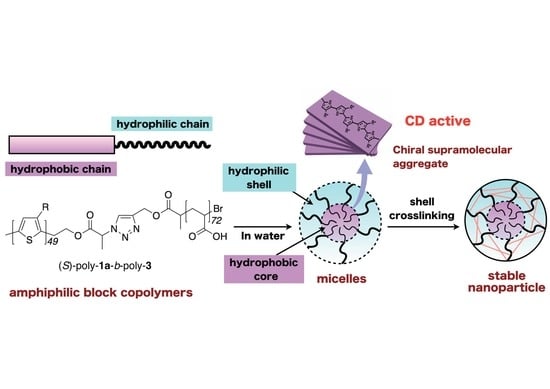
Synthesis of Amphiphilic Block Copolymers Containing Chiral Polythiophene Chains and Their Micelle Formation and Chiroptical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Instruments
2.3. Synthesis
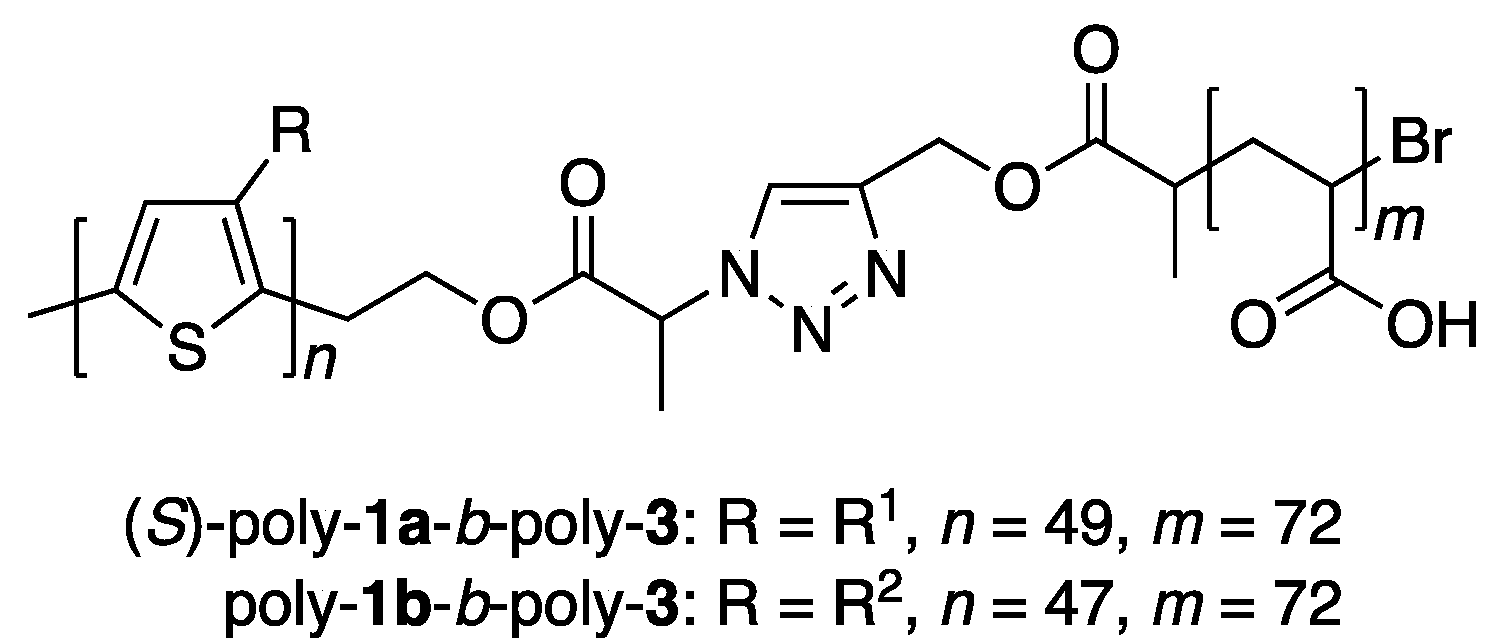
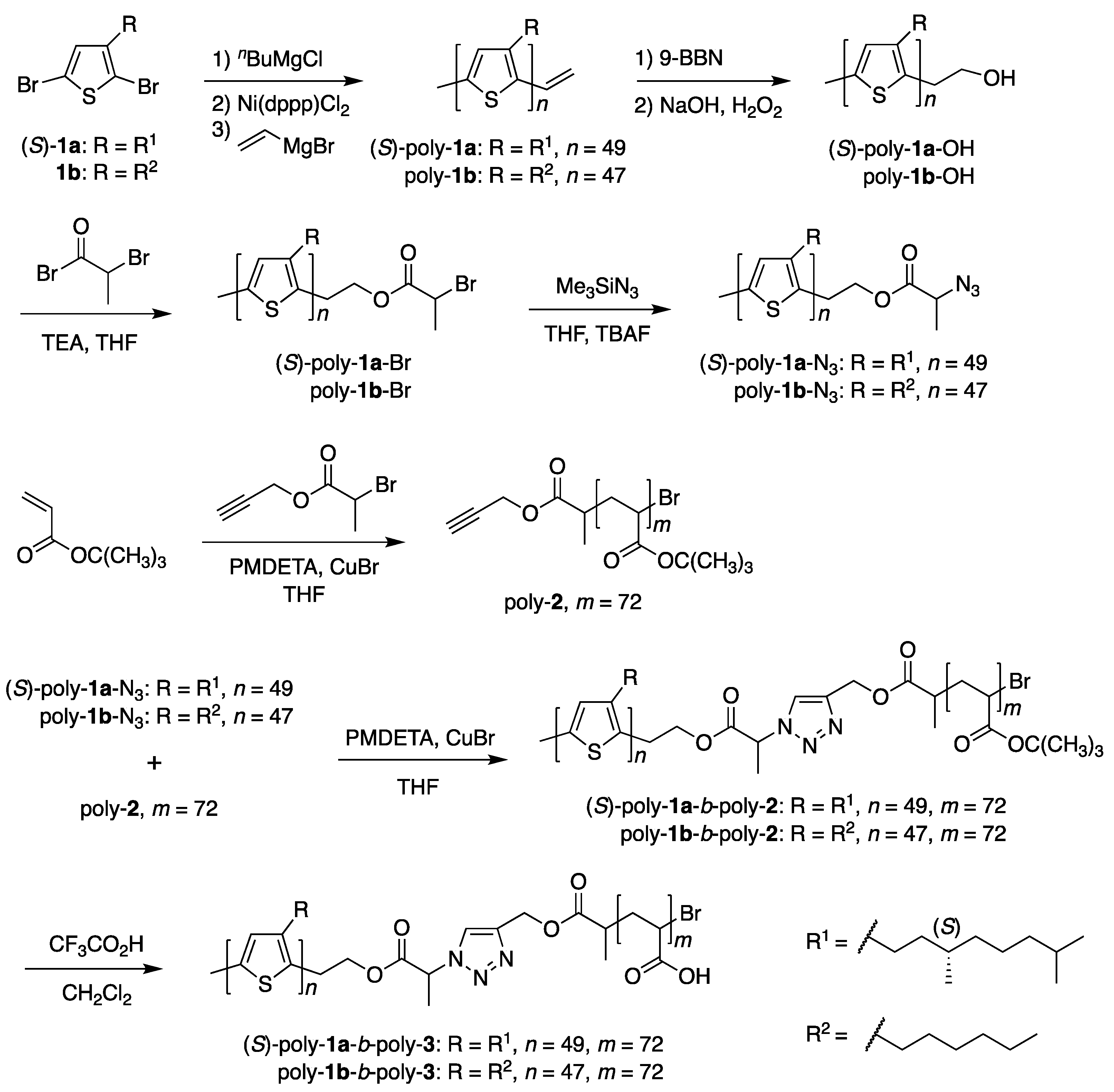
2.3.1. Synthesis of Amphiphilic Block Copolymers
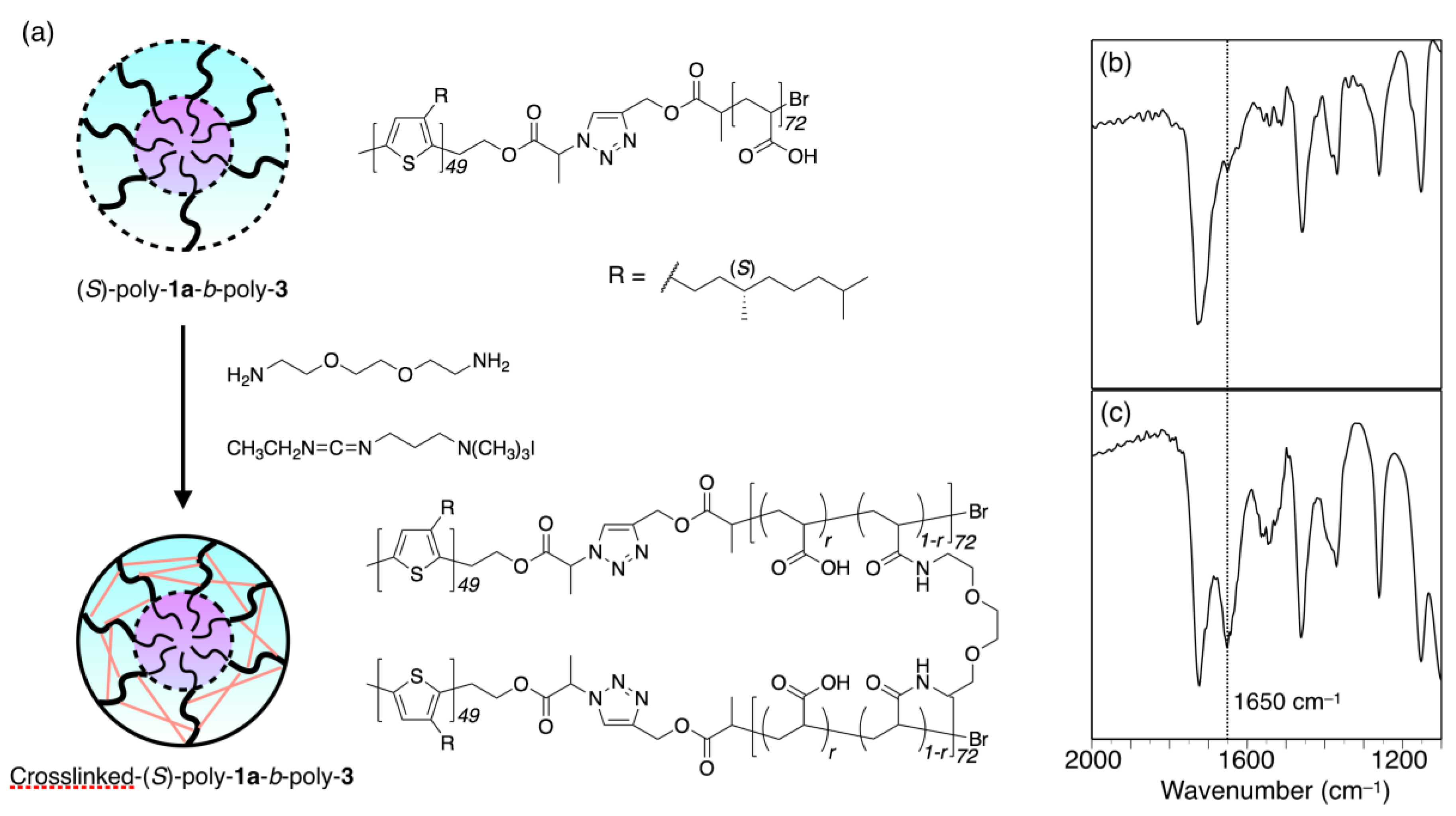
2.3.2. Crosslinking of (S)-Poly-1a-b-poly-3 Micelles
3. Results and Discussion
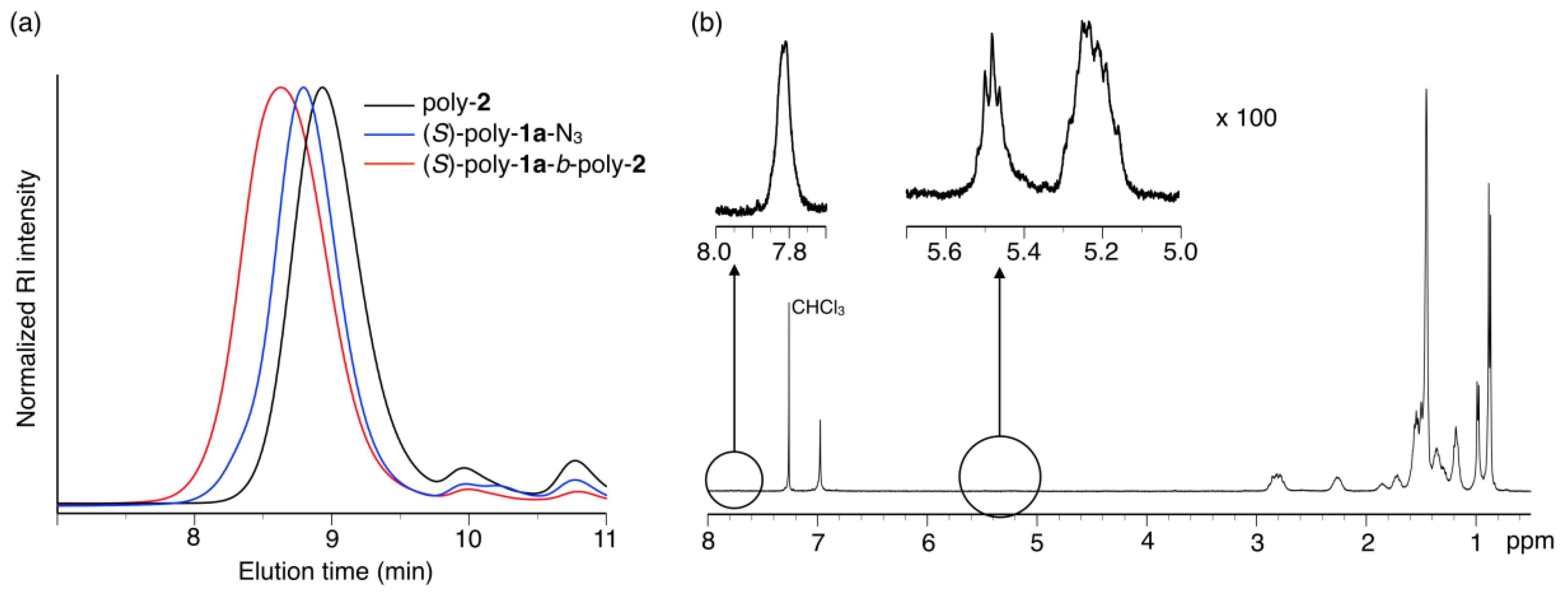
3.1. Synthesis of Amphiphilic Block Copolymers
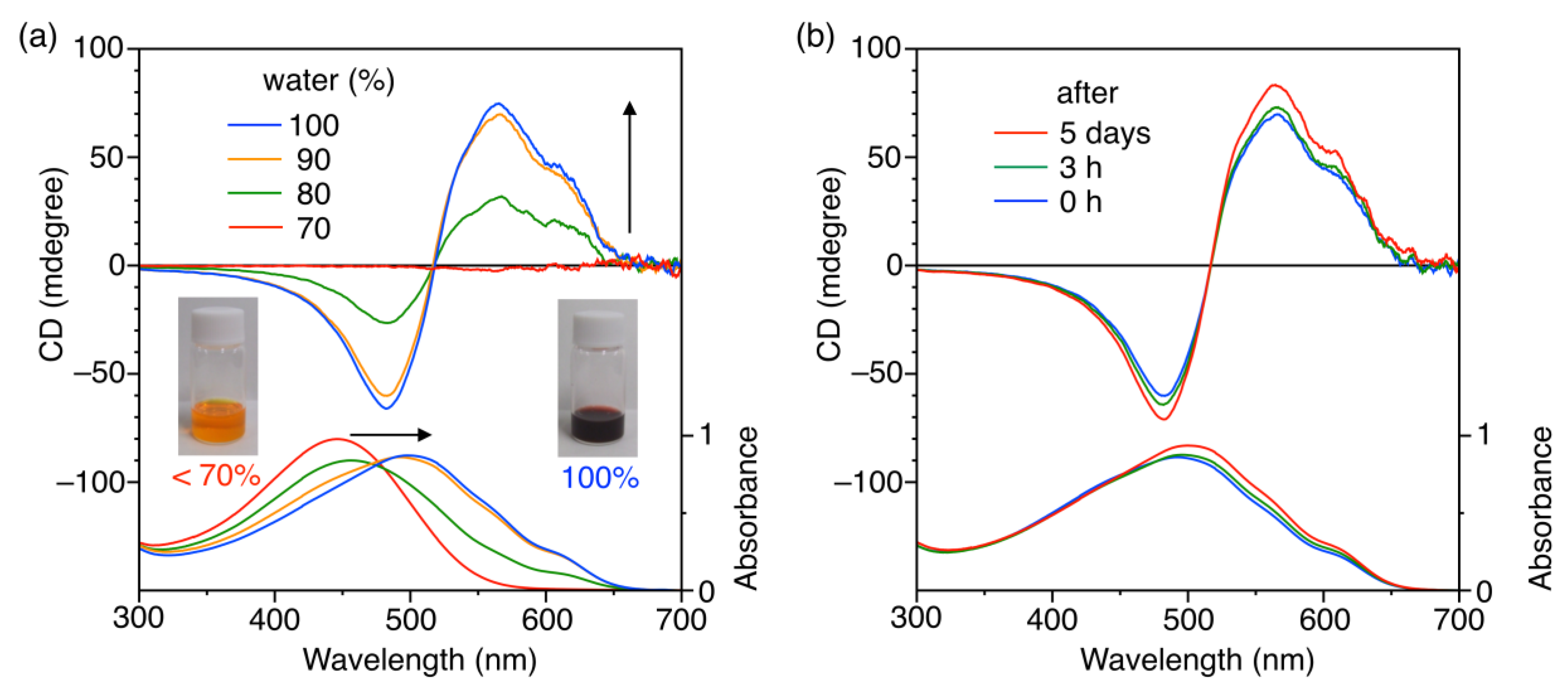
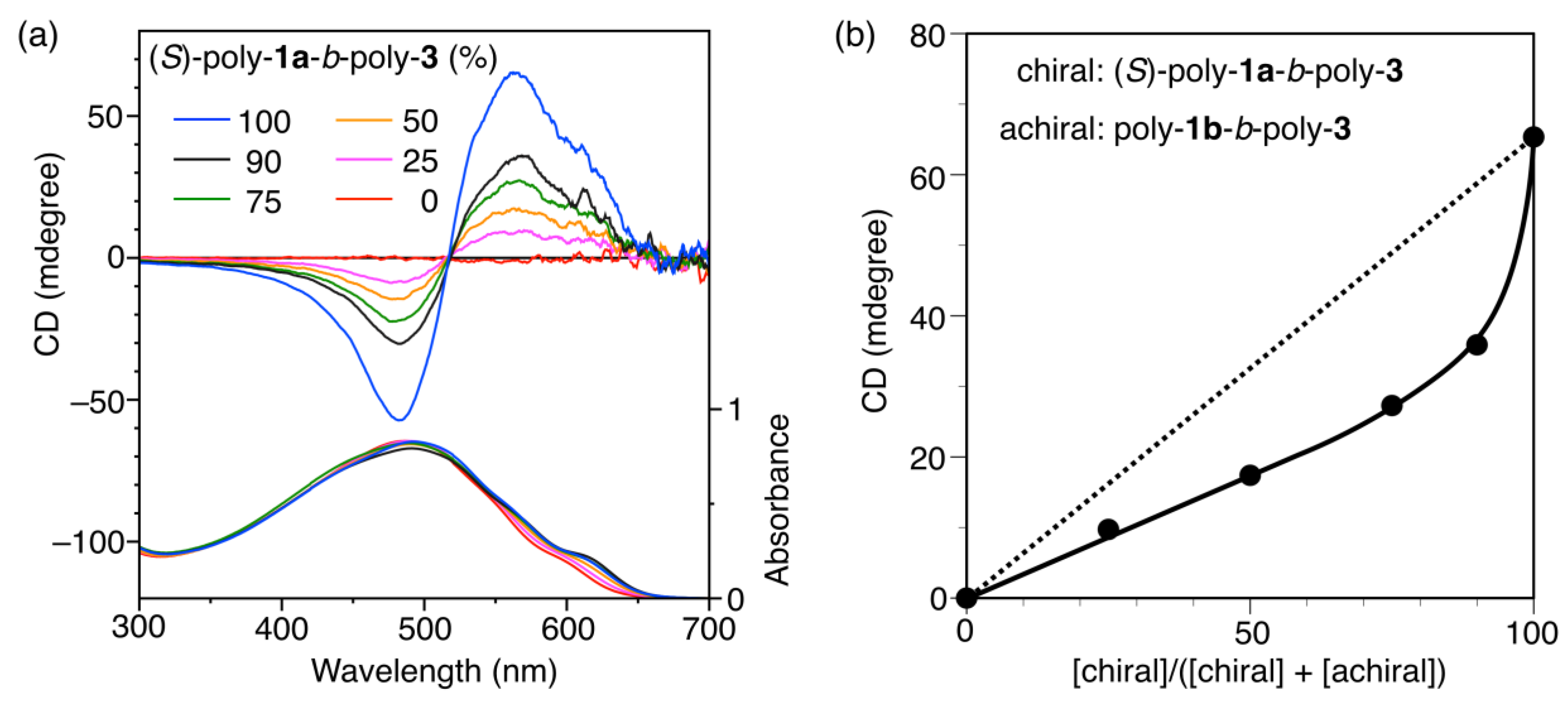
3.2. Chiroptical Properties
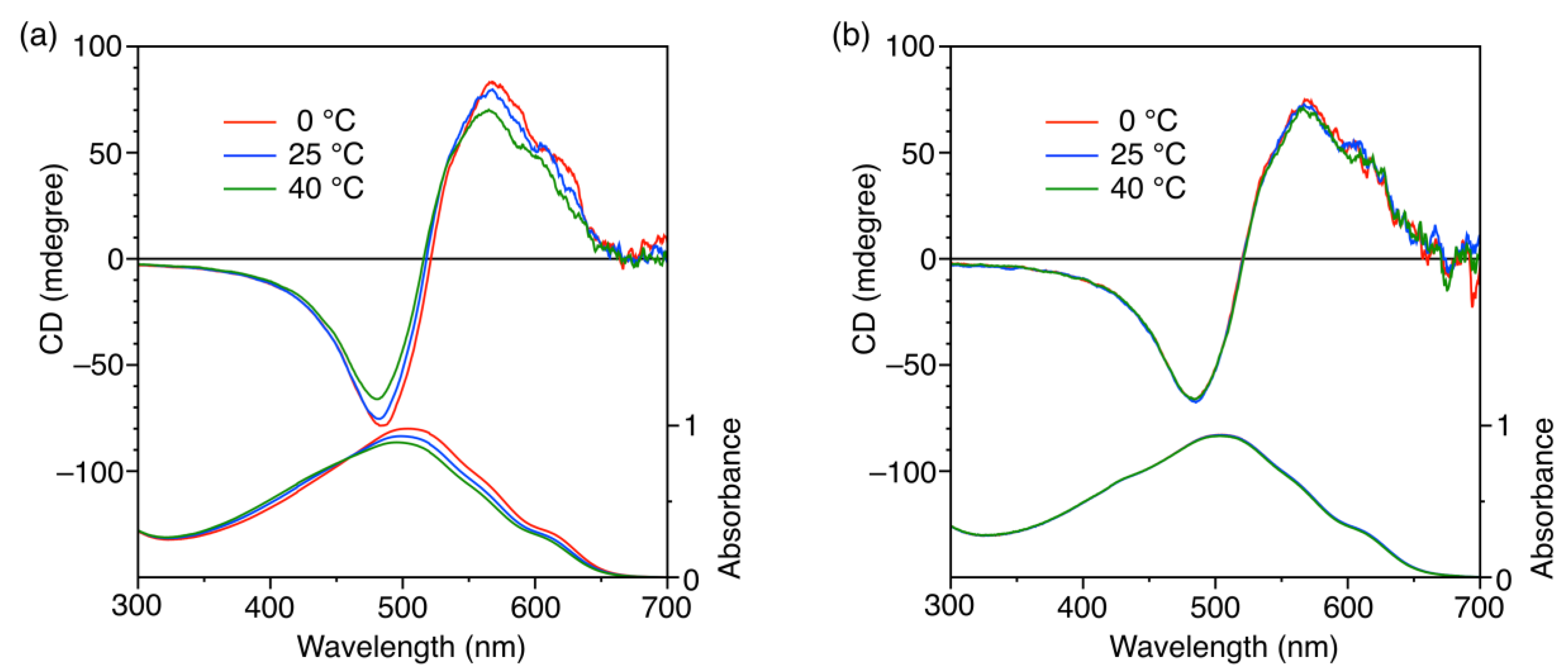
3.3. Micelle Stabilization through Crosslinking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Langeveld-Voss, B.M.W.; Janssen, R.A.J.; Meijer, E.W. On the origin of optical activity in polythiophenes. J. Mol. Struct. 2000, 521, 285–301. [Google Scholar] [CrossRef]
