A Biodegradable, Bio-Based Polymer for the Production of Tools for Aquaculture: Processing, Properties and Biodegradation in Sea Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Samples
2.3. Characterization
2.4. Biodegration
- Stabilization at 10 °C for 600 s;
- heating at 10 °C/s from 10 to 210 °C;
- maintaining at 210 °C for 600 s;
- cooling at 10 °C/s from 210 to 30 °C.
2.5. Statistical Analysis
3. Results and Discussion
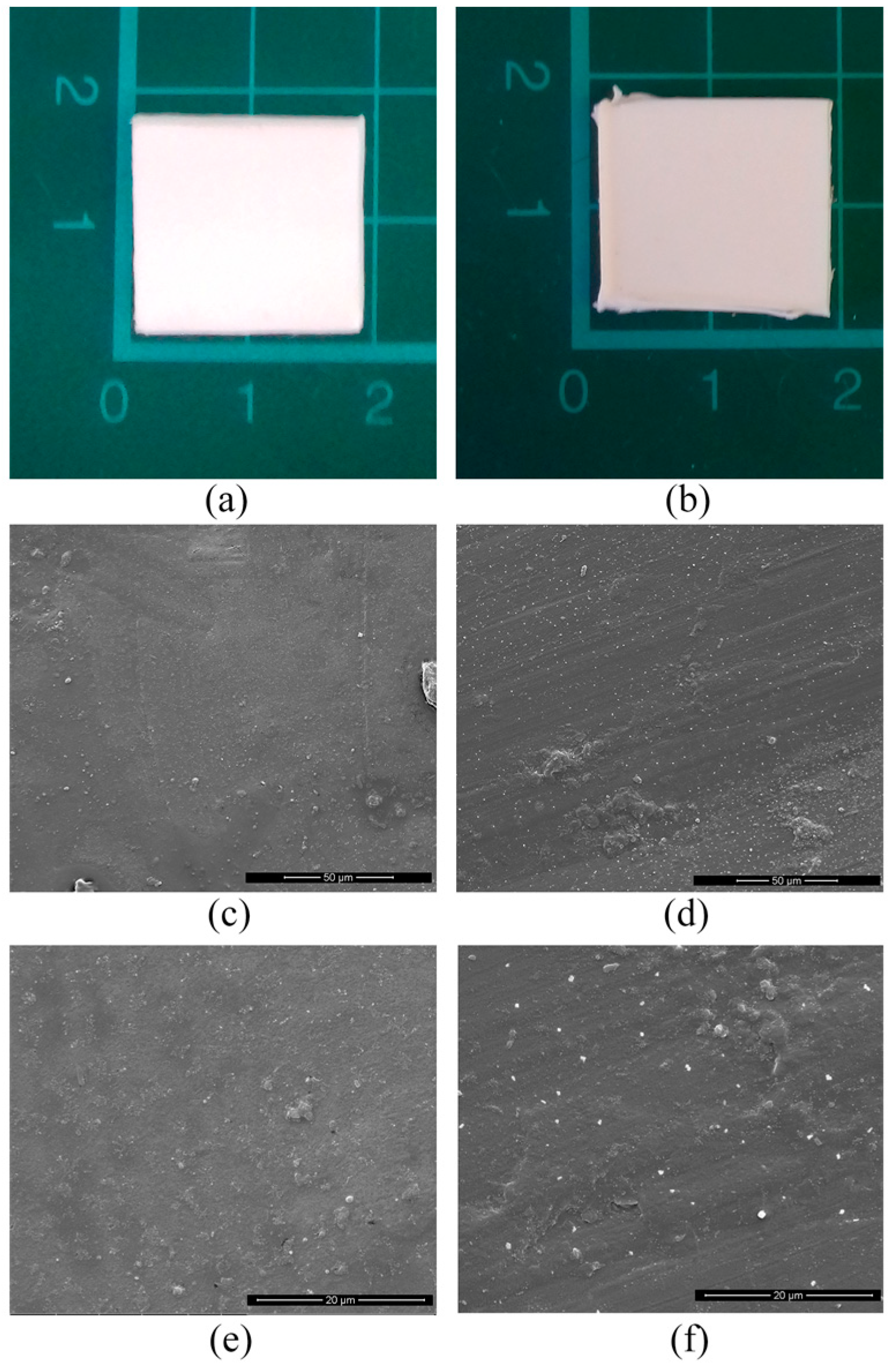
3.1. Characterization of the Biodegradable Polymer and of the Trays
3.2. Mechanical Characterization
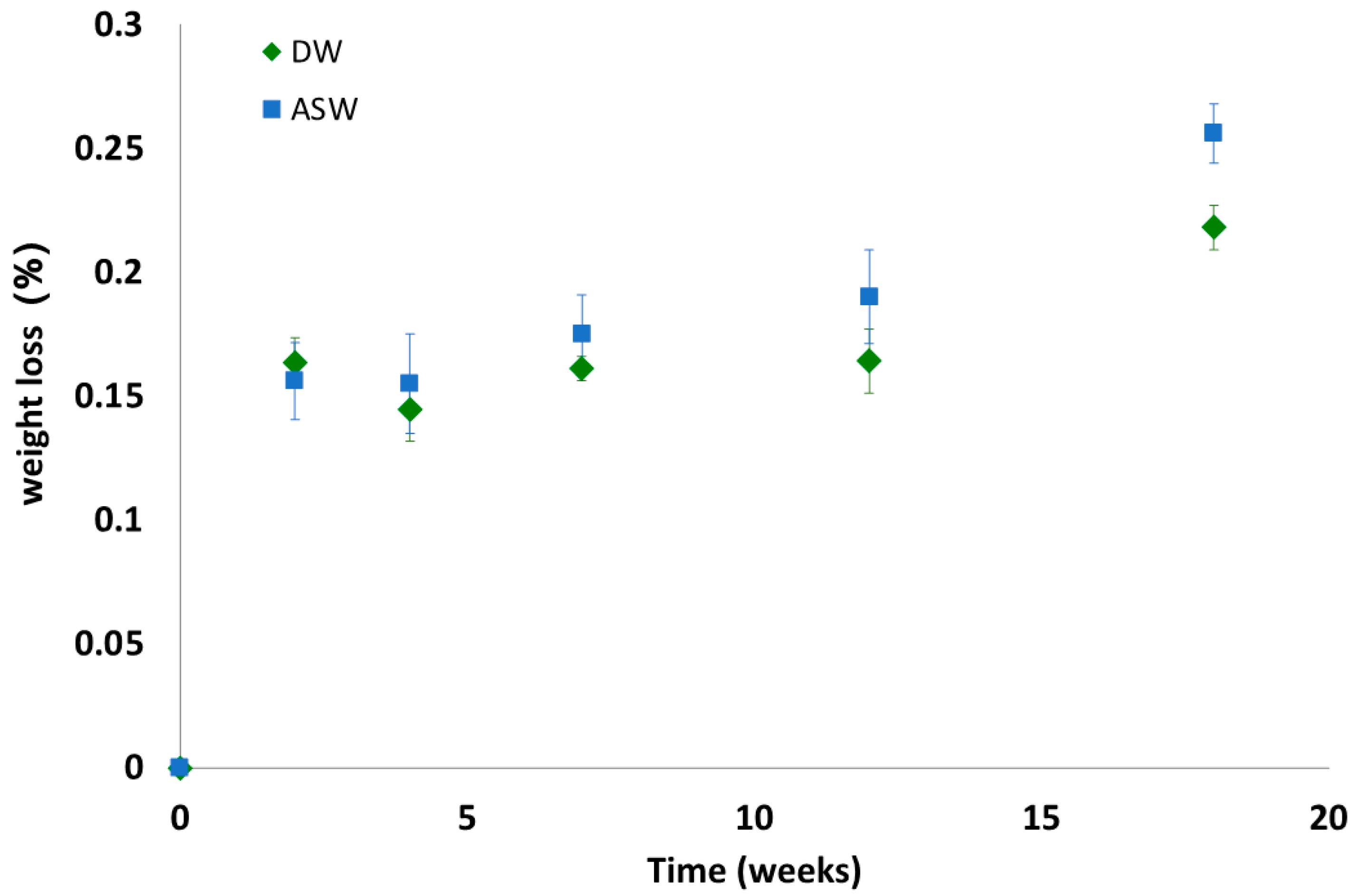
3.3. Biodegradation Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Monte, C.; Locritani, M.; Merlino, S.; Ricci, L.; Pistolesi, A.; Bronco, S. An In Situ Experiment to Evaluate the Aging and Degradation Phenomena Induced by Marine Environment Conditions on Commercial Plastic Granules. Polymers 2022, 14, 1111. [Google Scholar] [CrossRef]
- Wayman, C.; Niemann, H. The Fate of Plastic in the Ocean Environment-a Minireview. Environ. Sci. Process. Impacts 2021, 23, 198–212. [Google Scholar] [CrossRef]
