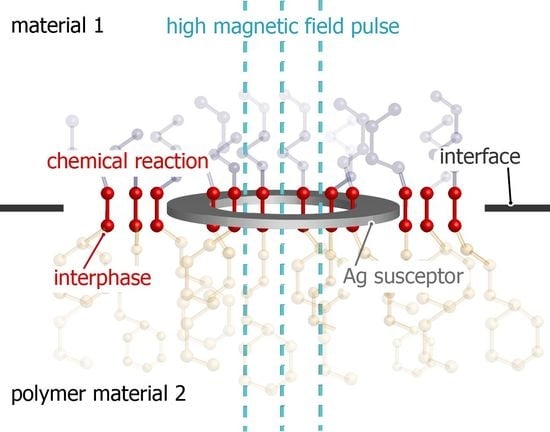
Inductive Heating Using a High-Magnetic-Field Pulse to Initiate Chemical Reactions to Generate Composite Materials
Abstract
:1. Introduction
1.1. Induction Heating for Materials Processing
1.2. Interaction of Polymers with Electromagnetic Radiation
1.3. Chemical Reactions at Polymer-Polymer Interfaces
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. High-Magnetic-Field Induced Heating Experiment
2.4. Atomic Force Microscopy
2.5. Resistance Measurements
2.6. Infrared Spectroscopic Imaging
2.7. Differential Scanning Calorimetry
3. Results and Discussions
3.1. Chemical Reaction between PC and ODA
3.2. Estimation of the Induced Temperature Change
3.3. Morphology of the Ag Loops
3.4. Verification of Chemical Reaction by FTIR Spectroscopic Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Grulke, N. Induction Heating in Light and Heavy-Metal Working Industry. Metallurgia 1975, 29, 22–27. [Google Scholar]
- Wilkinson, W.D. High-Frequency Induction Brazing, Soldering and Welding. Metallurgia 1983, 50, 26–27. [Google Scholar]
- Boulos, M.I. Materials Processing using Induction Plasma Technology. In Proceedings of the 121st TMS Annual Meeting, San Diego, CA, USA, 1–5 March 1992; pp. 295–308. [Google Scholar]
- Herlach, D.M.; Cochrane, R.F.; Egry, I.; Fecht, H.J.; Greer, A.L. Containerless Processing in the Study of Metallic Melts and their Solidification. Int. Mater. Rev. 1993, 38, 273–347. [Google Scholar] [CrossRef]
- Gagnoud, A.; Etay, J.; Garnier, M. The Levitation Melting using Cold Crucible Technique. Trans. Iron Steel Inst. Jpn. 1988, 28, 36–40. [Google Scholar] [CrossRef]
- Tanaka, T. A New induction cooking range for heating any kind of metal vessels. IEEE Trans. Consum. Electron. 1989, 35, 635–641. [Google Scholar] [CrossRef]
- Xu, C.; Zhou, N.; Xie, J.; Gong, X.; Chen, G.; Song, G. Investigation on eddy current pulsed thermography to detect hidden cracks on corroded metal surface. NDT E Int. 2016, 84, 27–35. [Google Scholar] [CrossRef]
- Netzelmann, U.; Walle, G.; Lugin, S.; Ehlen, A.; Bessert, S.; Valeske, B. Induction thermography: Principle, applications and first steps towards standardisation. Quant. Infrared Thermogr. J. 2016, 13, 170–181. [Google Scholar] [CrossRef]
- Abliz, D.; Duan, Y.; Steuernagel, L.; Xie, L.; Li, D.; Ziegmann, G. Curing methods for advanced polymer composites-a review. Polym. Polym. Compos. 2013, 21, 341–348. [Google Scholar] [CrossRef]
- Bayerl, T.; Duhovic, M.; Mitschang, P.; Bhattacharyya, D. The heating of polymer composites by electromagnetic induction—A review. Compos. Part A 2014, 57, 27–40. [Google Scholar] [CrossRef]
- Hubbard, J.W.; Orange, F.; Guinel, M.J.F.; Guenthner, A.J.; Mabry, J.M.; Sahagun, C.M.; Rinaldi, C. Curing of a bisphenol E based cyanate ester using magnetic nanoparticles as an internal heat source through induction heating. ACS Appl. Mater. Interfaces 2013, 5, 11329–11335. [Google Scholar] [CrossRef] [PubMed]
- Schieler, O.; Beier, U. Induction Welding of Hybrid Thermoplastic-thermoset Composite Parts. KMUTNB Int. J. Appl. Sci. Technol. 2016, 9, 27–36. [Google Scholar] [CrossRef]
- Caine, P. Resin applications using induction heat. In Proceedings of the Electrical Insulation Conference and Electrical-Manufacturing-and-Coil-Winding Technology Conference, Indianapolis, IN, USA, 23–25 September 2003; pp. 137–140. [Google Scholar]
