Glucose Oxidase Immobilized on a Functional Polymer Modified Glassy Carbon Electrode and Its Molecule Recognition of Glucose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
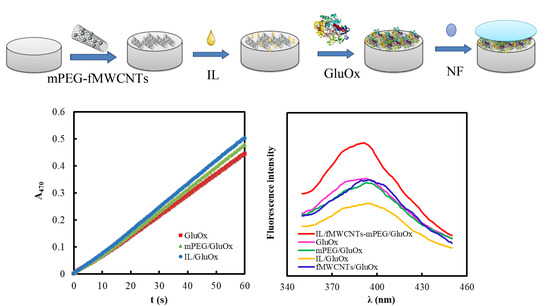
2.2. Preparation of Functional Polymer Modified GCE
2.3. Apparatus and Measurements
3. Results and Discussion
3.1. Electrochemical Behavior of the Different Modified GCEs
3.2. Electrocatalytic Behaviors of the NF/GluOx/IL/mPEG -fMWCNTs Modified GCE
3.3. Characterization of Composite Functional Polymer
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hecht, H.J.; Kalisz, H.M.; Hendle, J.; Schmid, R.D.; Schomburg, D. Crystal structure of glucose oxidase from aspergillus niger refined at 2·3 reslution. J. Mol. Biol. 1993, 229, 153–172. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.L.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 2010, 102, 29–45. [Google Scholar] [CrossRef]
- Wong, C.M.; Wong, K.H.; Chen, X.D. Glucose oxidase: Natural occurrence, function, properties and industrial applications. Appl. Microbiol. Biotechnol. 2008, 78, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Bankar, S.B.; Bule, M.V.; Singhal, R.S.; Ananthanarayan, L. Optimization of aspergillus niger fermentation for the production of glucose oxidase. Food Bioprocess Technol. 2009, 2, 344. [Google Scholar] [CrossRef]
- Hill, H.A.O. The development of bioelectrochemistry ☆. Coord. Chem. Rev. 1996, 151, 115–123. [Google Scholar] [CrossRef]
- Armstrong, F.A.; Wilson, G.S. Recent developments in faradaic bioelectrochemistry. Electrochim. Acta 2000, 45, 2623–2645. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification*. Anal. Lett. 2001, 34, 635–659. [Google Scholar]
- Gao, Y.F.; Yang, T.; Yang, X.L.; Zhang, Y.S.; Xiao, B.L.; Hong, J.; Sheibani, N.; Ghourchian, H.; Hong, T.; Moosavi-Movahedi, A.A. Direct electrochemistry of glucose oxidase and glucose biosensing on a hydroxyl fullerenes modified glassy carbon electrode. Biosens. Bioelectron. 2014, 60, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.N.; Safina, G.; Ludwig, R.; Gorton, L. Characteristics of third-generation glucose biosensors based on corynascus thermophilus cellobiose dehydrogenase immobilized on commercially available screen-printed electrodes working under physiological conditions. Anal. Biochem. 2012, 425, 36–42. [Google Scholar] [CrossRef]
- Yang, J.; Deng, S.; Lei, J.; Ju, H.; Gunasekaran, S. Electrochemical synthesis of reduced graphene sheet-aupd alloy nanoparticle composites for enzymatic biosensing. Biosens. Bioelectron. 2011, 29, 159–166. [Google Scholar] [CrossRef]
- Deng, C.; Chen, J.; Zhou, N.; Si, S. A sensitive and stable biosensor based on the direct electrochemistry of glucose oxidase assembled layer-by-layer at the multiwall carbon nanotube-modified electrode. Biosens. Bioelectron. 2011, 26, 213–219. [Google Scholar] [CrossRef]
- Cai, C.; Chen, J. Direct electron transfer of glucose oxidase promoted by carbon nanotubes. Anal. Biochem. 2004, 332, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Han, Z.; Pan, J.; Abdel-Halim, E.S.; Zhu, J.J. Direct electrochemistry of glucose oxidase and biosensing for glucose based on helical carbon nanotubes modified magnetic electrodes. Electrochim. Acta 2011, 58, 179–183. [Google Scholar] [CrossRef]
