Aliphatic Polyurethane Elastomers Quaternized with Silane-Functionalized TiO2 Nanoparticles with UV-Shielding Features
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Elastomeric Polyurethanes
2.3. TiO2 Nanoparticle Functionalization
2.4. Quaternization of PU-1/PU-2 with Functionalized TiO2 Nanoparticles in THF
2.5. Measurements
3. Results and Discussion
3.1. Functionalization and Characterization of TiO2 NPs
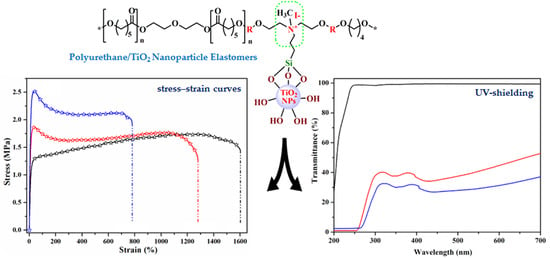
3.2. Synthesis and Characterization of Polyurethane/TiO2 Composites
3.3. Optical and UV-Shielding Properties of Polyurethane/TiO2 Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tatai, L.; Moore, T.G.; Adhikari, R.; Malherbe, F.; Jayasekara, R.; Griffiths, I.; Gunatillake, P.A. Thermoplastic biodegradable polyurethanes: The effect of chain extender structure on properties and in-vitro degradation. Biomaterials 2007, 28, 5407–5417. [Google Scholar] [CrossRef]
- Ge, C.; Zhai, W. Cellular thermoplastic polyurethane thin film: Preparation, elasticity, and thermal insulation performance. Ind. Eng. Chem. Res. 2018, 57, 4688–4696. [Google Scholar] [CrossRef]
- Wendels, S.; Averous, L. Biobased polyurethanes for biomedical applications. Bioact. Mater. 2021, 6, 1083–1106. [Google Scholar] [CrossRef]
- Mi, H.Y.; Jing, X.; Hagerty, B.S.; Chen, G.; Huang, A.; Turng, L.S. Post-crosslinkable biodegradable thermoplastic polyurethanes: Synthesis, and thermal, mechanical, and degradation properties. Mater. Design 2017, 127, 106–114. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Raju, K.V.S.N. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Kojio, K.; Nozaki, S.; Takahara, A.; Yamasaki, S. Influence of chemical structure of hard segments on physical properties of polyurethane elastomers: A review. J. Polym. Res. 2020, 27, 140. [Google Scholar] [CrossRef]
- Oprea, S.; Timpu, D.; Oprea, V. Design-properties relationships of polyurethanes elastomers depending on different chain extenders structures. J. Polym. Res. 2019, 26, 117. [Google Scholar] [CrossRef]
- Naheed, S.; Zuber, M.; Barikani, M.; Salman, M. Molecular engineering and morphology of polyurethane elastomers containing various molecular weight of macrodiol. Mat. Sci. Eng. B Adv. 2021, 264, 114960. [Google Scholar] [CrossRef]
- Cao, H.W.; Qi, F.X.Y.; Liu, R.W.; Wang, F.T.; Zhang, C.X.; Zhang, X.N.; Chai, Y.Y.; Zhai, L.L. The influence of hydrogen bonding on N-methyldiethanolamine-extended polyurethane solid–solid phase change materials for energy storage. RSC Adv. 2017, 7, 11244. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.J.; Wang, L.L.; Liu, H.; He, S.Q.; Liu, X.Y.; Liu, W.T.; Huang, M.M.; Zhu, C.S. Polyurethane as smart biocoatings: Effects of hard segments on phase structures and properties. Prog. Org. Coat. 2021, 150, 106000. [Google Scholar] [CrossRef]
- Lei, W.Q.; Fang, C.Q.; Zhou, X.; Cheng, Y.L.; Yang, R.; Liu, D.H. Morphology and thermal properties of polyurethane elastomer based on representative structural chain extenders. Thermochim. Acta 2017, 653, 116–125. [Google Scholar] [CrossRef]
- Dieterich, D.; Keberle, W.; Witt, H. Polyurethane ionomers, a new class of block polymers. Angew. Chem. Int. Edit. 1970, 9, 40–50. [Google Scholar] [CrossRef]
- Marx, C.L.; Caulfield, D.F.; Cooper, S.L. Morphology of ionomers. Macromolecules 1973, 6, 344–353. [Google Scholar] [CrossRef]
- Hwang, K.S.; Yang, C.Z.; Cooper, S.L. Properties of polyether-polyurethane zwitterionomers. Polym. Eng. Sci. 1981, 21, 1027–1036. [Google Scholar] [CrossRef]
- Nomula, S.; Cooper, S.L. Ionomer solution structure and solution diagram. Macromolecules 2001, 34, 2653–2659. [Google Scholar] [CrossRef]
