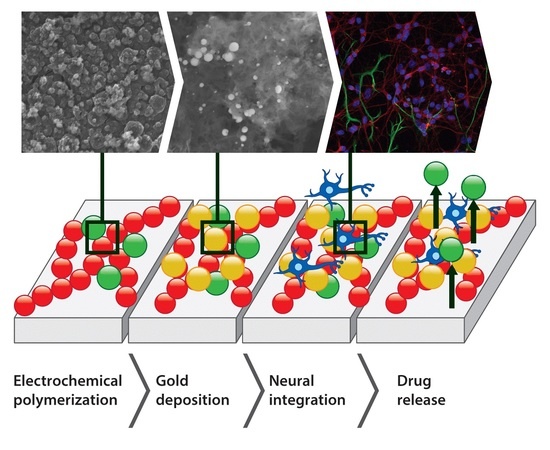
The Synergistic Effects of Gold Particles and Dexamethasone on the Electrochemical and Biological Performance of PEDOT Neural Interfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Poly(3,4-Ethylenedioxythiophene) (PEDOT)-Based Coatings
2.2. Electrochemical Characterization
2.3. Chemical and Morphological Characterization
2.4. In Vitro Drug Release
2.5. Biological Characterization
3. Results
3.1. Electrochemical Characterization
3.2. Surface Characterization
3.3. In Vitro Drug Release
3.4. Biological Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, X.L.; Xiong, Y.Y.; Xu, G.L.; Liu, X.F. Deep Brain Stimulation. Interv. Neurol. 2013, 1, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Shih, J.J.; Krusienski, D.J.; Wolpaw, J.R. Brain-computer interfaces in medicine. Mayo Clin. Proc. 2012, 87, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Vallejo-Giraldo, C.; Kelly, A.; Biggs, M.J.P. Biofunctionalisation of electrically conducting polymers. Drug Discov. Today 2014, 19, 88–94. [Google Scholar] [CrossRef] [PubMed]
- McConnell, G.C.; Rees, H.D.; Levey, A.I.; Gutekunst, C.A.; Gross, R.E.; Bellamkonda, R.V. Implanted neural electrodes cause chronic, local inflammation that is correlated with local neurodegeneration. J. Neural Eng. 2009, 6, 056003. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; McConnell, G.C.; Ross, J.D.; Deweerth, S.P.; Bellamkonda, R.V. A novel dexamethasone-releasing, anti-inflammatory coating for neural implants. In Proceedings of the 2nd International IEEE EMBS Conference on Neural Engineering, Washington, DC, USA, 16–19 March 2005. [Google Scholar]
- Svirskis, D.; Travas-Sejdic, J.; Rodgers, A.; Garg, S. Electrochemically controlled drug delivery based on intrinsically conducting polymers. J. Control. Release 2010, 146, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Krukiewicz, K.; Zak, J.K. Conjugated polymers as robust carriers for controlled delivery of anti-inflammatory drugs. J. Mater. Sci. 2014, 49, 5738–5745. [Google Scholar] [CrossRef] [Green Version]
- Carli, S.; Trapella, C.; Armirotti, A.; Fantinati, A.; Ottonello, G.; Scarpellini, A.; Prato, M.; Fadiga, L.; Ricci, D. Biochemically Controlled Release of Dexamethasone Covalently Bound to PEDOT. Chem.-A Eur. J. 2018, 24, 10300–10305. [Google Scholar] [CrossRef]
- Zhong, Y.; Bellamkonda, R.V. Dexamethasone-coated neural probes elicit attenuated inflammatory response and neuronal loss compared to uncoated neural probes. Brain Res. 2007, 1148, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Koehler, P.J. Use of corticosteroids in neuro-oncology. Anticancer Drugs 1995, 6, 19–33. [Google Scholar] [CrossRef]
- Wadhwa, R.; Lagenaur, C.F.; Cui, X.T. Electrochemically controlled release of dexamethasone from conducting polymer polypyrrole coated electrode. J. Control. Release 2006, 110, 531–541. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, G.; Moulton, S.E.; Innis, P.C.; Wallace, G.G. Polyterthiophene as an electrostimulated controlled drug release material of therapeutic levels of dexamethasone. Synth. Met. 2010, 160, 1107–1114. [Google Scholar] [CrossRef]