- Dai, Z.; Shao, G.; Hong, J.; Bao, J.; Shen, J. Immobilization and direct electrochemistry of glucose oxidase on a tetragonal pyramid-shaped porous zno nanostructure for a glucose biosensor. Biosens. Bioelectron. 2009, 24, 1286–1291. [Google Scholar] [CrossRef]
- Deng, C.; Chen, J.; Chen, X.; Xiao, C.; Nie, L.; Yao, S. Direct electrochemistry of glucose oxidase and biosensing for glucose based on boron-doped carbon nanotubes modified electrode. Biosens. Bioelectron. 2008, 23, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Yang, W.; Chen, X.; Evans, D.G. Direct electrochemistry of glucose oxidase on a graphite nanosheet–nafion composite film modified electrode. Electrochem. Commun. 2009, 11, 997–1000. [Google Scholar] [CrossRef]
- Gu, T.; Zhang, Y.; Deng, F.; Zhang, J.; Hasebe, Y. Direct electrochemistry of glucose oxidase and biosensing for glucose based on DNA/chitosan film. J. Environ. Sci. 2011, 23, S66–S69. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, X.; Li, J.; Yao, X.; Li, J. Direct electrochemistry of glucose oxidase and electrochemical biosensing of glucose on quantum dots/carbon nanotubes electrodes. Biosens. Bioelectron. 2007, 22, 3203–3209. [Google Scholar] [CrossRef]
- Periasamy, A.P.; Chang, Y.J.; Chen, S.M. Amperometric glucose sensor based on glucose oxidase immobilized on gelatin-multiwalled carbon nanotube modified glassy carbon electrode. Bioelectrochemistry 2011, 80, 114–120. [Google Scholar] [CrossRef]
- Qiu, C.; Wang, X.; Liu, X.; Hou, S.; Ma, H. Direct electrochemistry of glucose oxidase immobilized on nanostructured gold thin films and its application to bioelectrochemical glucose sensor. Electrochim. Acta 2012, 67, 140–146. [Google Scholar] [CrossRef]
- Tasviri, M.; Rafiee-Pour, H.A.; Ghourchian, H.; Gholami, M.R. Amine functionalized tio–carbon nanotube composite: Synthesis, characterization and application to glucose biosensing. Appl. Nanosci. 2011, 1, 189–195. [Google Scholar] [CrossRef]
- Wang, K.; Hua, Y.; Lin, Z.; Ma, Z.; Xing, S.; Qiang, L.; Liao, J.; Liu, C.; Wei, X. Direct electron transfer and electrocatalysis of glucose oxidase immobilized on glassy carbon electrode modified with nafion and mesoporous carbon fdu-15. Electrochim. Acta 2009, 54, 4626–4630. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, R.; Xu, Y.; He, P.; Fang, Y. Direct electrochemistry study of glucose oxidase on pt nanoparticle-modified aligned carbon nanotubes electrode by the assistance of chitosan–cds and its biosensoring for glucose. Electrochem. Commun. 2008, 10, 1889–1892. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Y.; Dai, H.; Wang, X.; Fan, C.; Li, G. Tuning the redox and enzymatic activity of glucose oxidase in layered organic films and its application in glucose biosensors. Anal. Biochem. 2004, 329, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Meng, Z.; Wang, Q.; Zheng, J. A novel glucose biosensor based on direct electrochemistry of glucose oxidase incorporated in biomediated gold nanoparticles–carbon nanotubes composite film. Sens. Actuators B Chem. 2011, 158, 23–27. [Google Scholar] [CrossRef]
