The II–I Phase Transition Behavior of Butene-1 Copolymers with Hydroxyl Groups
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Copolymerization of Butene-1 with 6-Iodo-1-Hexene
2.3. Synthesis of Functionalized Polybutene-1
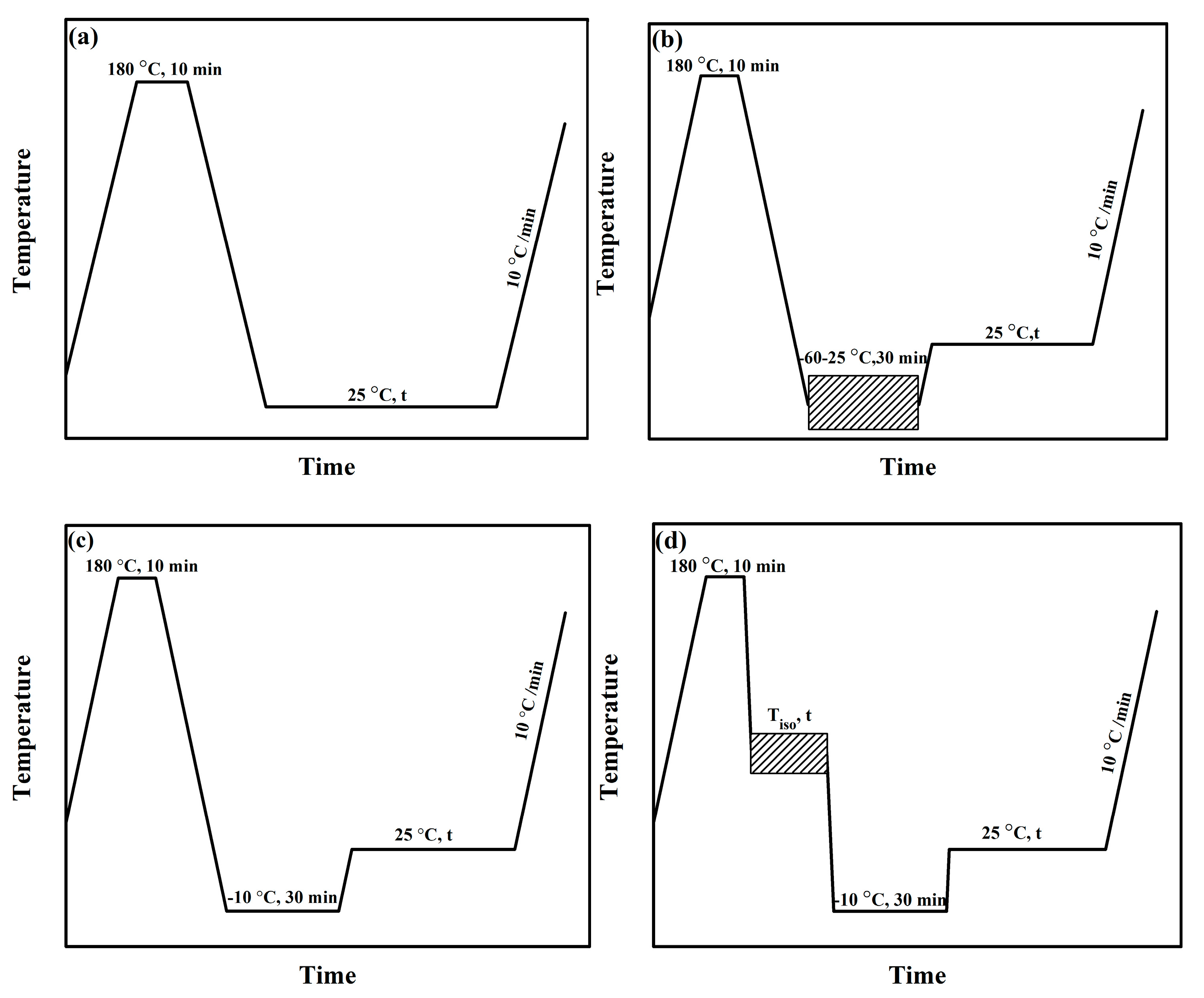
2.4. Methods
3. Results and Discussion
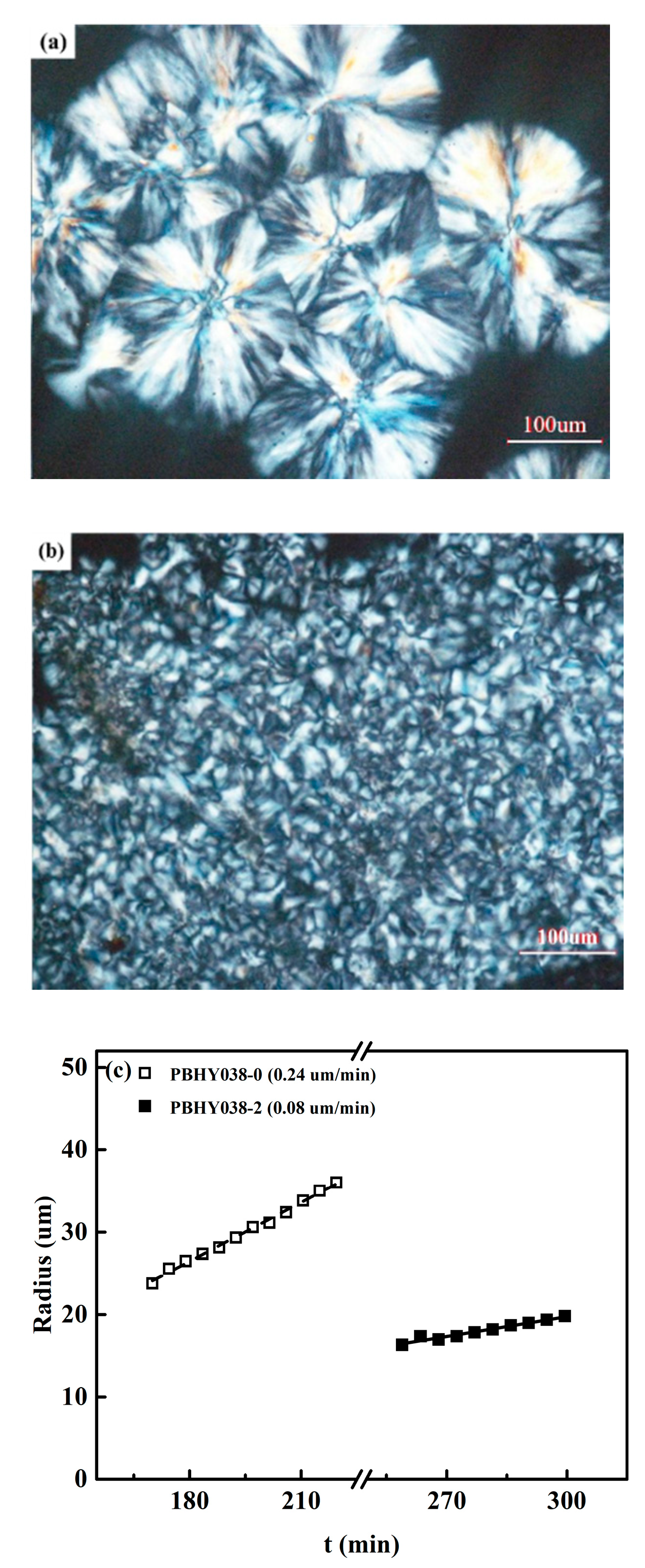
3.1. Influence of the Hydroxyl Groups on Crystallization
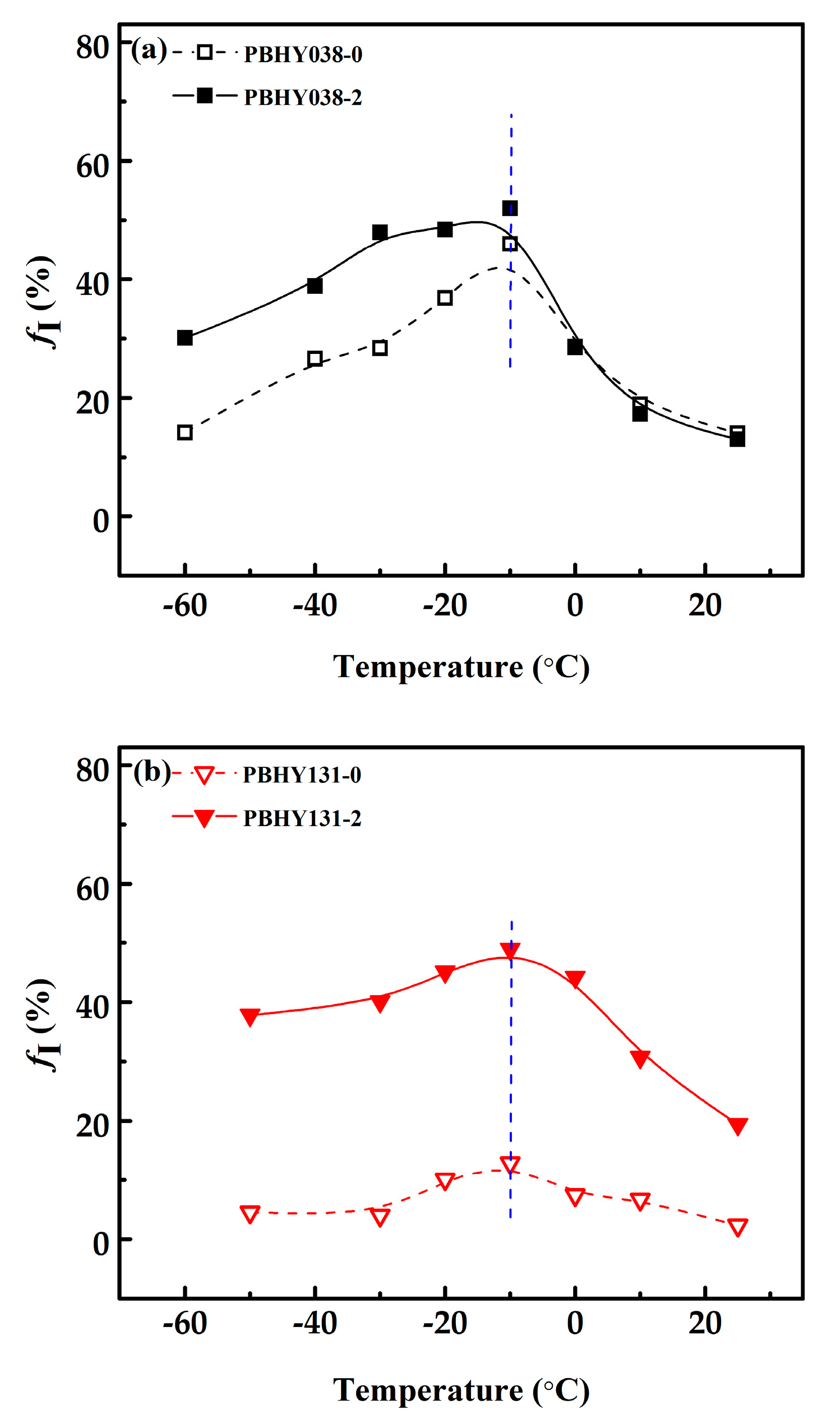
3.2. Influence of the Hydroxyl Groups in Phase Transition
3.3. The Relationship between Phase Transition and Transition Temperatures
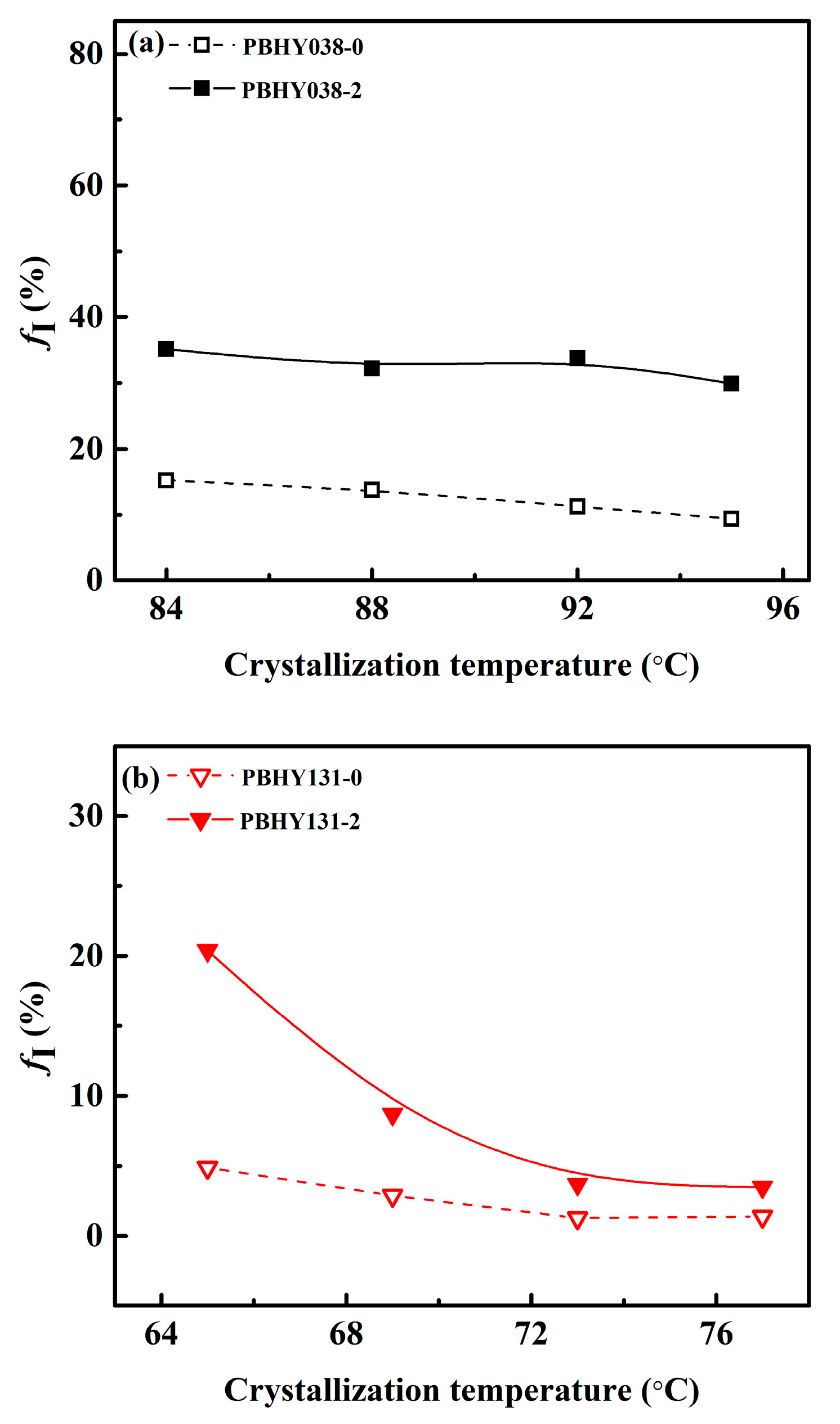
3.4. The Relationship between Crystallization Temperature and Phase Transition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zou, C.; Zhang, H.; Tan, C.; Cai, Z. Polyolefins with intrinsic antimicrobial properties. Macromolecules 2021, 54, 64–70. [Google Scholar] [CrossRef]
- Zou, C.; Chen, C.L. Polar-functionalized, crosslinkable, self-healing, and photoresponsive polyolefins. Angew. Chem. Int. Ed. 2020, 59, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Walsh, D.J.; Su, E.; Guironnet, D. Catalytic synthesis of functionalized (polar and non-polar) polyolefin block copolymers. Chem. Sci. 2018, 9, 4703–4707. [Google Scholar] [CrossRef] [Green Version]
- Na, Y.; Chen, C.L. Catechol-functionalized polyolefins. Angew. Chem. Int. Ed. 2020, 59, 7953–7959. [Google Scholar] [CrossRef]
- Yuan, X.P.; Matsuyama, Y.; Chung, T.C.M. Synthesis of functionalized isotactic polypropylene dielectrics for electric energy storage applications. Macromolecules 2010, 43, 4011–4015. [Google Scholar] [CrossRef]
- Gupta, S.; Yuan, X.P.; Chung, T.C.M.; Kumar, S.; Cakmak, M.; Weiss, R.A. Effect of hydroxyl-functionalization on the structure and properties of polypropylene. Macromolecules 2013, 46, 5455–5463. [Google Scholar] [CrossRef]