- da Costa, J.P.; Nunes, A.R.; Santos, P.S.M.; Girão, A.V.; Duarte, A.C.; Rocha-Santos, T. Degradation of Polyethylene Microplastics in Seawater: Insights into the Environmental Degradation of Polymers. J. Environ. Sci. Health 2018, 53, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Gewert, B.; Plassmann, M.M.; Macleod, M. Pathways for Degradation of Plastic Polymers Floating in the Marine Environment. Environ. Sci. Process. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef]
- Oliveira, J.; Belchior, A.; da Silva, V.D.; Rotter, A.; Petrovski, Ž.; Almeida, P.L.; Lourenço, N.D.; Gaudêncio, S.P. Marine Environmental Plastic Pollution: Mitigation by Microorganism Degradation and Recycling Valorization. Front. Mar. Sci. 2020, 7, 567126. [Google Scholar] [CrossRef]
- Min, K.; Cuiffi, J.D.; Mathers, R.T. Ranking Environmental Degradation Trends of Plastic Marine Debris Based on Physical Properties and Molecular Structure. Nat. Commun. 2020, 11, 727. [Google Scholar] [CrossRef]
- Masry, M.; Rossignol, S.; Gardette, J.L.; Therias, S.; Bussière, P.O.; Wong-Wah-Chung, P. Characteristics, Fate, and Impact of Marine Plastic Debris Exposed to Sunlight: A Review. Mar. Pollut. Bull. 2021, 171, 112701. [Google Scholar] [CrossRef]