- Hoeben, F.J.M.; Jonkheijm, P.; Meijer, E.W.; Schenning, A.P.H.J. About supramolecular assemblies of pi-conjugated systems. Chem. Rev. 2005, 105, 1491–1546. [Google Scholar] [CrossRef] [PubMed]
- Kane-Maguire, L.A.P.; Wallace, G.G. Chiral conducting polymers. Chem. Soc. Rev. 2010, 39, 2545–2576. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Yashima, E.; Okamoto, Y. Unusual solvent effects on chiroptical properties of an optically active regioregular polythiophene in solution. Chirality 2000, 12, 396–399. [Google Scholar] [CrossRef]
- Goto, H.; Yashima, E. Electron-induced switching of the supramolecular chirality of optically active polythiophene aggregates. J. Am. Chem. Soc. 2002, 124, 7943–7949. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Okamoto, Y.; Yashima, E. Solvent-induced chiroptical changes in supramolecular assemblies of an optically active, regioregular polythiophene. Macromolecules 2002, 35, 4590–4601. [Google Scholar] [CrossRef]
- Leysen, P.; Teyssandier, J.; De Feyter, S.; Koeckelberghs, G. Controlled Synthesis of a Helical Conjugated Polythiophene. Macromolecules 2018, 51, 3504–3514. [Google Scholar] [CrossRef]
- Liu, Y.L.; Pauloehrl, T.; Presolski, S.I.; Albertazzi, L.; Palmans, A.R.A.; Meijer, E.W. Modular Synthetic Platform for the Construction of Functional Single-Chain Polymeric Nanoparticles: From Aqueous Catalysis to Photosensitization. J. Am. Chem. Soc. 2015, 137, 13096–13105. [Google Scholar] [CrossRef] [PubMed]
- Elsabahy, M.; Heo, G.S.; Lim, S.M.; Sun, G.R.; Wooley, K.L. Polymeric Nanostructures for Imaging and Therapy. Chem. Rev. 2015, 115, 10967–11011. [Google Scholar] [CrossRef] [PubMed]
- O'Reilly, R.K.; Joralemon, M.J.; Wooley, K.L.; Hawker, C.J. Functionalization of micelles and shell cross-linked nanoparticles using click chemistry. Chem. Mater. 2005, 17, 5976–5988. [Google Scholar] [CrossRef]