- Gupta, S.; Yuan, X.P.; Chung, T.C.M.; Cakmak, M.; Weiss, R.A. Isothermal and non-isothermal crystallization kinetics of hydroxyl-functionalized polypropylene. Polymer 2014, 55, 924–935. [Google Scholar] [CrossRef]
- Misra, M.; Agarwal, M.; Sinkovits, D.W.; Kumar, S.K.; Wang, C.; Pilania, G.; Ramprasad, R.; Weiss, R.A.; Yuan, X.P.; Chung, T.C.M. Enhanced polymeric dielectrics through incorporation of hydroxyl groups. Macromolecules 2014, 47, 1122–1129. [Google Scholar] [CrossRef]
- Dai, S.Y.; Li, S.K.; Xu, G.Y.; Chen, C.L. Direct synthesis of Polar functionalized polyethylene thermoplastic elastomer. Macromolecules 2020, 53, 2539–2546. [Google Scholar] [CrossRef]
- Shang, R.; Gao, H.; Luo, F.L.; Li, Y.L.; Wang, B.; Ma, Z.; Pan, L.; Li, Y.S. Functional isotactic polypropylenes via efficient direct copolymerizations of propylene with various amino-functionalized α-olefins. Macromolecules 2019, 52, 9280–9290. [Google Scholar] [CrossRef]
- Luciani, L.; Seppälä, J.; Löfgren, B. Poly-1-butene: Its preparation, properties and challenges. Prog. Polym. Sci. 1988, 13, 37–62. [Google Scholar] [CrossRef]
- Miller, R.L.; Holland, V.F. On transformations in isotactic polybutene-1. J. Polym. Sci. Part B Polym. Lett. 1964, 2, 519–521. [Google Scholar] [CrossRef]
- Boor, J.; Youngman, E.A. Polymorphsim in poly-1-butene: Appearent direct formation of mofification I. J. Polym. Sci. Part B Polym. Lett. 1964, 2, 903–907. [Google Scholar] [CrossRef]
- Turner-Jones, A.; Aizlewood, J.M. Crystalline form of polypentene-1. J. Polym. Sci. Part B Polym. Lett. 1963, 1, 471–476. [Google Scholar] [CrossRef]
- Wang, Y.T.; Liu, P.R.; Lu, Y.; Men, Y.F. Mechanism of polymorph selection during crystallization of random butene-1/ethylene copolymer. Chin. J. Polym. Sci. 2016, 34, 1014–1020. [Google Scholar] [CrossRef]