- Alwarappan, S.; Liu, C.; Kumar, A.; Li, C.Z. Enzyme-doped graphene nanosheets for enhanced glucose biosensing. J. Phys. Chem. C 2010, 114, 1599–1608. [Google Scholar] [CrossRef]
- Shangguan, X.; Zhang, H.; Zheng, J. Direct electrochemistry of glucose oxidase based on its direct immobilization on carbon ionic liquid electrode and glucose sensing. Electrochem. Commun. 2008, 10, 1140–1143. [Google Scholar] [CrossRef]
- Salimi, A.; Sharifi, E.; Noorbakhsh, A.; Soltanian, S. Direct electrochemistry and electrocatalytic activity of catalase immobilized onto electrodeposited nano-scale islands of nickel oxide. Biophys. Chem. 2007, 125, 540–548. [Google Scholar] [CrossRef]
- German, N.; Ramanaviciene, A.; Voronovic, J.; Ramanavicius, A. Glucose biosensor based on graphite electrodes modified with glucose oxidase and colloidal gold nanoparticles. Microchim. Acta 2010, 168, 221–229. [Google Scholar] [CrossRef]
- Du, J.; Yu, X.; Di, J. Comparison of the direct electrochemistry of glucose oxidase immobilized on the surface of au, cds and zns nanostructures. Biosens. Bioelectron. 2012, 37, 88–93. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhai, S.; Chen, G.; Zhang, K.; Yue, Q.; Wang, L.; Liu, J.; Jia, J. Effects of morphology of nanostructured zno on direct electrochemistry and biosensing properties of glucose oxidase. J. Electroanal. Chem. 2011, 656, 198–205. [Google Scholar] [CrossRef]
- Calzolai, L.; Franchini, F.; Gilliland, D.; Rossi, F. Protein—Nanoparticle interaction: Identification of the ubiquitin—Gold nanoparticle interaction site. Nano Lett. 2010, 10, 3101–3105. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Wang, Y.; Jiang, J.; Dong, S. Ph-dependent protein conformational changes in albumin: Gold nanoparticle bioconjugates: A spectroscopic study. Langmuir 2007, 23, 2714–2721. [Google Scholar] [CrossRef] [PubMed]
- Ajayan, P.M. Nanotubes from carbon. Chem. Rev. 1999, 99, 1787–1800. [Google Scholar] [CrossRef] [PubMed]
- Ebbesen, T.W.; Ajayan, P.M. Large-scale synthesis of carbon nanotubes. Nature 1992, 358, 220–222. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Yong, C.J.; So, H.H.; Cho, J.W. Polypyrrole coated carbon nanotubes: Synthesis, characterization, and enhanced electrical properties. Synth. Met. 2007, 157, 374–379. [Google Scholar] [CrossRef]
- Xie, X.-L.; Mai, Y.-W.; Zhou, X.-P. Dispersion and alignment of carbon nanotubes in polymer matrix: A review. Mater. Sci. Eng. R 2005, 49, 89–112. [Google Scholar] [CrossRef]
- Hong, J.; Moosavi-Movahedi, A.A.; Ghourchian, H.; Rad, A.M.; Rezaei-Zarchi, S. Direct electron transfer of horseradish peroxidase on nafion-cysteine modified gold electrode. Electrochim. Acta 2007, 52, 6261–6267. [Google Scholar] [CrossRef]
- Hong, J.; Ghourchian, H.; Moosavi–Movahedi, A.A. Direct electron transfer of redox proteins on a nafion-cysteine modified gold electrode. Electrochem. Commun. 2006, 8, 1572–1576. [Google Scholar] [CrossRef]
- Mogharrab, N.; Ghourchian, H. Anthraquinone 2-carboxylic acid as an electron shuttling mediator and attached electron relay for horseradish peroxidase. Electrochem. Commun. 2005, 7, 466–471. [Google Scholar] [CrossRef]