- Mavila, S.; Eivgi, O.; Berkovich, I.; Lemcoff, N.G. Intramolecular Cross-Linking Methodologies for the Synthesis of Polymer Nanoparticles. Chem. Rev. 2016, 116, 878–961. [Google Scholar] [CrossRef] [PubMed]
- Gois, J.R.; Popov, A.V.; Guliashvili, T.; Serra, A.C.; Coelho, J.F.J. Synthesis of functionalized poly(vinyl acetate) mediated by alkyne-terminated RAFT agents. RSC Adv. 2015, 5, 91225–91234. [Google Scholar] [CrossRef]
- Vandeleene, S.; Jivanescu, M.; Stesmans, A.; Cuppens, J.; Van Bael, M.J.; Verbiest, T.; Koeckelberghs, G. Influence of the Supramolecular Organization on the Magnetic Properties of Poly(3-alkylthiophene)s in Their Neutral State. Macromolecules 2011, 44, 4911–4919. [Google Scholar] [CrossRef] [Green Version]
- Hammer, B.A.G.; Bokel, F.A.; Hayward, R.C.; Emrick, T. Cross-Linked Conjugated Polymer Fibrils: Robust Nanowires from Functional Polythiophene Diblock Copolymers. Chem. Mater. 2011, 23, 4250–4256. [Google Scholar] [CrossRef]
- Iovu, M.C.; Jeffries-El, M.; Sheina, E.E.; Cooper, J.R.; McCullough, R.D. Regioregular poly(3-alkylthiophene) conducting block copolymers. Polymer 2005, 46, 8582–8586. [Google Scholar] [CrossRef]