- Wu, X.; Liu, P.; Wang, H.; Huang, H.; Shi, Y.; Yang, C.; Gao, S. Photo Aging of Polypropylene Microplastics in Estuary Water and Coastal Seawater: Important Role of Chlorine Ion. Water Res. 2021, 202, 117396. [Google Scholar] [CrossRef] [PubMed]
- Bonifazi, G.; Fiore, L.; Pelosi, C.; Serranti, S. Evaluation of Plastic Packaging Waste Degradation in Seawater and Simulated Solar Radiation by Spectroscopic Techniques. Polym. Degrad. Stab. 2023, 207, 110215. [Google Scholar] [CrossRef]
- Brucato, V.; CarfìPavia, F.; la Carrubba, V. Demixing Time and Temperature Influence on Porosity and Interconnection of PLLA Scaffolds Prepared via TIPS. Macromol. Symp. 2009, 286, 49–52. [Google Scholar] [CrossRef]
- Niaounakis, M. Degradation of Plastics in the Marine Environment. In Management of Marine Plastic Debris; William Andrew Publishing: Norwich, NY, USA, 2017. [Google Scholar]
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of Bioplastics in Natural Environments. Waste Manag. 2017, 59, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Frącz, W.; Janowski, G.; Smusz, R.; Szumski, M. The Influence of Chosen Plant Fillers in PHBV Composites on the Processing Conditions, Mechanical Properties and Quality of Molded Pieces. Polymers 2021, 13, 3934. [Google Scholar] [CrossRef] [PubMed]
- Ceraulo, M.; la Mantia, F.P.; Mistretta, M.C.; Titone, V. The Use of Waste Hazelnut Shells as a Reinforcement in the Development of Green Biocomposites. Polymers 2022, 14, 2151. [Google Scholar] [CrossRef] [PubMed]
- Chinaglia, S.; Tosin, M.; Degli-Innocenti, F. Biodegradation Rate of Biodegradable Plastics at Molecular Level. Polym. Degrad. Stab. 2018, 147, 237–244. [Google Scholar] [CrossRef]
- Tosin, M.; Pischedda, A.; Degli-Innocenti, F. Biodegradation Kinetics in Soil of a Multi-Constituent Biodegradable Plastic. Polym. Degrad. Stab. 2019, 166, 213–218. [Google Scholar] [CrossRef]
- Eich, A.; Weber, M.; Lott, C. Disintegration Half-Life of Biodegradable Plastic Films on Different Marine Beach Sediments. PeerJ 2021, 9, e11981. [Google Scholar] [CrossRef]
- Tosin, M.; Weber, M.; Siotto, M.; Lott, C.; Innocenti, F.D. Laboratory Test Methods to Determine the Degradation of Plastics in Marine Environmental Conditions. Front. Microbiol. 2012, 3, 225. [Google Scholar] [CrossRef]
