 1352 |
| 1606 | 1600 vw | na |
| 1752 | 1765 s | 1738 ⊥ |
| 3567 | 3500 vw | 3450 // |
| 3575 m | 3497 ⊥ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cozzolino, A.; Monaco, G.; Rizzo, P.; Guerra, G. Segregation of Benzoic Acid in Polymer Crystalline Cavities. Polymers 2023, 15, 177. https://doi.org/10.3390/polym15010177
Cozzolino A, Monaco G, Rizzo P, Guerra G. Segregation of Benzoic Acid in Polymer Crystalline Cavities. Polymers. 2023; 15(1):177. https://doi.org/10.3390/polym15010177
Chicago/Turabian StyleCozzolino, Antonietta, Guglielmo Monaco, Paola Rizzo, and Gaetano Guerra. 2023. "Segregation of Benzoic Acid in Polymer Crystalline Cavities" Polymers 15, no. 1: 177. https://doi.org/10.3390/polym15010177