- Verheyen, L.; Leysen, P.; Van den Eede, M.P.; Ceunen, W.; Hardeman, T.; Koeckelberghs, G. Advances in the controlled polymerization of conjugated polymers. Polymer 2017, 108, 521–546. [Google Scholar] [CrossRef] [Green Version]
- Van Camp, W.; Germonpre, V.; Mespouille, L.; Dubois, P.; Goethals, E.J.; Du Prez, F.E. New poly(acrylic acid) containing segmented copolymer structures by combination of “Click” chemistry and atom transfer radical polymerization. React. Funct. Polym. 2007, 67, 1168–1180. [Google Scholar] [CrossRef]
- Craley, C.R.; Zhang, R.; Kowalewski, T.; McCullough, R.D.; Stefan, M.C. Regioregular Poly(3-hexylthiophene) in a Novel Conducting Amphiphilic Block Copolymer. Macromol. Rapid Commun. 2009, 30, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Jeffries-El, M.; Sauve, G.; McCullough, R.D. Facile synthesis of end-functionalized regioregular poly(3-alkylthiophene)s via modified Grignard metathesis reaction. Macromolecules 2005, 38, 10346–10352. [Google Scholar] [CrossRef]
- Miyakoshi, R.; Yokoyama, A.; Yokozawa, T. Catalyst-transfer polycondensation. Mechanism of Ni-catalyzed chain-growth polymerization leading to well-defined poly(3-hexylthiophene). J. Am. Chem. Soc. 2005, 127, 17542–17547. [Google Scholar] [CrossRef] [PubMed]
- Kamigaito, M.; Ando, T.; Sawamoto, M. Metal-Catalyzed Living Radical Polymerization. Chem. Rev. 2001, 101, 3689–3746. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K.; Xia, J. Atom Transfer Radical Polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef] [PubMed]
- Huisgen, R. Cycloadditions—Definition, Classification, and Characterization. Angew.Chem. Int. Ed. Engl. 1968, 7, 321–328. [Google Scholar] [CrossRef]
- Kolb Hartmuth, C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef] [Green Version]
- Binder Wolfgang, H.; Sachsenhofer, R. ‘Click’ Chemistry in Polymer and Materials Science. Macromol. Rapid Commun. 2007, 28, 15–54. [Google Scholar] [CrossRef]