- Nomula, S.; Cooper, S.L. Influence of ionic content in polyurethane ionomer solutions. J. Phys. Chem. B 2000, 104, 6963–6972. [Google Scholar] [CrossRef]
- Capek, I. Nature and properties of ionomer assemblies. II. Adv. Colloid Interface Sci. 2005, 118, 73–112. [Google Scholar] [CrossRef]
- Jaudouin, O.; Robin, J.J.; Lopez-Cuesta, J.M.; Perrin, D.; Imbert, C. Ionomer-based polyurethanes: A comparative study of properties and applications. Polym. Int. 2012, 61, 495–510. [Google Scholar] [CrossRef]
- Buruiana, T.; Melinte, V.; Buruiana, E.C.; Mihai, A. Synthesis and characterization of polyurethane cationomer/MMT hybrid composite. Polym. Int. 2009, 58, 1181–1189. [Google Scholar] [CrossRef]
- Francolini, I.; D’Ilario, L.; Guaglianone, E.; Donelli, G.; Martinelli, A.; Piozzi, A. Polyurethane anionomers containing metal ions with antimicrobial properties: Thermal, mechanical and biological characterization. Acta Biomater. 2010, 6, 3482–3490. [Google Scholar] [CrossRef]
- Buruiana, T.; Buruiana, E.C.; Diaconu, I.; Robila, G. Sulfobetaine polyurethane ionomers-salts effects on the properties of dilute-solutions and polymer-films. Angew. Makromol. Chem. 1992, 200, 173–181. [Google Scholar] [CrossRef]
- Król, P.; Król, B. Structures, properties and applications of the polyurethane ionomers. J. Mater. Sci. 2020, 55, 73–87. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Z.; Luo, S.; Yang, K.; Wu, X.J.; Ren, T.B. High-performance anionic waterborne polyurethane/Ag nanocomposites with excellent antibacterial property via in situ synthesis of Ag nanoparticles. RSC Adv. 2017, 7, 42296. [Google Scholar] [CrossRef] [Green Version]
- Saadat-Monfareda, A.; Mohsenia, M.; Hashemi Tabatabaei, M. Polyurethane nanocomposite films containing nano-cerium oxide as UV absorber. Part 1. Static and dynamic light scattering, small angle neutron scattering and optical studies. Colloid Surf. A-Physicochem. Eng. Asp. 2012, 408, 64–70. [Google Scholar] [CrossRef]
- Zhang, S.W.; Zhang, D.D.; Bai, H.Y.; Ming, W.H. ZnO nanoparticles coated with amphiphilic polyurethane for transparent polyurethane nanocomposites with enhanced mechanical and UV-shielding performance. ACS Appl. Nano Mater. 2020, 3, 59–67. [Google Scholar] [CrossRef]
- Liu, H.; Gao, J.C.; Huang, W.J.; Dai, K.; Zheng, G.Q.; Liu, C.T.; Shen, C.Y.; Yan, X.R.; Guo, J.; Guo, Z.H. Electrically conductive strain sensing polyurethane nanocomposites with synergistic carbon nanotubes and graphene bifillers. Nanoscale 2016, 8, 12977. [Google Scholar] [CrossRef]
- Verma, G. Weathering, salt spray corrosion and mar resistance mechanism of clay (nano-platelet) reinforced polyurethane nanocomposite coatings. Prog. Org. Coat. 2019, 129, 260–270. [Google Scholar] [CrossRef]
- Du, W.N.; Zhang, Z.T.; Su, H.; Lin, H.; Li, Z.J. Urethane-functionalized graphene oxide for improving compatibility and thermal conductivity of waterborne polyurethane composites. Ind. Eng. Chem. Res. 2018, 57, 7146–7155. [Google Scholar] [CrossRef]
- Ireni, N.G.; Karuppaiah, M.; Narayan, R.; Raju, K.V.S.N.; Basak, P. TiO2/Poly(thiourethane-urethane)-urea nanocomposites: Anticorrosion materials with NIR-reflectivity and high refractive index. Polymer 2017, 119, 142–151. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Dao, P.H.; Duong, K.L.; Duong, Q.H.; Vu, Q.T.; Nguyen, A.H.; Mac, V.P.; Le, T.L. Effect of R-TiO2 and ZnO nanoparticles on the UV-shielding efficiency of water-borne acrylic coating. Prog. Org. Coat. 2017, 110, 114–121. [Google Scholar] [CrossRef]
- Melinte, V.; Buruiana, T.; Rosca, I.; Chibac, A.L. TiO2-based photopolymerized hybrid catalysts with visible light catalytic activity induced by in situ generated Ag/Au NPs. ChemistrySelect 2019, 4, 5138–5149. [Google Scholar] [CrossRef]