- Magni, S.; Bonasoro, F.; della Torre, C.; Parenti, C.C.; Maggioni, D.; Binelli, A. Plastics and Biodegradable Plastics: Ecotoxicity Comparison between Polyvinylchloride and Mater-Bi® Micro-Debris in a Freshwater Biological Model. Sci. Total Environ. 2020, 720, 137602. [Google Scholar] [CrossRef]
- Ibáñez-García, A.; Martínez-García, A.; Ferrándiz-Bou, S. Recyclability Analysis of Starch Thermoplastic/Almond Shell Biocomposite. Polymers 2021, 13, 1159. [Google Scholar] [CrossRef]
- Deroiné, M.; le Duigou, A.; Corre, Y.M.; le Gac, P.Y.; Davies, P.; César, G.; Bruzaud, S. Accelerated Ageing of Polylactide in Aqueous Environments: Comparative Study between Distilled Water and Seawater. Polym. Degrad. Stab. 2014, 108, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yao, L.; Wang, Y.; Wang, L.; Jiang, Z.; Qiu, D.; Weng, Y. Degradation Kinetics and Performances of Poly(Lactic Acid) Films in Artificial Seawater. Chem. Pap. 2022, 76, 5929–5941. [Google Scholar] [CrossRef]
- Niaounakis, M.; Kontou, E.; Pispas, S.; Kafetzi, M.; Giaouzi, D. Aging of Packaging Films in the Marine Environment. Polym. Eng. Sci. 2019, 59, E432–E441. [Google Scholar] [CrossRef]