- Xin, R.; Zhang, J.; Sun, X.L.; Li, H.H.; Ren, Z.J.; Yan, S.K. Polymorphic behavior and phase transition of poly(1-butene) and its copolymers. Polymers 2018, 10, 556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natta, G.; Corradini, P.; Bassi, I.W. Crystal structure of isotactic poly-alpha-butene. Nuovo Cim. 1960, 15, 52–56. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, D.C.; Yan, S.K. Direct formation of form I poly(1-butene) single crystals from melt crystallization in ultrathin films. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 2641–2645. [Google Scholar] [CrossRef]
- Su, F.M.; Li, X.Y.; Zhou, W.M.; Zhu, S.S.; Ji, Y.X.; Wang, Z.; Qi, Z.; Li, L.B. Direct formation of isotactic poly(1-butene) form I crystal from memorized ordered melt. Macromolecules 2013, 46, 7399–7405. [Google Scholar] [CrossRef]
- Ji, Y.X.; Su, F.M.; Cui, K.P.; Huang, N.D.; Qi, Z.M.; Li, L.B. Mixing assisted direct formation of isotactic poly(1-butene) form I′ crystals from blend melt of isotactic poly(1-butene)/polypropylene. Macromolecules 2016, 49, 1761–1769. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Ruiz de Ballesteros, O.; Esposito, F.; Laguzza, D.; Di Girolamo, R.; Resconi, L. Crystallization properties and polymorphic behavior of isotactic poly(1-Butene) from metallocene catalysts: The crystallization of form I from the melt. Macromolecules 2009, 42, 8286–8297. [Google Scholar] [CrossRef]
- Cavallo, D.; Zhang, L.; Sics, I.; Alfonso, G.C.; Dumas, P.; Marco, C.; Ellis, G. The morphology and polymorphism of self-nucleated trigonal isotactic poly(1-butene) studied by synchrotron IR microspectroscopy. Cryst. Eng. Comm. 2016, 18, 816–828. [Google Scholar] [CrossRef]
- Androsch, R.; Hohlfeld, R.; Frank, W.; Nase, M.; Cavallo, D. Transition from two-stage to direct melt-crystallization in isotactic random butene-1/propene copolymers. Polymer 2013, 54, 2528–2534. [Google Scholar] [CrossRef]
- Li, Y.P.; Guo, Z.X.; Xue, M.; Yan, S.K. Epitaxial recrystallization of iPBu in Form II on an oriented iPS film initially induced by oriented form I iPBu. Macromolecules 2019, 52, 4232–4239. [Google Scholar] [CrossRef]