- Deng, S.Q.; Djukic, L.; Paton, R.; Ye, L. Thermoplastic-epoxy interactions and their potential applications in joining composite structures-a review. Compos. A 2015, 68, 121–132. [Google Scholar] [CrossRef]
- Devillers, S.; Barthélémy, B.; Delhalle, J.; Mekhalif, Z. Induction heating Vs conventional heating for the hydrothermal treatment of nitinol and its subsequent 2-(Methacryloyloxy)ethyl 2-(trimethlyammonio)ethyl phosphate coating by surfaceinitiated atom transfer radical polymerization. ACS Appl. Mater. Interfaces 2011, 3, 4059–4066. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Cramer, N.B.; Stevens, B.E.; Sani, R.L.; Bowman, C.N. Induction curing of thiol–acrylate and thiol–ene composite systems. Macromolecules 2011, 44, 4988–4996. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.P.; de Campos Silva, P.; Marques, F.D.; Gomes de Souza, F., Jr. Glycerin-Based Polyurethane Obtained by Inverse Emulsion: Comparison between Magnetic Induction and Conventional Heating. Macromol. Symp. 2018, 380, 1800091. [Google Scholar] [CrossRef]
- Jo, W.J.; Baek, S.K.; Park, J.H. A wireless actuating drug delivery system. IOP Publ. J. Micromech. Microeng. 2015, 25, 4. [Google Scholar] [CrossRef]
- Marques, F.D.; Nele de Souza, M.; Gomes de Souza, F., Jr. Sealing system activated by magnetic induction polymerization. J. Appl. Polym. Sci. 2017, 134, 45549. [Google Scholar] [CrossRef]
- Liu, C.W.; Qu, C.Y.; Han, L.; Wang, D.Z.; Xiao, W.B.; Hou, X. Preparation of carbon fiber-reinforced polyimide composites via in situ induction heating. High Perform. Polym. 2017, 29, 1027–1036. [Google Scholar] [CrossRef]
- Zimmerer, C.; Heinrich, G.; Wolff-Fabris, F.; Koch, E.; Steiner, G. Chemical reactions between poly(carbonate) and poly(vinyl amine) thermally induced by a high magnetic field pulse. Polymer 2013, 54, 6732–6738. [Google Scholar] [CrossRef]
- Weigel, T.; Mohr, R.; Lendlein, A. Investigation of parameters to achieve temperatures required to initiate the shape-memory effect of magnetic nanocomposites by inductive heating. Smart Mater. Struct. 2009, 18, 025011–025020. [Google Scholar] [CrossRef]
- Vialle, G.; Di Prima, M.; Hocking, E.; Gall, K.; Garmestani, H.; Sanderson, T.; Arzberger, S.C. Remote activation of nanomagnetite reinforced shape memory polymer foam. Smart Mater. Struct. 2009, 18, 115014. [Google Scholar] [CrossRef]
- Suwanwatana, W.; Yarlagadda, S.; Gillespie, J.W., Jr. Hysteresis heating based induction bonding of thermoplastic composites. Compos. Sci. Technol. 2006, 66, 1713–1723. [Google Scholar] [CrossRef]
- Bowman, C.; Adzima, B.; Kloxin, C. Radio Frequency Magnetic Responsive Polymer Composites. U.S. Patent 9044902, 2 June 2015. [Google Scholar]
- Al-Harbi, L.M.; Darwish, M.S.A.; Khowdiary, M.M.; Stibor, I. Controlled Preparation of Thermally Stable Fe-Poly(dimethylsiloxane) Composite by Magnetic Induction Heating. Polymers 2018, 10, 507. [Google Scholar] [CrossRef]
- Bae, D.H.; Shon, M.Y.; Oh, S.T.; Kim, G.N. Study on the Heating Behavior of Fe3O4-Embedded Thermoplastic Polyurethane Adhesive Film via Induction heating. Bull. Korean Chem. Soc. 2016, 37, 1211–1218. [Google Scholar] [CrossRef]
- Javadi, A.; Mehr, H.S.; Sobani, M.; Soucek, M.D. Cure-on-command technology: A review of the current state of the art. Prog. Org. Coat. 2016, 100, 2–31. [Google Scholar] [CrossRef]
- Zimmerer, C.; Steiner, G.; Heinrich, G. Method for Bonding Plastics and Method for Releasing a Bond in the Plastic Composite and a Plastic Composite. U.S. Patent US20150111042A1, 20 September 2016. [Google Scholar]