- Tao, P.; Li, Y.; Rungta, A.; Viswanath, A.; Gao, J.; Benicewicz, B.C.; Siegel, R.W.; Schadler, L.S. TiO2 nanocomposites with high refractive index and transparency. J. Mat. Chem. 2011, 21, 18623–18629. [Google Scholar] [CrossRef]
- Bet-moushoul, E.; Mansourpanah, Y.; Farhadi, K.; Tabatabaei, M. TiO2 nanocomposite based polymeric membranes: A review on performance improvement for various applications in chemical engineering processes. Chem. Eng. J. 2016, 283, 29–46. [Google Scholar] [CrossRef]
- Barba-Nieto, I.; Caudillo-Flores, U.; Fernández-García, M.; Kubacka, A. Sunlight-operated TiO2-based photocatalysts. Molecules 2020, 25, 4008. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Barati, A. Efficient preparation of hybrid nanocomposite coatings based on poly(vinyl alcohol) and silane coupling agent modified TiO2 nanoparticles. Prog. Org. Coat. 2011, 71, 391–398. [Google Scholar] [CrossRef]
- Loste, J.; Lopez-Cuesta, J.M.; Billon, L.; Garay, H.; Save, M. Transparent polymer nanocomposites: An overview on their synthesis and advanced properties. Prog. Polym. Sci. 2019, 89, 133–158. [Google Scholar] [CrossRef]
- Gong, Z.L.; Tang, D.Y.; Guo, Y.D. The fabrication and self-flocculation effect of hybrid TiO2 nanoparticles grafted with poly(N-isopropylacrylamide) at ambient temperature via surface-initiated atom transfer radical polymerization. J. Mater. Chem. 2012, 22, 16872. [Google Scholar] [CrossRef]
- Dalod, A.R.M.; Henriksen, L.; Grande, T.; Einarsrud, M.A. Functionalized TiO2 nanoparticles by single-step hydrothermal synthesis: The role of the silane coupling agents. Beilstein J. Nanotechnol. 2017, 8, 304–312. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wang, X.; Li, N.; Xia, J.; Meng, Q.; Ding, J.; Lu, J. Synthesis and characterization of TiO2/graphene oxide nanocomposites for photoreduction of heavy metal ions in reverse osmosis concentrate. RSC Adv. 2018, 8, 34241. [Google Scholar] [CrossRef] [Green Version]
- Wanag, G.A.; Sienkiewicz, A.; Rokicka-Konieczna, P.; Kusiak-Nejman, E.; Morawski, A.W. Influence of modification of titanium dioxide by silane coupling agents on the photocatalytic activity and stability. J. Environ. Chem. Eng. 2020, 8, 103917. [Google Scholar] [CrossRef]
- Barrioni, B.R.; de Carvalho, S.M.; Orefice, R.L.; de Oliveira, A.A.R.; Pereira, M.D. Synthesis and characterization of biodegradable polyurethane films based on HDI with hydrolysable crosslinked bonds and a homogeneous structure for biomedical applications. Mater. Sci. Eng. C-Mater. Biol. Appl. 2015, 52, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kim, B.S. Shape memory polyurethane biocomposites based on toughened polycaprolactone promoted by nano-chitosan. Nanomaterials 2019, 9, 225. [Google Scholar] [CrossRef] [Green Version]
- Gupta, K.K.; Kundan, A.; Mishra, P.K.; Srivastava, P.; Mohanty, S.; Singh, N.K.; Mishra, A.; Maiti, P. Polycaprolactone composites with TiO2 for potential nanobiomaterials: Tunable properties using different phases. Phys. Chem. Chem. Phys. 2012, 14, 12844–12853. [Google Scholar] [CrossRef] [PubMed]
- Serkis, M.; Spirkova, M.; Poreba, R.; Hodan, J.; Kredatusova, J.; Kubies, D. Hydrolytic stability of polycarbonate-based polyurethane elastomers tested in physiologically simulated conditions. Polym. Degrad. Stabil. 2015, 119, 23–34. [Google Scholar] [CrossRef]