- O’Brine, T.; Thompson, R.C. Degradation of Plastic Carrier Bags in the Marine Environment. Mar. Pollut. Bull. 2010, 60, 2279–2283. [Google Scholar] [CrossRef]
- la Mantia, F.P.; Baiamonte, M.; Santangelo, S.; Scaffaro, R.; Mistretta, M.C. Influence of Different Environments and Temperatures on the Photo-Oxidation Behaviour of the Polypropylene. Polymers 2022, 15, 74. [Google Scholar] [CrossRef] [PubMed]
- Ruggero, F.; Onderwater, R.C.A.; Carretti, E.; Roosa, S.; Benali, S.; Raquez, J.M.; Gori, R.; Lubello, C.; Wattiez, R. Degradation of Film and Rigid Bioplastics During the Thermophilic Phase and the Maturation Phase of Simulated Composting. J. Polym. Env. 2021, 29, 3015–3028. [Google Scholar] [CrossRef]
- Andrady, A.L. The Plastic in Microplastics: A Review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef]
- Jacquin, J.; Callac, N.; Cheng, J.; Giraud, C.; Gorand, Y.; Denoual, C.; Pujo-Pay, M.; Conan, P.; Meistertzheim, A.L.; Barbe, V.; et al. Microbial Diversity and Activity During the Biodegradation in Seawater of Various Substitutes to Conventional Plastic Cotton Swab Sticks. Front. Microbiol. 2021, 12, 604395. [Google Scholar] [CrossRef]