- Hu, J.; Tashiro, K. Time-resolved imaging of the phase transition in the melt-grown spherulites of isotactic polybutene-1 as detected by the two-dimensional polarized IR imaging technique. J. Phys. Chem. B 2016, 120, 4689–4698. [Google Scholar] [CrossRef]
- Azzurri, F.; Flores, A.; Alfonso, G.C.; Calleja, F.J.B. Polymorphism of isotactic poly(1-butene) as revealed by microindentation hardness. 1. Kinetics of the transformation. Macromolecules 2002, 35, 9069–9073. [Google Scholar] [CrossRef]
- Azzurria, F.; Flores, A.; Alfonso, G.C.; Sicsc, I.; Hsiao, B.S.; Calleja, F.J.B. Polymorphism of isotactic polybutene-1 as revealed by microindentation hardness. Part II: Correlations to microstructure. Polymer 2003, 44, 1641–1645. [Google Scholar] [CrossRef]
- Rubin, I.D. Relative stabilities of polymorphs of polybutene-1 obtained from the melt. J. Polym. Sci. Part B Polym. Lett. 1964, 2, 747–749. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, X.; Cavallo, D.; Müller, A.J.; Wang, D. Promotion of self-nucleation with latent form I nuclei in polybutene-1 and its copolymer. Macromolecules 2018, 51, 6037–6046. [Google Scholar] [CrossRef]
- Danusso, F.; Gianotti, G. Isotactic polybutene-1: Formation and transformation of modification 2. Die Makromol. Chem. 1965, 88, 149–158. [Google Scholar] [CrossRef]
- Tarallo, O.; Ruiz de Ballesteros, O.; Bellissimo, A.; Scoti, M.; Malafronte, A.; Auriemma, F.; De Rosa, C. Crystallization and mechanical properties of metallocene made 1-butene-pentene and 1-butene-hexene isotactic copolymers. Polymer 2018, 158, 231–242. [Google Scholar] [CrossRef]
- Tashiro, K.; Hu, J.; Wang, H.; Hanesaka, M.; Saiani, A. Refinement of the crystal structures of forms I and II of isotactic polybutene-1 and a proposal of phase transition mechanism between Them. Macromolecules 2016, 49, 1392–1404. [Google Scholar] [CrossRef]