- Gu, Y.; Kornev, K.G. Ferromagnetic Nanorods in Applications to Control of the In-Plane Anisotropy of Composite Films and for In Situ Characterization of the Film Rheology. Adv. Funct. Mater. 2016, 26, 3796–3808. [Google Scholar] [CrossRef]
- Cebers, A.; Erglis, K. Flexible Magnetic Filaments and their Applications. Adv. Funct. Mater. 2016, 26, 3783–3795. [Google Scholar] [CrossRef]
- Farahani, R.D.; Dubé, M. Novel heating elements for induction welding of carbon fiber/polyphenylene sulfide thermoplastic composites. Adv. Eng. Mater. 2017, 19, 1700294. [Google Scholar] [CrossRef]
- Salski, B.; Gwarek, W.; Korpas, P. Electromagnetic inspection of carbon-fiber-reinforced polymer composites with coupled spiral inductors. IEEE Trans. Microw. Theory Tech. 2014, 62, 1535–1544. [Google Scholar] [CrossRef]
- Moser, L.; Mitschang, P.; Schlarb, A.K. Induction welding of thermoplastic polymer composites using robotic techniques. Sampe J. 2008, 44, 43–48. [Google Scholar]
- Zimmerer, C.; Steiner, G.; Heinrich, G. Method for Bonding Plastics and Method for Releasing a Bond in the Plastic Composite and a Plastic Composite. 2012. [Google Scholar]
- Sung, Y.; Hwang, S.; Lee, H.; Huang, D. Study on Induction Heating Coil for Uniform Mold Cavity Surface Heating. Adv. Mech. Eng. 2014, 6, 349078. [Google Scholar] [CrossRef]
- Nian, S.C.; Tsai, T.H.; Huang, M.S. Novel inductive hot embossing for increasing micromolding efficiency. Int. Commun. Heat Mass Transf. 2016, 70, 38–46. [Google Scholar] [CrossRef]
- Miller, K.J.; Collier, K.N.; Soll-Morris, H.B.; Swaminathan, R.; McHenry, M.E. Induction heating of FeCo nanoparticles for rapid rf curing of epoxy composites. J. Appl. Phys. 2009, 105, 07E714. [Google Scholar] [CrossRef]
- Qiao, R.; Yang, C.; Gao, M. Superparamagnetic iron oxide nanoparticles: From preparations to in vivo MRI applications. J. Mater. Chem. 2009, 19, 6274–6293. [Google Scholar] [CrossRef]
- Lee, H.; Thirunavukkarasu, G.K.; Kim, S.; Lee, J.Y. Remote induction of in situ hydrogelation in a deep tissue, using an alternating magnetic field and superparamagnetic nanoparticles. Nano Res. 2018, 11, 5997–6009. [Google Scholar] [CrossRef]
- Lin, K.; Gu, Y.; Zhang, H.; Qiang, Z.; Vogt, B.D.; Zacharia, N.S. Accelerated Amidization of Branched Poly(ethylenimine)/Poly(acrylic acid) Multilayer Films by Microwave Heating. Langmuir 2016, 32, 9118–9125. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Qiang, Z.; Lee, B.; Becker, M.L.; Vogt, B.D. Solid state microwave synthesis of highly crystalline ordered mesoporous hausmannite Mn3O4 films. CrystEngComm 2017, 19, 4294–4303. [Google Scholar] [CrossRef]
- Nirmalraj, P.N.; Lutz, T.; Kumar, S.; Duesberg, G.S.; Boland, J.J. Nanoscale Mapping of Electrical Resistivity and Connectivity in Graphene Strips and Networks. Nano Lett. 2011, 11, 16–22. [Google Scholar] [CrossRef]
- Alves da Silva, C.; Pötschke, P.; Simon, F.; Holzschuh, M.; Pionteck, J.; Heinrich, G.; Wießner, S.; Zimmerer, C. Synthesis and Characterization of Graphene Derivatives for Application in Magnetic High-Field Induction Heating. AIP Conf. Proc. 2019, 2055, 130006. [Google Scholar]
- Pionteck, J.; Melchor, E.M.V.; Piana, F.; Omastova, M.; Luyt, A.S.; Voit, B. Reduced percolation concentration in polypropylene/expanded graphite composites: Effect of viscosity and polypyrrole. J. Appl. Polym. Sci. 2015, 132, 41994. [Google Scholar] [CrossRef]