- Satyanarayama, T.; Abraham, S.; Kagan, H.B. Nonlinear Effects in Asymmetric Catalysis. Angew. Chem. Int. Ed. 2009, 48, 456–494. [Google Scholar] [CrossRef] [PubMed]
- Langeveld-Voss, B.M.W.; Waterval, R.J.M.; Janssen, R.A.J.; Meijer, E.W. Principles of “majority rules” and “sergeants and soldiers” applied to the aggregation of optically active polythiophenes: Evidence for a multichain phenomenon. Macromolecules 1999, 32, 227–230. [Google Scholar] [CrossRef]
- Mahadevi, A.S.; Sastry, G.N. Cooperativity in Noncovalent Interactions. Chem. Rev. 2016, 116, 2775–2825. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Qi, K.; Khoshdel, E.; Wooley, K.L. Tandem synthesis of core-shell brush copolymers and their transformation to peripherally cross-linked and hollowed nanostructures. J. Am. Chem. Soc. 2006, 128, 6808–6809. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Kowalewski, T.; Remsen, E.E.; Gertzmann, R.; Wooley, K.L. Hydrogel-coated glassy nanospheres: A novel method for the synthesis of shell cross-linked knedels. J. Am. Chem. Soc. 1997, 119, 11653–11659. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirose, D.; Nozaki, S.; Kanoh, S.; Maeda, K. Synthesis of Amphiphilic Block Copolymers Containing Chiral Polythiophene Chains and Their Micelle Formation and Chiroptical Properties. Polymers 2018, 10, 718. https://doi.org/10.3390/polym10070718
Hirose D, Nozaki S, Kanoh S, Maeda K. Synthesis of Amphiphilic Block Copolymers Containing Chiral Polythiophene Chains and Their Micelle Formation and Chiroptical Properties. Polymers. 2018; 10(7):718. https://doi.org/10.3390/polym10070718
Chicago/Turabian StyleHirose, Daisuke, Satoru Nozaki, Shigeyoshi Kanoh, and Katsuhiro Maeda. 2018. "Synthesis of Amphiphilic Block Copolymers Containing Chiral Polythiophene Chains and Their Micelle Formation and Chiroptical Properties" Polymers 10, no. 7: 718. https://doi.org/10.3390/polym10070718