- Qiao, Y.N.; Wang, H.; Men, Y.F. Retardance of form II to form I transition in polybutene-1 at late stage: A proposal of a new mechanism. Macromolecules 2018, 51, 2232–2239. [Google Scholar] [CrossRef]
- Lotz, B.; Thierry, A. Spherulite morphology of form III isotactic poly(1-butene). Macromolecules 2003, 36, 286–290. [Google Scholar] [CrossRef]
- Zheng, L.R.; Liu, L.; Shao, C.G.; Wang, W.; Wang, B.; Pan, L.; Li, Y.S.; Ma, Z. Phase transition from tetragonal form II to hexagonal form I of butene-1/4-Methyl-1-pentene random copolymers: Molecular factor versus stretching stimuli. Macromolecules 2019, 52, 1188–1199. [Google Scholar] [CrossRef]
- Nakafuku, C.; Miyaki, T. Effect of pressure on the melting and crystallization behavior of isotactic polybutene-1. Polymer 1983, 24, 141–148. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, T.; Li, L.; Li, D.C.; Yuan, W.K.; Zhao, L. Controlling crystal phase transition from form II to I in isotactic poly-1-butene using CO2. Polymer 2012, 53, 6102–6111. [Google Scholar] [CrossRef]
- Shi, J.Y.; Wu, P.Y.; Li, L.; Liu, T.; Zhao, L. Crystalline transformation of isotactic polybutene-1 in supercritical CO2 studied by in-situ fourier transform infrared spectroscopy. Polymer 2009, 50, 5598–5604. [Google Scholar] [CrossRef]
- Li, L.; Liu, T.; Zhao, L.; Yuan, W.K. CO2-induced crystal phase transition from form II to form I in isotactic poly-1-butene. Macromolecules 2009, 42, 2286–2290. [Google Scholar] [CrossRef]
- Liu, Y.P.; Cui, K.P.; Tian, N.; Zhou, W.Q.; Meng, L.P.; Li, L.B.; Ma, Z.; Wang, X.L. Stretch-induced crystal–crystal transition of polybutene-1: An in situ synchrotron radiation wide-angle x-ray scattering study. Macromolecules 2012, 45, 2764–2772. [Google Scholar] [CrossRef]
- Tanaka, A.; Sugimoto, N.; Aasada, T.; Onogi, S. Orientation and crystal transformation in polybutene-1 under stress relaxation. Polym. J. 1975, 7, 529–537. [Google Scholar] [CrossRef]