- Shourian, M.; Ghourchian, H. Biosensing improvement of horseradish peroxidase towards hydrogen peroxide upon modifying the accessible lysines. Sens. Actuators B Chem. 2010, 145, 607–612. [Google Scholar] [CrossRef]
- Hong, J.; Yang, W.Y.; Zhao, Y.X.; Xiao, B.L.; Gao, Y.F.; Yang, T.; Ghourchian, H.; Moosavi-Movahedi, Z.; Sheibani, N.; Li, J.G. Catalase immobilized on a functionalized multi-walled carbon nanotubes–gold nanocomposite as a highly sensitive bio-sensing system for detection of hydrogen peroxide. Electrochim. Acta 2013, 89, 317–325. [Google Scholar] [CrossRef]
- Unnikrishnan, B.; Palanisamy, S.; Chen, S.M. A simple electrochemical approach to fabricate a glucose biosensor based on graphene–glucose oxidase biocomposite. Biosens. Bioelectron. 2013, 39, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Xu, B.; Hu, C.; Shao, H.B.; Qu, L. Direct electrochemistry and electrocatalysis of glucose oxidase on three-dimensional interpenetrating, porous graphene modified electrode. Electrochim. Acta 2013, 98, 48–53. [Google Scholar] [CrossRef]
- Mani, V.; Devadas, B.; Chen, S.M. Direct electrochemistry of glucose oxidase at electrochemically reduced graphene oxide-multiwalled carbon nanotubes hybrid material modified electrode for glucose biosensor. Biosens. Bioelectron. 2013, 41, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Yuwen, L.; Han, Y.; Tian, J.; Zhu, X.; Weng, L.; Wang, L. Reduced graphene oxide/pamam-silver nanoparticles nanocomposite modified electrode for direct electrochemistry of glucose oxidase and glucose sensing. Biosens. Bioelectron. 2012, 36, 179–185. [Google Scholar] [CrossRef]
- Xu, Q.; Gu, S.-X.; Jin, L.; Zhou, Y.-E.; Yang, Z.; Wang, W.; Hu, X. Graphene/polyaniline/gold nanoparticles nanocomposite for the direct electron transfer of glucose oxidase and glucose biosensing. Sens. Actuators B Chem. 2014, 190, 562–569. [Google Scholar]
- Xu, C.X.; Huang, K.J.; Xiong, X.Q. Direct electrochemistry of glucose oxidase immobilized on tio–graphene/nickel oxide nanocomposite film and its application. J. Solid State Electrochem. 2012, 16, 3747–3752. [Google Scholar] [CrossRef]
- Vilian, A.T.; Chen, S.M. Direct electrochemistry and electrocatalysis of glucose oxidase based poly(l-arginine)-multi-walled carbon nanotubes. RSC Adv. 2014, 4, 50771–50781. [Google Scholar] [CrossRef]
- Liu, X.; Shi, L.; Niu, W.; Li, H.; Xu, G. Amperometric glucose biosensor based on single-walled carbon nanohorns. Biosens. Bioelectron. 2008, 23, 1887–1890. [Google Scholar] [CrossRef] [PubMed]
- Madhu, R.; Devadas, B.; Chen, S.M.; Rajkumar, M. An enhanced direct electrochemistry of glucose oxidase at poly(taurine) modified glassy carbon electrode for glucose biosensor. Anal. Methods-UK 2014, 6, 9053–9058. [Google Scholar] [CrossRef]
- Palanisamy, S.; Karuppiah, C.; Chen, S.M. Direct electrochemistry and electrocatalysis of glucose oxidase immobilized on reduced graphene oxide and silver nanoparticles nanocomposite modified electrode. Colloids Surf. B 2014, 114, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Zhen-Zhen, M.A.; Hui-Cheng, Y.U.; Zhao-Yang, W.U.; Yan, W.U.; Xiao, F.B. A highly sensitive amperometric glucose biosensor based on a nano-cube cu 2 o modified glassy carbon electrode. Chin. J. Anal. Chem. 2016, 44, 822–827. [Google Scholar]
