A Comparative Study on Physiochemical, Thermomechanical, and Electrochemical Properties of Sulfonated Poly(Ether Ether Ketone) Block Copolymer Membranes with and without Fe3O4 Nanoparticles
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. SPEEK (X) and Hydrophobic Oligomer(Y) Synthesis
2.3. XY Block Copolymer Synthesis
2.4. Membrane Preparation
3. Characterizations
3.1. Structural Characterizations
3.2. Morphological Characterizations
3.3. Thermal and Mechanical Characterizations
4. Measurements
4.1. Water Uptake
4.2. Swelling Ratio
4.3. Ion Exchange Capacity
4.4. Contact Angle
4.5. Proton Conductivity
4.6. Fabrication of Membrane Electrode Assembly and PEFC Test
5. Results and Discussion
5.1. Structural Properties
5.2. Morphological Properties
5.3. Thermal and Mechanical Properties
5.4. Water Uptake, Swelling Ratio, IEC, and Contact Angle
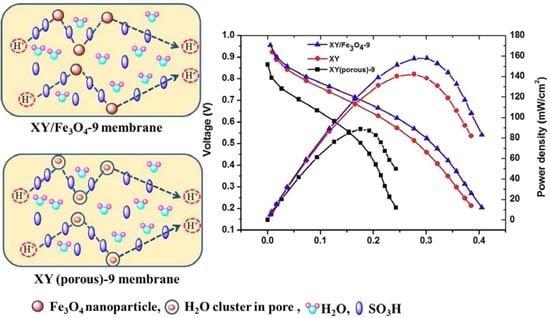
5.5. Proton Conductivity and PEFC Performance and Durability
6. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Tang, Y.; Bi, L.; Mortimer, R.J.G.; Pan, G. Cryogenic circulation for indoor air pollution control. Sci. Total Environ. 2019, 651, 1451–1456. [Google Scholar] [CrossRef]
- Bruce, N.; Padilla, R.P.; Albalak, R. Indoor air pollution in developing countries: A major environmental and public health challenge. B World Health Organ. 2000, 78, 1078–1092. [Google Scholar]
- Sivanjineyulu, V.; Chang, Y.-H.; Chiu, F.-C. Characterization of carbon nanotube- and organoclay-filled polypropylene/poly(butylene succinate) blend-based nanocomposites with enhance rigidity and electrical conductivity. J. Polym. Res. 2017, 24, 130. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nature 2001, 114, 332–337. [Google Scholar] [CrossRef]
- Mehta, V.; Cooper, J.S. Review and analysis of PEM fuel cell design and manufacturing. J. Power Source 2003, 114, 32–53. [Google Scholar] [CrossRef]
- Chen, H.; Cong, T.N.; Yang, W.; Tan, C.; Li, Y.; Ding, Y. Progress in electrical energy storage system: A critical review. Prog. Nat. Sci. 2009, 19, 291–312. [Google Scholar] [CrossRef]
- Mishra, A.K.; Kim, N.H.; Jung, D.; Lee, J.H. Enhanced mechanical properties and proton conductivity of Nafion–SPEEK–GO composite membranes for fuel cell applications. J. Membr. Sci. 2014, 458, 128–135. [Google Scholar] [CrossRef]
- Matsumoto, K.; Higashihara, T.; Ueda, M. Locally and densely sulfonated poly(ether sulfone)s as proton exchange membrane. Macromolecules 2009, 42, 1161–1166. [Google Scholar] [CrossRef]
- Hickner, M.A.; Ghassemi, H.; Kim, Y.S.; Einsla, B.R.; McGrath, J.E. Alternative polymer systems for proton exchange membranes (PEMs). Chem. Rev. 2004, 104, 4587–4612. [Google Scholar] [CrossRef]
- Peckham, T.J.; Holdcroft, S. Structure-morphology-property relationships of non-perfluorinated proton-conducting membranes. Adv. Mater. 2010, 22, 4667–4690. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Kothapalli, R.V. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Robert, C.L.; Valle, K.; Pereir, F.; Sanchez, C. Design and properties of functional hybrid organic-inorganic membranes for fuel cells. Chem. Soc. Rev. 2011, 40, 961–1005. [Google Scholar] [CrossRef] [PubMed]
- Nagarale, R.K.; Shin, W.; Singh, P.K. Progress in ionic organic-inorganic composite membranes for fuel cell applications. Polym. Chem. 2010, 1, 388–408. [Google Scholar] [CrossRef]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef] [Green Version]