- Krukiewicz, K. Tailorable drug capacity of dexamethasone-loaded conducting polymer matrix. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Istanbul, Turkey, 8 August 2018. [Google Scholar]
- Castagnola, E.; Carli, S.; Vomero, M.; Scarpellini, A.; Prato, M.; Goshi, N.; Fadiga, L.; Kassegne, S.; Ricci, D. Multilayer poly(3,4-ethylenedioxythiophene)-dexamethasone and poly(3,4-ethylenedioxythiophene)-polystyrene sulfonate-carbon nanotubes coatings on glassy carbon microelectrode arrays for controlled drug release. Biointerphases 2017, 12, 031002. [Google Scholar] [CrossRef] [PubMed]
- Boehler, C.; Kleber, C.; Martini, N.; Xie, Y.; Dryg, I.; Stieglitz, T.; Hofmann, U.G.; Asplund, M. Actively controlled release of Dexamethasone from neural microelectrodes in a chronic in vivo study. Biomaterials 2017, 129, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Goding, J.A.; Gilmour, A.D.; Martens, P.J.; Poole-Warren, L.A.; Green, R.A. Small bioactive molecules as dual functional co-dopants for conducting polymers. J. Mater. Chem. B 2015, 3, 5058–5069. [Google Scholar] [CrossRef]
- Abidian, M.R.; Kim, D.H.; Martin, D.C. Conducting-polymer nanotubes for controlled drug release. Adv. Mater. 2006, 18, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Massoumi, B.; Entezami, A. Electrochemically controlled binding and release of dexamethasone from conducting polymer bilayer films. J. Bioact. Compat. Polym. 2002, 17, 51–62. [Google Scholar] [CrossRef]
- Baranes, K.; Shevach, M.; Shefi, O.; Dvir, T. Gold Nanoparticle-Decorated Scaffolds Promote Neuronal Differentiation and Maturation. Nano Lett. 2016, 16, 2916–2920. [Google Scholar] [CrossRef]
- Demir, U.S.; Shahbazi, R.; Calamak, S.; Ozturk, S.; Gultekinoglu, M.; Ulubayram, K. Gold nano-decorated aligned polyurethane nanofibers for enhancement of neurite outgrowth and elongation. J. Biomed. Mater. Res. Part A 2018, 106, 1604–1613. [Google Scholar] [CrossRef]
- Shamaeli, E.; Alizadeh, N. Kinetic studies of electrochemically controlled release of salicylate from nanostructure conducting molecularly imprinted polymer. Electrochim. Acta 2013, 114, 409–415. [Google Scholar] [CrossRef]
- Vallejo-Giraldo, C.; Pampaloni, N.P.; Pallipurath, A.R.; Mokarian-Tabari, P.; O’Connell, J.; Holmes, J.D.; Trotier, A.; Krukiewicz, K.; Orpella-Aceret, G.; Pugliese, E.; et al. Preparation of Cytocompatible ITO Neuroelectrodes with Enhanced Electrochemical Characteristics Using a Facile Anodic Oxidation Process. Adv. Funct. Mater. 2017, 28, 1605035. [Google Scholar] [CrossRef] [Green Version]
- Krukiewicz, K.; Chudy, M.; Vallejo-Giraldo, C.; Skorupa, M.; Więcławska, D.; Turczyn, R.; Biggs, M. Fractal form PEDOT/Au assemblies as thin-film neural interface materials. Biomed. Mater. 2018, 13, 54102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallejo-Giraldo, C.; Pugliese, E.; Larrañaga, A.; Fernandez-Yague, M.A.; Britton, J.J.; Trotier, A.; Tadayyon, G.; Kelly, A.; Rago, I.; Sarasua, J.R.; Dowd, E.; Quinlan, L.R.; Pandit, A.; Biggs, M.J.P. Polyhydroxyalkanoate/carbon nanotube nanocomposites: Flexible electrically conducting elastomers for neural applications. Nanomedicine 2016, 11, 2547–2563. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, G.W.; Dockery, P.; Sullivan, A.M. Effects of growth/differentiation factor 5 on the survival and morphology of embryonic rat midbrain dopaminergic neurones in vitro. J. Neurocytol. 