| Material | Manufacturer | MFI, g/10 min | Sample Code |
|---|---|---|---|
| Yuplene BH3821 | SK Global Chemical (Korea) | 28 | PP |
| Mater-Bi EI51N0 | Novamont (Italy) | 35 | MB |
| Sample | Temperature Profile, °C | Injection Pressure, Bar | Holding Pressure, Bar | Mold Temperature, °C | Flow Rate, cm3/s | Holding Time, s |
|---|---|---|---|---|---|---|
| PP | 170/185/215/235 | 80 | 35 | 30 | 400 | 60 |
| MB | 170/180/205/235 | 90 | 40 | 30 | 330 | 60 |
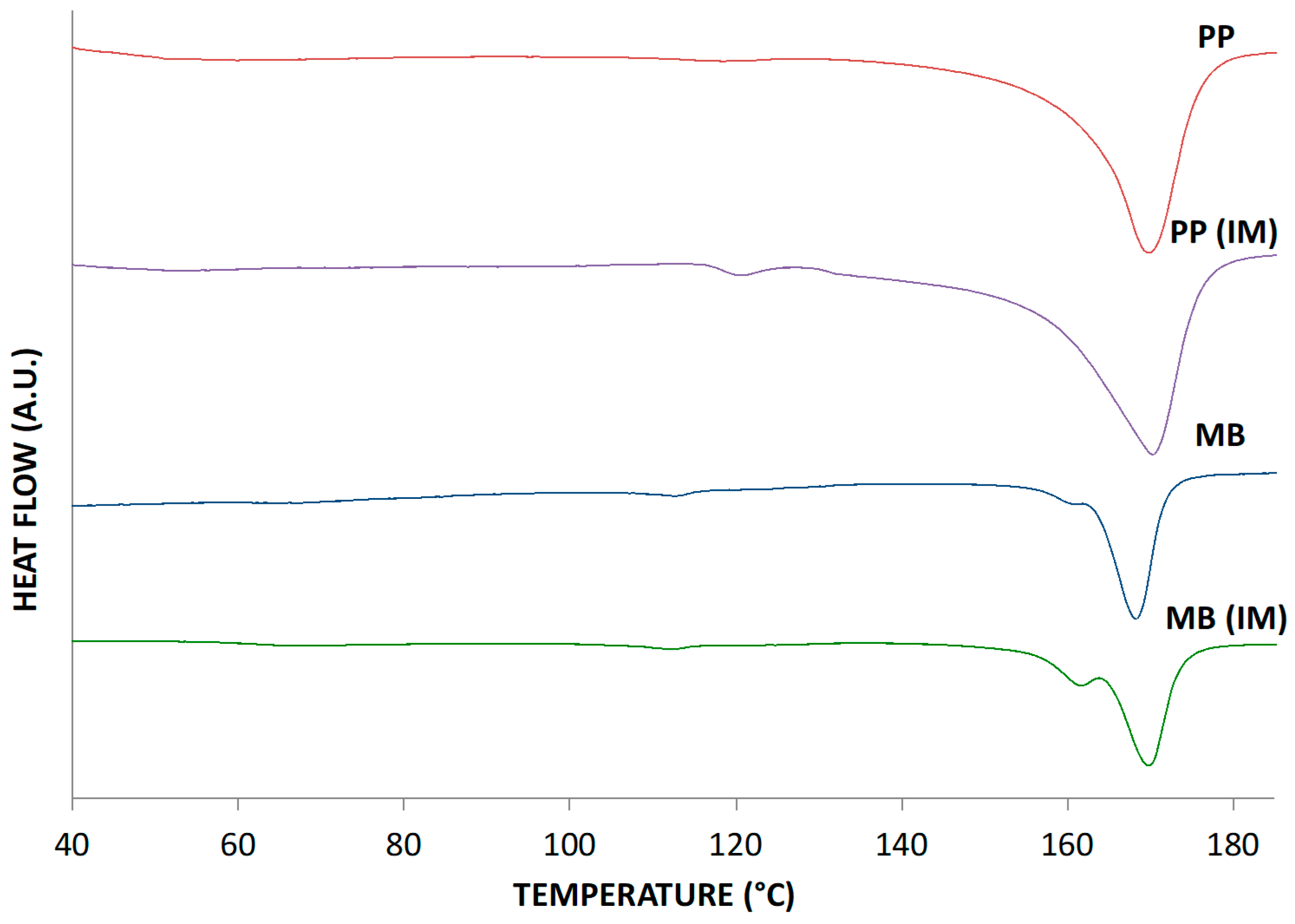
| Sample | Enthalpy of Fusion, J/g | Melting Temperature, °C |
|---|---|---|
| PP | 64.1 | 169.6 |
| PP (IM) | 75.5 | 170.1 |
| MB | 27.1 | 168.2 |
| MB (IM) | 29.5 | 169.8 |
| Sample | E, MPa | TS, MPa | EB, % |
|---|---|---|---|
| PP | 1040 ± 66 | 20.6 ± 1.2 | 6.9 ± 0.7 |
| PP (IM) | 1154 ± 61 | 21.8 ± 1.5 | 5.8 ± 0.5 |
| MB | 1910 ± 142 | 36.60 ± 2.2 | 3.4 ± 0.5 |
| MB (IM) | 2120 ± 131 | 38.20 ± 2.8 | 2.8 ± 0.3 |
| Sample | IS, J |
|---|---|
| PP (IM) | 7.45 |
| MB (IM) | 4.84 |
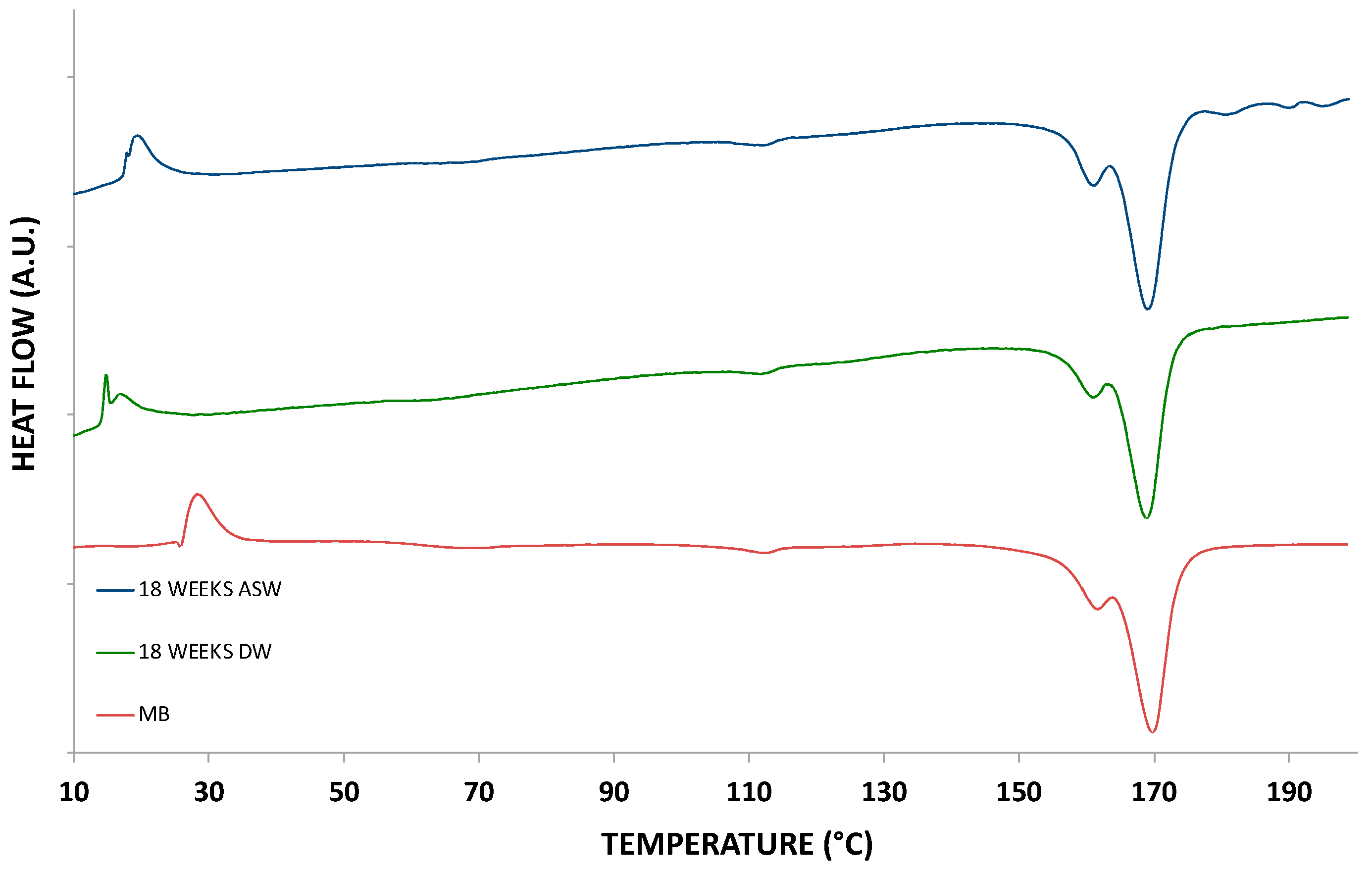
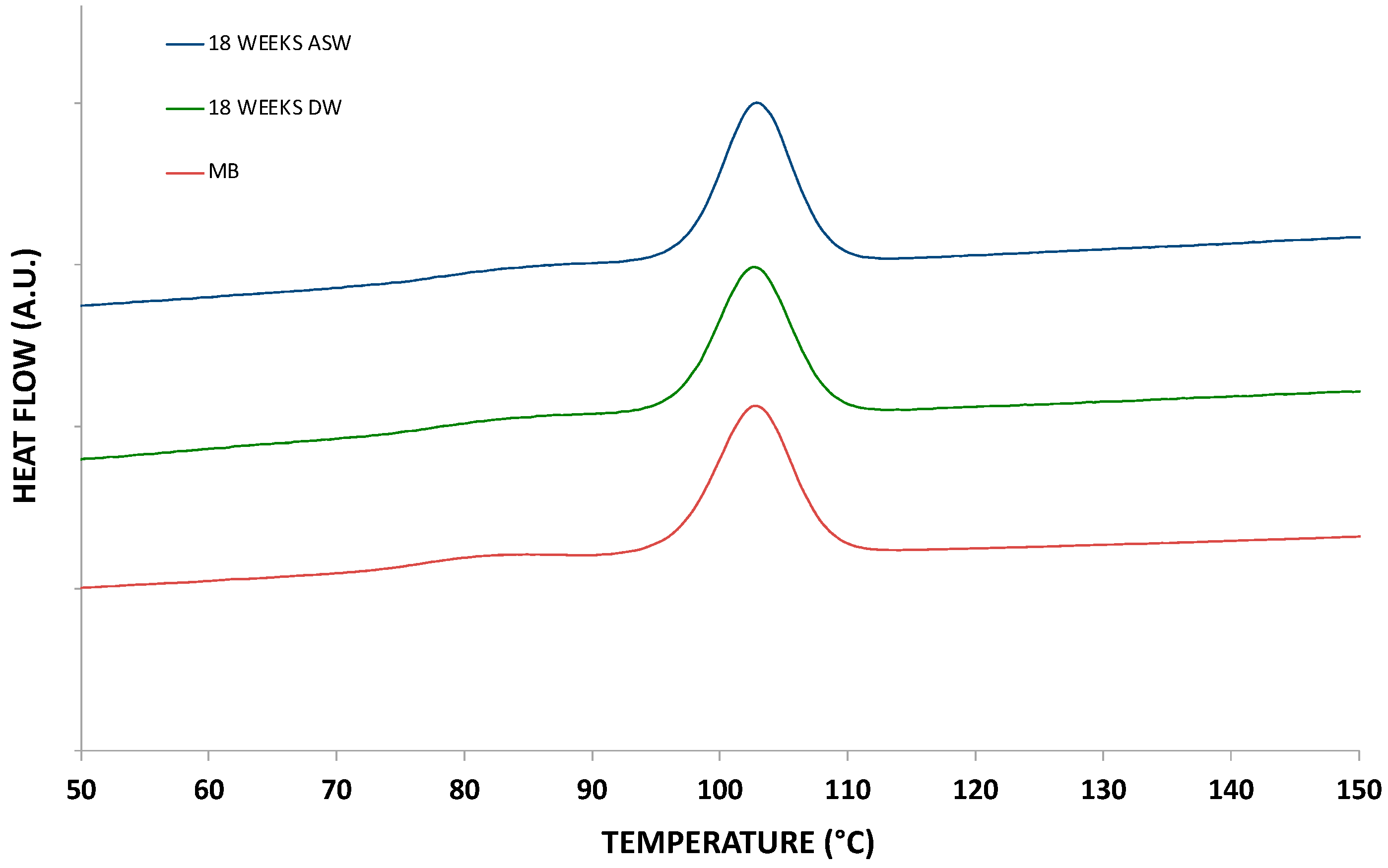