- Yadav, M. Study on thermal and mechanical properties of cellulose/iron oxide bionanocomposites film. Compos. Commun. 2018, 10, 1–5. [Google Scholar] [CrossRef]
- Yadava, M.; Muna, S.; Hyunb, J.; Kima, J. Synthesis and characterization of iron oxide/cellulose nanocomposite film. Int. J. Biol. Macromolecules 2015, 74, 142–149. [Google Scholar] [CrossRef]
- Jain, M.; Yadav, M.; Kohout, T.; Lahtinen, M.; Garg, V.K.; Sillanpa, M. Development of iron oxide/activated carbon nanoparticle composite for the removal of Cr(VI), Cu(II) and Cd(II) ions from aqueous solution. Water Resour. Ind. 2018, 20, 54–74. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Kim, A.R.; Nahm, K.S.; Yoo, D.J. Ternary hybrid (SPEEK/SPVdF-HFP/GO) based membrane electrolyte for the applications of fuel cells: Profile of improved mechanical strength, thermal stability and proton conductivity. RSC Adv. 2016, 6, 108851–108863. [Google Scholar] [CrossRef]
- Kim, A.R.; Vinothkannan, M.; Park, C.J.; Yoo, D.J. Alleviating the Mechanical and Thermal Degradations of Highly Sulfonated Poly(Ether Ether Ketone) Blocks via Copolymerization with Hydrophobic Unit for Intermediate Humidity Fuel Cells. Polymers 2018, 10, 1346. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Kim, A.R.; Gnana Kumar, G.; Yoo, D.J. Sulfonated graphene oxide/Nafion composite membranes for high temperature and low humidity proton exchange membrane fuel cells. RSC Adv. 2018, 8, 7494–7508. [Google Scholar] [CrossRef] [Green Version]
- Hickner, M.A.; Pivovar, B.S. The chemical and structural nature of proton exchange membrane fuel cell properties. Fuel Cells 2005, 5, 213–227. [Google Scholar] [CrossRef]
- Hibbs, M.R.; Hickner, M.A.; Alam, T.M.; McIntyre, S.K.; Fujimoto, C.H.; Cornelius, C.J. Transport properties of hydroxide and proton conducting membranes. Chem. Mater. 2008, 20, 2566–2573. [Google Scholar] [CrossRef]
- Janssen, G.J.M.; Overvelde, M.L.J. Water transport in the proton exchange membrane fuel cell: Measurement of effective drag coefficient. J. Power Source 2011, 101, 117–125. [Google Scholar] [CrossRef]
- Rowe, A.; Li, X. Mathematical model of proton exchange membrane fuel cell. J. Power Sourc. 2011, 102, 82–96. [Google Scholar] [CrossRef]
- Kreuer, K.D. On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells. J. Membrane Sci. 2001, 185, 29–39. [Google Scholar] [CrossRef]
- Lee, C.H.; Park, H.B.; Lee, Y.M.; Lee, R.D. Importance of proton conductivity measurement in polymer electrolyte membrane for fuel cell application. Ind. Eng. Chem. Res. 2005, 44, 7617–7626. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, H.; Sun, J.C.; Zhang, J. AC impedance technique in PEM fuel cell diagnosis A review. Int. J. Hydrogen Energ. 2007, 32, 4365–4380. [Google Scholar] [CrossRef]
- Gomadam, P.M.; Weidner, J.W. Analysis of electrochemical impedance spectroscopy in proton exchange membrane fuel cells. Int. J. Energy Res. 2005, 29, 1133–1151. [Google Scholar] [CrossRef]
- Chu, J.Y.; Kim, A.R.; Nahm, K.S.; Lee, K.H.; Yoo, D.J. Synthesis and characterization of partially fluorinated sulfonated poly(arylene biphenylsulfone ketone) block copolymers containing 6F-BPA and perfluorobiphenylene units. Int. J. Hydrogen Eng. 2013, 38, 6268–6274. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Kim, A.R.; Gnana Kumar, G.; Yoon, J.M.; Yoo, D.J. Toward improved mechanical strength, oxidative stability and proton conductivity of an aligned quadratic hybrid (SPEEK/FPAPB/Fe3O4-FGO) membrane for application in high temperature and low humidity fuel cells. RSC Adv. 2017, 7, 39034–39048. [Google Scholar] [CrossRef]