- Chen, W.; Li, X.Y.; Li, H.L.; Su, F.M.; Zhou, W.; Li, L.B. Deformation-induced crystal–crystal transition of polybutene-1: An in situ FTIR imaging study. J. Mater. Sci. 2013, 48, 4925–4933. [Google Scholar] [CrossRef]
- Cavallo, D.; Kanters, M.J.W.; Caelers, H.J.M.; Portale, G.; Govaert, L.E. Kinetics of the polymorphic transition in isotactic poly(1-butene) under uniaxial extension. New insights from designed mechanical histories. Macromolecules 2014, 47, 3033–3040. [Google Scholar] [CrossRef] [Green Version]
- Lou, Y.H.; Liao, Y.L.; Pan, L.; Wang, B.; Li, Y.S.; Ma, Z. Effect of linear and ring-like co-units on the temperature dependence of nucleation and growth in II-I phase transition of butene-1 copolymers. Chin. J. Polym. Sci. 2018, 36, 1269–1276. [Google Scholar] [CrossRef]
- Gianotti, G.; Capizzi, A. Butene-1/propylene copolymers. Influence of the comonomerie units on polymorphism. Die Makromol. Chem. 1969, 124, 152–159. [Google Scholar] [CrossRef]
- De Rosa, C.; Scoti, M.; Ruiz de Ballesteros, O.; Di Girolamo, R.; Auriemma, F.; Malafronte, A. Propylene–butene copolymers: Tailoring mechanical properties from isotactic polypropylene to polybutene. Macromolecules 2020, 53, 4407–4421. [Google Scholar] [CrossRef]
- Qiao, Y.N.; Men, Y.F. Intercrystalline links determined kinetics of form II to I polymorphic transition in polybutene-1. Macromolecules 2017, 50, 5490–5497. [Google Scholar] [CrossRef]
- An, C.B.; Lou, Y.H.; Li, Y.L.; Wang, B.; Pan, L.; Ma, Z.; Li, Y.S. Unusual II–I phase transition behavior of polybutene-1 ionomers in the presence of long-chain branch and ionic functional groups. Macromolecules 2019, 52, 4634–4645. [Google Scholar] [CrossRef]