2004, 33, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, E.T.; Loughlin, J.P.; Herbert, K.R.; Dockery, P.; Samali, A.; Doyle, K.M.; Gorman, A.M. Functionality of NGF-protected PC12 cells following exposure to 6-hydroxydopamine. Biochem. Biophys. Res. Commun. 2006, 351, 890–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estrany, F.; Oliver, R.; Armelin, E.; Iribaren, J.I.; Liesa, F.; Aleman, C. Electroactive Properties and Electrochemical Stability of Poly(3,4-ethylenedioxythiophene) and Poly(N-methylpyrrole) Multi-layered Films Generated by Anodic Oxidation. Port. Electrochim. Acta 2007, 25, 55–65. [Google Scholar] [CrossRef]
- Hariri, M.B.; Dolati, A.; Moakhar, R.S. The Potentiostatic Electrodeposition of Gold Nanowire/Nanotube in HAuCl4 Solutions Based on the Model of Recessed Cylindrical Ultramicroelectrode Array. J. Electrochem. Soc. 2013, 160, D279–D288. [Google Scholar] [CrossRef]
- Cui, Z.; Coletta, C.; Rebois, R.; Baiz, S.; Gervais, M.; Goubard, F.; Aubert, P.H.; Dazzi, A.; Remita, S. Radiation-induced reduction-polymerization route for the synthesis of PEDOT conducting polymers. Radiat. Phys. Chem. 2016, 119, 157–166. [Google Scholar] [CrossRef]
- Coletta, C.; Cui, Z.; Dazzi, A.; Guigner, J.M.; Néron, S.; Marignier, J.L.; Remita, S. A pulsed electron beam synthesis of PEDOT conducting polymers by using sulfate radicals as oxidizing species. Radiat. Phys. Chem. 2016, 126, 21–31. [Google Scholar] [CrossRef]
- Rodrigues, L.B.; Leite, H.F.; Yoshida, M.I.; Saliba, J.B.; Junior, A.S.C.; Faraco, A.A.G. In vitro release and characterization of chitosan films as dexamethasone carrier. Int. J. Pharm. 2009, 368, 1–6. [Google Scholar] [CrossRef]
- Wang, C.; Hou, H.; Nan, K.; Sailor, M.J.; Freeman, W.R.; Cheng, L. Intravitreal controlled release of dexamethasone from engineered microparticles of porous silicon dioxide. Exp. Eye Res. 2014, 129, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, G.R.; Da Silva-Cunha, A.; Behar-Cohen, F.; Ayres, E.; Oréfice, R.L. Biodegradable polyurethane nanocomposites containing dexamethasone for ocular route. Mater. Sci. Eng. C 2011, 31, 414–422. [Google Scholar] [CrossRef]
- Krukiewicz, K.; Kruk, A.; Turczyn, R. Evaluation of drug loading capacity and release characteristics of PEDOT/naproxen system: Effect of doping ions. Electrochim. Actarochim. Acta. 2018, 289, 218–227. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, L.; Li, C.; Shi, G. Electrosynthesis of poly(3,4-ethylenedioxythiophene) microcups in the aqueous solution of LiClO4 and tri(ethylene glycol). Polymer 2006, 47, 4953–4958. [Google Scholar] [CrossRef]
- Mumtaz, M.; Ibarboure, E.; Labrugère, C.; Cloutet, E.; Cramail, H. Synthesis of PEDOT Nano-objects Using Poly(vinyl alcohol)-Based Reactive Stabilizers in Aqueous Dispersion. Macromolecules 2008, 41, 8964–8970. [Google Scholar] [CrossRef]
- Alizadeh, N.; Shamaeli, E. Electrochemically controlled release of anticancer drug methotrexate using nanostructured polypyrrole modified with cetylpyridinium: Release kinetics investigation. Electrochim. Acta 2014, 130, 488–496. [Google Scholar] [CrossRef]