- Lee, K.H.; Chu, J.Y.; Kim, A.R.; Yoo, D.J. Enhanced performance of a sulfonated poly(arylene ether ketone) block copolymer bearing pendant sulfonic acid groups for polymer electrolyte membrane fuel cells operating at 80% relative humidity. ACS Appl. Mater. Interfaces 2018, 10, 20835–20844. [Google Scholar] [CrossRef] [PubMed]
- Doyle, C.D. Estimating thermal stability of experimental polymers by empirical thermogravimetric analysis. Anal. Chem. 1961, 33, 77–79. [Google Scholar] [CrossRef]
- Gil, M.; Ji, X.; Li, X.; Nab, H.; Hampsey, J.E.; Lua, Y. Direct synthesis of sulfonated aromatic poly(ether ether ketone) proton exchange membranes for fuel cell applications. J. Membrane Sci. 2004, 234, 75–81. [Google Scholar] [CrossRef]
- Wang, F.; Hickner, M.; Kim, Y.S. Direct polymerization of sulfonated poly(arylene ether sulfone) random (statistical) copolymers: Candidates for new proton exchange membranes. J. Membrane Sci. 2002, 197, 232–241. [Google Scholar] [CrossRef]
- Kim, A.R.; Vinothkannan, M.; Yoo, D.J. Artificially designed, low humidifying organic-inorganic (SFBC-50/FSiO2) composite membrane for electrolyte applications of fuel cells. Compos. Part B-Eng. 2017, 130, 103–118. [Google Scholar] [CrossRef]
- Eikerling, M.; Kornyshev, A.A.; Kuznetsov, A.M.; Ulstrup, J.; Walbran, S. Mechanisms of proton conductance in polymer electrolyte membranes. J. Phys. Chem. B 2001, 105, 3646–3662. [Google Scholar] [CrossRef]
- Sagarik, K.; Phonyiem, M.; Ngama, C.L.; Chaiwongwattana, S. Mechanisms of proton transfer in Nafions: Elementary reactions at the sulfonic acid groups. Phys. Chem. Chem. Phys. 2008, 10, 2098–2112. [Google Scholar] [CrossRef]
- Kreuer, K.D.; Rabenau, A.; Weppner, W. Vehicle mechanism, a new model for the interpretation of the conductivity of fast proton conductors. Angew. Chem. Int. Ed. Engl. 1982, 21, 206–209. [Google Scholar] [CrossRef]













| Polymer | Mn | Mw | Mz | Mw/Mn (PDI) |
|---|---|---|---|---|
| X | 62,600 | 142,800 | 282,000 | 2.2 |
| Y | 4800 | 20,100 | 47,800 | 4.1 |
| XY | 48,900 | 164,100 | 455,400 | 3.3 |
| Membrane | Water Uptake (%) | Swelling Ratio (%) | IEC (meq g−1) | ||
|---|---|---|---|---|---|
| Mass | Length (SL) | Area (SA) | Volume (SV) | ||
| XY | 11.9 | 4.6 | 9.7 | 12.9 | 1.24 |
| XY/Fe3O4-3 | 14.2 | 5.0 | 10.2 | 13.9 | 1.33 |
| XY/Fe3O4-9 | 16.7 | 6.7 | 13.7 | 16.4 | 1.41 |
| XY/porous-3 | 22.3 | 7.3 | 15.0 | 18.7 | 1.60 |
| XY/porous-9 | 26.6 | 7.5 | 16.0 | 20.9 | 1.81 |
| Nafion-117 | 13.8 | 9.2 | 18.9 | 24.7 | 0.94 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, A.R.; Yoo, D.J. A Comparative Study on Physiochemical, Thermomechanical, and Electrochemical Properties of Sulfonated Poly(Ether Ether Ketone) Block Copolymer Membranes with and without Fe3O4 Nanoparticles. Polymers 2019, 11, 536. https://doi.org/10.3390/polym11030536
Kim AR, Yoo DJ. A Comparative Study on Physiochemical, Thermomechanical, and Electrochemical Properties of Sulfonated Poly(Ether Ether Ketone) Block Copolymer Membranes with and without Fe3O4 Nanoparticles. Polymers. 2019; 11(3):536. https://doi.org/10.3390/polym11030536
Chicago/Turabian StyleKim, Ae Rhan, and Dong Jin Yoo. 2019. "A Comparative Study on Physiochemical, Thermomechanical, and Electrochemical Properties of Sulfonated Poly(Ether Ether Ketone) Block Copolymer Membranes with and without Fe3O4 Nanoparticles" Polymers 11, no. 3: 536. https://doi.org/10.3390/polym11030536