- Wang, X.Y.; Long, Y.Y.; Wang, Y.X.; Li, Y.S. Insights into propylene/ω-halo-α-alkenes copolymerization promoted by rac-Et(Ind)2 ZrCl2 and (pyridyl-amido)hafnium catalysts. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 3421–3428. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, Y.X.; Shi, X.C.; Liu, J.Y.; Chen, C.L.; Li, Y.S. Syntheses of well-defined functional isotactic polypropylenes via efficient copolymerization of propylene with ω-halo-α-alkenes by post-metallocene hafnium catalyst. Macromolecules 2014, 47, 552–559. [Google Scholar] [CrossRef]
- Wang, X.Y.; Li, Y.G.; Mu, H.L.; Pan, L.; Li, Y.S. Efficient synthesis of diverse well-defined functional polypropylenes with high molecular weights and high functional group contents via thiol–halogen click chemistry. Polym. Chem. 2015, 6, 1150–1158. [Google Scholar] [CrossRef]
- Zhang, S.J.; Han, J.R.; Gao, Y.; Guo, B.H.; Reiter, G.; Xu, J. Determination of the critical size of secondary nuclei on the lateral growth front of lamellar polymer crystals. Macromolecules 2019, 52, 7439–7447. [Google Scholar] [CrossRef]
- Zhuravlev, E.; Schmelzer, J.W.P.; Abyzov, A.S.; Fokin, V.M.; Androsch, R.; Schick, C. Experimental test of tammann’s nuclei development approach in crystallization of macromolecules. Cryst. Growth Des. 2015, 15, 786–798. [Google Scholar] [CrossRef]
- Cheng, S.Z.D. Chapter 2: Thermodynamics and kinetics of phase transitions. In Phase Transitions in Polymers; Elsevier: Amsterdam, The Netherlands, 2008; pp. 17–59. [Google Scholar]
- Androsch, R.; Schick, C.; Rhoades, A.M. Application of tammann’s two-stage crystal nuclei development method for analysis of the thermal stability of homogeneous crystal nuclei of poly(ethylene terephthalate). Macromolecules 2015, 48, 8082–8089. [Google Scholar] [CrossRef]
- Qiao, Y.N.; Wang, Q.; Men, Y.F. Kinetics of nucleation and growth of form II to I polymorphic transition in polybutene-1 as revealed by stepwise annealing. Macromolecules 2016, 49, 5126–5136. [Google Scholar] [CrossRef]