- Howe, E.J.; Okesola, B.O.; Smith, D.K. Self-assembled sorbitol-derived supramolecular hydrogels for the controlled encapsulation and release of active pharmaceutical ingredients. Chem. Commun. 2015, 51, 7451–7454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamaeli, E.; Alizadeh, N. Nanostructured biocompatible thermal/electrical stimuli-responsive biopolymer-doped polypyrrole for controlled release of chlorpromazine: Kinetics studies. Int. J. Pharm. 2014, 472, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, N.; Shamaeli, E.; Fazili, M. Online Spectroscopic Monitoring of Drug Release Kinetics from Nanostructured Dual-Stimuli-Responsive Conducting Polymer. Pharm. Res. 2017, 34, 113–120. [Google Scholar] [CrossRef]
- Demir, E.; Inam, O.; Inam, R.; Aboul-Enein, H.Y. Voltammetric Determination of Ophthalmic Drug Dexamethasone Using Poly-glycine Multi Walled Carbon Nanotubes Modified Paste Electrode. Curr. Anal. Chem. 2018, 14, 83–89. [Google Scholar] [CrossRef]
- Rebuffat, A.G.; Tam, S.; Nawrocki, A.R.; Baker, M.E.; Frey, B.M.; Frey, F.J.; Odermatt, A. The 11-ketosteroid 11-ketodexamethasone is a glucocorticoid receptor agonist. Mol. Cell. Endocrinol. 2004, 214, 27–37. [Google Scholar] [CrossRef]
- Abidian, M.R.; Martin, D.C. Experimental and theoretical characterization of implantable neural microelectrodes modified with conducting polymer nanotubes. Biomaterials 2008, 29, 1273–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsabahy, M.; Wooley, K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Martin, D.C. Fuzzy gold electrodes for lowering impedance and improving adhesion with electrodeposited conducting polymer films. Sens. Actuators A Phys. 2003, 103, 384–394. [Google Scholar] [CrossRef]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef]
- Shain, W.; Spataro, L.; Dilgen, J.; Haverstick, K.; Retterer, S.; Isaacson, M.; Saltzman, M.; Turner, J.N. Controlling cellular reactive responses around neural prosthetic devices using peripheral and local intervention strategies. IEEE Trans. Neural Syst. Rehabil. Eng. 2003, 11, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Moulton, S.E.; Imisides, M.D.; Shepherd, R.L.; Wallace, G.G. Galvanic coupling conducting polymers to biodegradable Mg initiates autonomously powered drug release. J. Mater. Chem. 2008, 18, 3608–3613. [Google Scholar] [CrossRef]





| Polymer Matrix | k, 1/h | n | R2 |
|---|---|---|---|
| PEDOT-Dex | 0.95 | 0.28 | 0.94 |
| PEDOT-Dex/Au | 0.18 | 0.49 | 0.99 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krukiewicz, K.; Chudy, M.; Gregg, S.; Biggs, M.J.P. The Synergistic Effects of Gold Particles and Dexamethasone on the Electrochemical and Biological Performance of PEDOT Neural Interfaces. Polymers 2019, 11, 67. https://doi.org/10.3390/polym11010067
Krukiewicz K, Chudy M, Gregg S, Biggs MJP. The Synergistic Effects of Gold Particles and Dexamethasone on the Electrochemical and Biological Performance of PEDOT Neural Interfaces. Polymers. 2019; 11(1):67. https://doi.org/10.3390/polym11010067
Chicago/Turabian StyleKrukiewicz, Katarzyna, Magdalena Chudy, Stephen Gregg, and Manus J. P. Biggs. 2019. "The Synergistic Effects of Gold Particles and Dexamethasone on the Electrochemical and Biological Performance of PEDOT Neural Interfaces" Polymers 11, no. 1: 67. https://doi.org/10.3390/polym11010067