- Laviron, E. Surface linear potential sweep voltammetry: Equation of the peaks for a reversible reaction when interactions between the adsorbed molecules are taken into account. J. Electroanal. Chem. 1974, 52, 395–402. [Google Scholar] [CrossRef]
- Ma, H.; Hu, N.; Rusling, J.F. Electroactive myoglobin films grown layer-by-layer with poly(styrenesulfonate) on pyrolytic graphite electrodes. Langmuir 2000, 16, 4969–4975. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]












| Modified Electrode | Eº′ (V) | Ks (s−1) | Γ (mol cm−2) | Kmapp (μmol/L) | Linear Range (μmol/L) | Detection Limit (μmol/L) | Ref |
|---|---|---|---|---|---|---|---|
| NF/GluOx/IL/mPEG-fMWCNTs/GCE | −0.27 | 6.49 | 6.23 × 10−10 | 143 | 20–9.5 × 102 | 0.2 | This work |
| CHI/GluOx-HFs/GCE | −0.35 | 2.72 | 8.29 × 10−11 | 694 | 50–500 | 5 | [8] |
| CHI/DNA/GluOx/CHI/GCE | −0.47 | 0.91 | - | 6.91 × 103 | 40–2.28 × 103 | 40 | [17] |
| BCNTs/GluOx/GCE | −0.37 | - | 8.77 × 10−11 | 651 | 5 × 102–7 × 102 | 500 | [18] |
| CHI–GluOx–CdS/ACNTs–Pt nano | −0.45 | 3.8 | 8.0 × 10−10 | 11.9 × 103 | 4 × 102–2.1 × 104 | 46.8 | [23] |
| NF/ZnO/(PSS/PDDA)3/GluOx/ITO/GCE | −0.11 | 2.16 | 3.57 × 10−11 | 3.12 × 103 | 1 × 102–9 × 103 | 1.94 | [31] |
| RGO–GluOx/GCE | −0.45 | 4.8 | 1.22 × 10−10 | - | 1 × 102–2.7 × 104 | - | [44] |
| CHI-GluOx-RGO/GCE | −0.50 | 6.05 | 1.67 × 10−10 | - | 20–3.2 × 103 | 1.7 | [45] |
| NF/GluOx/ RGO–MWCNT/GCE | −0.43 | 3.02 | 3.58 × 10−10 | - | 10–6.5 × 103 | 4.7 | [46] |
| CHI/ GluOx/RGO–PAMAM–Ag/GCE | - | 8.59 | - | - | 32–1.89 × 103 | 4.5 | [47] |
| GluOx-GR/PANI/AuNPs/GCE | −0.48 | 4.8 | - | 6 × 102 | 4–1.12 × 103 | 0.6 | [48] |
| GluOx/NiO/TiO2–GR/GCE | −0.44 | - | - | 7.3 × 103 | 1 × 103–1.2 × 104 | 1.2 | [49] |
| GluOx/P-L-Arg/f-MWCNTs/GCE | −0.45 | 5.16 | 1.76 × 10−10 | 2.2 × 103 | 4–6 × 103 | 0.1 | [50] |
| NF–GluOx–SWCNTs/GCE | −0.45 | 3 | - | 8.5 × 103 | 0–6 × 103 | 6 | [51] |
| p-taurine/GluOx/NF/GCE | −0.37 | 1.39 | 1.31 × 10−10 | - | 9 × 102–1.5 × 104 | 60 | [52] |
| RGO/Ag/GluOx/GCE | −0.42 | 5.27 | - | - | 500–1.25 × 104 | 160 | [53] |
| GluOx/Cu2O/GCE | - | - | - | - | 50–4 × 103 | 0.68 | [54] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, Y.-N.; Xiao, B.-L.; Niu, N.-N.; Moosavi-Movahedi, A.A.; Hong, J. Glucose Oxidase Immobilized on a Functional Polymer Modified Glassy Carbon Electrode and Its Molecule Recognition of Glucose. Polymers 2019, 11, 115. https://doi.org/10.3390/polym11010115
Ning Y-N, Xiao B-L, Niu N-N, Moosavi-Movahedi AA, Hong J. Glucose Oxidase Immobilized on a Functional Polymer Modified Glassy Carbon Electrode and Its Molecule Recognition of Glucose. Polymers. 2019; 11(1):115. https://doi.org/10.3390/polym11010115
Chicago/Turabian StyleNing, Yan-Na, Bao-Lin Xiao, Nan-Nan Niu, Ali Akbar Moosavi-Movahedi, and Jun Hong. 2019. "Glucose Oxidase Immobilized on a Functional Polymer Modified Glassy Carbon Electrode and Its Molecule Recognition of Glucose" Polymers 11, no. 1: 115. https://doi.org/10.3390/polym11010115