- Rudnev, V.; Loveless, D.; Cook, R.; Black, M. Handbook of Induction Heating; Marcel Dekker AG: Basel, Switzerland; New York, NY, USA, 2003. [Google Scholar]
- Rapoport, E.; Pleshivtseva, Y. Optimal Control of Induction Heating Processes; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Zimmerer, C.; Nagel, J.; Steiner, G.; Heinrich, G. Nondestructive molecular characterization of polycarbonate—Polyvinylamine composites after thermally induced aminolysis. Macromol. Mater. Eng. 2016, 301, 648–652. [Google Scholar] [CrossRef]
- Wedgewood, A.; Hardy, P. Induction welding of thermoset composite adherends using thermoplastic interlayers and susceptors. Technol. Transf. A Glob. Community 1996, 28, 850–861. [Google Scholar]
- Zimmerer, C.; Matulaitiene, I.; Niaura, G.; Reuter, U.; Janke, A.; Boldt, R.; Sablinskas, V.; Steiner, G. Nondestructive Characterization of the Polycarbonate—Octadecylamine Interface by Surface Enhanced Raman Spectroscopy. Polym. Test. 2019, 73, 152–158. [Google Scholar] [CrossRef]
- European Magnetic Field Laboratory. Available online: https//emfl.eu (accessed on 1 February 2019).
- Helmholtz-Zentrum Dresden-Rossendorf. Available online: https://www.hzdr.de (accessed on 1 February 2019).
- Zherlitsy, S.; Herrmannsdörfer, T.; Wustmann, B.; Wosnitza, J. Design and performance of non-destructive pulsed magnets at the Dresden High Magnetic Field Laboratory. IEEE Trans. Appl. Supercond. 2010, 20, 672–675. [Google Scholar] [CrossRef]
- Gmelins Handbuch der Anorganischen Chemie, Bd. 61 Silber, 8th ed.; Verlag Chemie: Weinheim, Germany, 1973.












| Heating Effect | Susceptor Material | Remarks | Literature | |
|---|---|---|---|---|
| resistive heating | eddy current | electrically conductive | [2,6,21] | |
| junction heating | local dielectric heating | particulate, electrically conductive | various factors in complex relationship | [2] |
| magnetic polarization | hysteresis heating | particulate, ferro- and ferrimagnetic | various factors in complex relationship | [3] |
| skin effect | current flow at outer surface | electrically conductive | increases with frequency | [5,46] |
| proximity effect | current crowding | electrically conductive | increases with frequency, thermal hot spot formation | [47] |
| edge effect | diffraction on arras | electrically conductive | defined by susceptors geometry | [9] |
| ring effect | variation in field line density | all susceptor materials | inhomogeneous field, which depends on the coupling distance | [2,47] |
| Ring Material | Specific Heat (J/kg K) | Electrical Resistivity (Ω m) | Density (kg/m3) |
|---|---|---|---|
| Ag | 235 | 1.6 × 10−8 | 10.5 × 103 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmerer, C.; Mejia, C.S.; Utech, T.; Arnhold, K.; Janke, A.; Wosnitza, J. Inductive Heating Using a High-Magnetic-Field Pulse to Initiate Chemical Reactions to Generate Composite Materials. Polymers 2019, 11, 535. https://doi.org/10.3390/polym11030535
Zimmerer C, Mejia CS, Utech T, Arnhold K, Janke A, Wosnitza J. Inductive Heating Using a High-Magnetic-Field Pulse to Initiate Chemical Reactions to Generate Composite Materials. Polymers. 2019; 11(3):535. https://doi.org/10.3390/polym11030535
Chicago/Turabian StyleZimmerer, Cordelia, Catalina Salazar Mejia, Toni Utech, Kerstin Arnhold, Andreas Janke, and Joachim Wosnitza. 2019. "Inductive Heating Using a High-Magnetic-Field Pulse to Initiate Chemical Reactions to Generate Composite Materials" Polymers 11, no. 3: 535. https://doi.org/10.3390/polym11030535