| Sample | Melting Enthalpy (J/g) | Melting Temperature (°C) | Crystallization Enthalpy (J/g) | Crystallization Temperature (°C) |
|---|---|---|---|---|
| MB (IM) | 29.5 | 169.8 | 27.7 | 102.7 |
| 18 WEEKS DW | 29.5 | 168.9 | 27.7 | 102.8 |
| 18 WEEKS ASW | 29.7 | 169.1 | 28.0 | 102.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavia, F.C.; Brucato, V.; Mistretta, M.C.; Botta, L.; La Mantia, F.P. A Biodegradable, Bio-Based Polymer for the Production of Tools for Aquaculture: Processing, Properties and Biodegradation in Sea Water. Polymers 2023, 15, 927. https://doi.org/10.3390/polym15040927
Pavia FC, Brucato V, Mistretta MC, Botta L, La Mantia FP. A Biodegradable, Bio-Based Polymer for the Production of Tools for Aquaculture: Processing, Properties and Biodegradation in Sea Water. Polymers. 2023; 15(4):927. https://doi.org/10.3390/polym15040927
Chicago/Turabian StylePavia, F. Carfì, V. Brucato, M. C. Mistretta, L. Botta, and F. P. La Mantia. 2023. "A Biodegradable, Bio-Based Polymer for the Production of Tools for Aquaculture: Processing, Properties and Biodegradation in Sea Water" Polymers 15, no. 4: 927. https://doi.org/10.3390/polym15040927