- Deboer, J.H. Molecular mechanism of rate processes in solids. C. Steady-State Processes Involving Lattice Re-Arrangement Introductory Paper. Discuss. Faraday Soc. 1957, 23, 171–182. [Google Scholar] [CrossRef]
- Burgers, W.G.; Groen, L.J. Mechanisim and kinetics of the allotropic transformation of tin. Faraday Soc. 1957, 23, 171–182. [Google Scholar]
- Powers, J.; Hoffman, J.D.; Week, J.J.; Quinn, F.A. Crystallization kinetics and polymorphic transformations in polybutene-1. J. Res. Natl. Bur. Stand. Sect. A 1965, 69A, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Gohil, R.M.; Miles, M.J.; Petermann, J. On the molecular mechanism of the crystal transformation (tetragonal-hexagonal) in polybutene-1. J. Macromol. Sci. Part B 2006, 21, 189–201. [Google Scholar] [CrossRef]
- Maruyama, M.; Sakamoto, Y.; Nozaki, K.; Yamamoto, T.; Kajioka, H.; Toda, A.; Yamada, K. Kinetic study of the II–I phase transition of isotactic polybutene-1. Polymer 2010, 51, 5532–5538. [Google Scholar] [CrossRef]





| Sample Code | Incorp. (mol%) | Number of Hydroxyl Groups in Each Branch | Mw (105 Da) | Mw/Mn |
|---|---|---|---|---|
| PBHY038-0 | 0.38 | 0 | 6.9 | 2.02 |
| PBHY038-2 | 0.38 | 2 | 6.9 | 2.02 |
| PBHY131-0 | 1.31 | 0 | 5.2 | 2.23 |
| PBHY131-2 | 1.31 | 2 | 5.2 | 2.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, T.; Li, W.; Lou, Y.; Liu, L.; Ma, Z. The II–I Phase Transition Behavior of Butene-1 Copolymers with Hydroxyl Groups. Polymers 2021, 13, 1315. https://doi.org/10.3390/polym13081315
Li Y, Li T, Li W, Lou Y, Liu L, Ma Z. The II–I Phase Transition Behavior of Butene-1 Copolymers with Hydroxyl Groups. Polymers. 2021; 13(8):1315. https://doi.org/10.3390/polym13081315
Chicago/Turabian StyleLi, Yuanyuan, Tao Li, Wei Li, Yahui Lou, Liyuan Liu, and Zhe Ma. 2021. "The II–I Phase Transition Behavior of Butene-1 Copolymers with Hydroxyl Groups" Polymers 13, no. 8: 1315. https://doi.org/10.3390/polym13081315