- Duran, I.R.; Laroche, G. Current trends, challenges, and perspectives of anti-fogging technology: Surface and material design, fabrication strategies, and beyond. Prog. Mater. Sci. 2019, 99, 106–186. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Kumar, R. Superhydrophilic TiO2 thin film by nanometer scale surface roughness and dangling bonds. Appl. Surf. Sci. 2016, 364, 51–60. [Google Scholar] [CrossRef]
- Zheng, Z.Y.; Liu, Y.P.; Wang, L.; Yu, L.; Cen, Y.; Zhu, T.T.; Yu, D.M.; Chen, C.G. A novel organic-inorganic zwitterionic acrylate polymer for high performance anti-fog coating. Prog. Org. Coat. 2020, 142, 105578. [Google Scholar] [CrossRef]
- da Silva, G.R.; da Silva-Cunha, A.; Behar-Cohen, F.; Ayres, E.; Orefice, R.L. Biodegradation of polyurethanes and nanocomposites to non-cytotoxic degradation products. Polym. Degrad. Stabil. 2010, 95, 491–499. [Google Scholar] [CrossRef]
- Rogulska, M.; Kultys, A.; Lubczak, J. New thermoplastic polyurethane elastomers based on aliphatic–aromatic chain extenders with different content of sulfur atoms. J. Therm. Anal. Calorim. 2015, 121, 397–410. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Development of nanocomposites reinforced with carboxylatedpoly(ether ether ketone) grafted to zinc oxide with superior antibacterial properties. ACS Appl. Mater. Interfaces 2014, 6, 3729–3741. [Google Scholar] [CrossRef] [Green Version]
- Anancharoenwong, E.; Chueangchayaphan, W.; Rakkapao, N.; Marthosa, S.; Chaisrikhwun, B. Thermo-mechanical and antimicrobial properties of natural rubber-based polyurethane nanocomposites for biomedical applications. Polym. Bull. 2021, 78, 833–848. [Google Scholar] [CrossRef]
- Cao, X.; Habibi, Y.; Lucia, L.A. One-pot polymerization, surface grafting, and processing of waterborne polyurethane-cellulose nanocrystal nanocomposites. J. Mater. Chem. 2009, 19, 7137–7145. [Google Scholar] [CrossRef]
- Chirayil, C.J.; Mathew, M.; Hassan, P.A.; Mozetic, M.; Thomas, S. Rheological behaviour of nanocellulose reinforced unsaturated polyester nanocomposites. Int. J. Biol. Macromol. 2014, 69, 274–281. [Google Scholar] [CrossRef]
- Molkenova, A.; Khamkhash, L.; Zhussupbekova, A.; Zhussupbekov, K.; Sarsenov, S.; Taniguchi, I.; Shvets, I.V.; Atabaev, T.S. Solution-based deposition of transparent Eu-doped titanium oxide thin films for potential security labeling and UV screening. Nanomaterials 2020, 10, 1132. [Google Scholar] [CrossRef]
- Johansson, W.; Peralta, A.; Jonson, B.; Anand, S.; Österlund, L.; Karlsson, S. Transparent TiO2 and ZnO thin films on glass for UV protection of PV modules. Front. Mater. 2019, 6, 259. [Google Scholar] [CrossRef]











| Sample | PCL (mol) | PEG (mol) | IPDI (mol) | NMDA (mol) | 1,4-BD (mol) | TiO2-f | |
|---|---|---|---|---|---|---|---|
| (mol) | (wt.%) | ||||||
| PU-1 | 1 | 0 | 3 | 1 | 1 | 0 | 0 |
| PU-1-0.5 | 1 | 0 | 3 | 1 | 1 | 0.5 | 8.5 |
| PU-1-1.0 | 1 | 0 | 3 | 1 | 1 | 1 | 17 |
| PU-2 | 0.8 | 0.2 | 3 | 1 | 1 | 0 | 0 |
| PU-2-0.5 | 0.8 | 0.2 | 3 | 1 | 1 | 0.5 | 8.5 |
| PU-2-1.0 | 0.8 | 0.2 | 3 | 1 | 1 | 1 | 17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stroea, L.; Chibac-Scutaru, A.-L.; Melinte, V. Aliphatic Polyurethane Elastomers Quaternized with Silane-Functionalized TiO2 Nanoparticles with UV-Shielding Features. Polymers 2021, 13, 1318. https://doi.org/10.3390/polym13081318
Stroea L, Chibac-Scutaru A-L, Melinte V. Aliphatic Polyurethane Elastomers Quaternized with Silane-Functionalized TiO2 Nanoparticles with UV-Shielding Features. Polymers. 2021; 13(8):1318. https://doi.org/10.3390/polym13081318
Chicago/Turabian StyleStroea, Lenuta, Andreea-Laura Chibac-Scutaru, and Violeta Melinte. 2021. "Aliphatic Polyurethane Elastomers Quaternized with Silane-Functionalized TiO2 Nanoparticles with UV-Shielding Features" Polymers 13, no. 8: 1318. https://doi.org/10.3390/polym13081318