Precise Synthesis, Properties, and Structures of Cyclic Poly(ε-caprolactone)s
Abstract
:1. Introduction
2. Synthesis
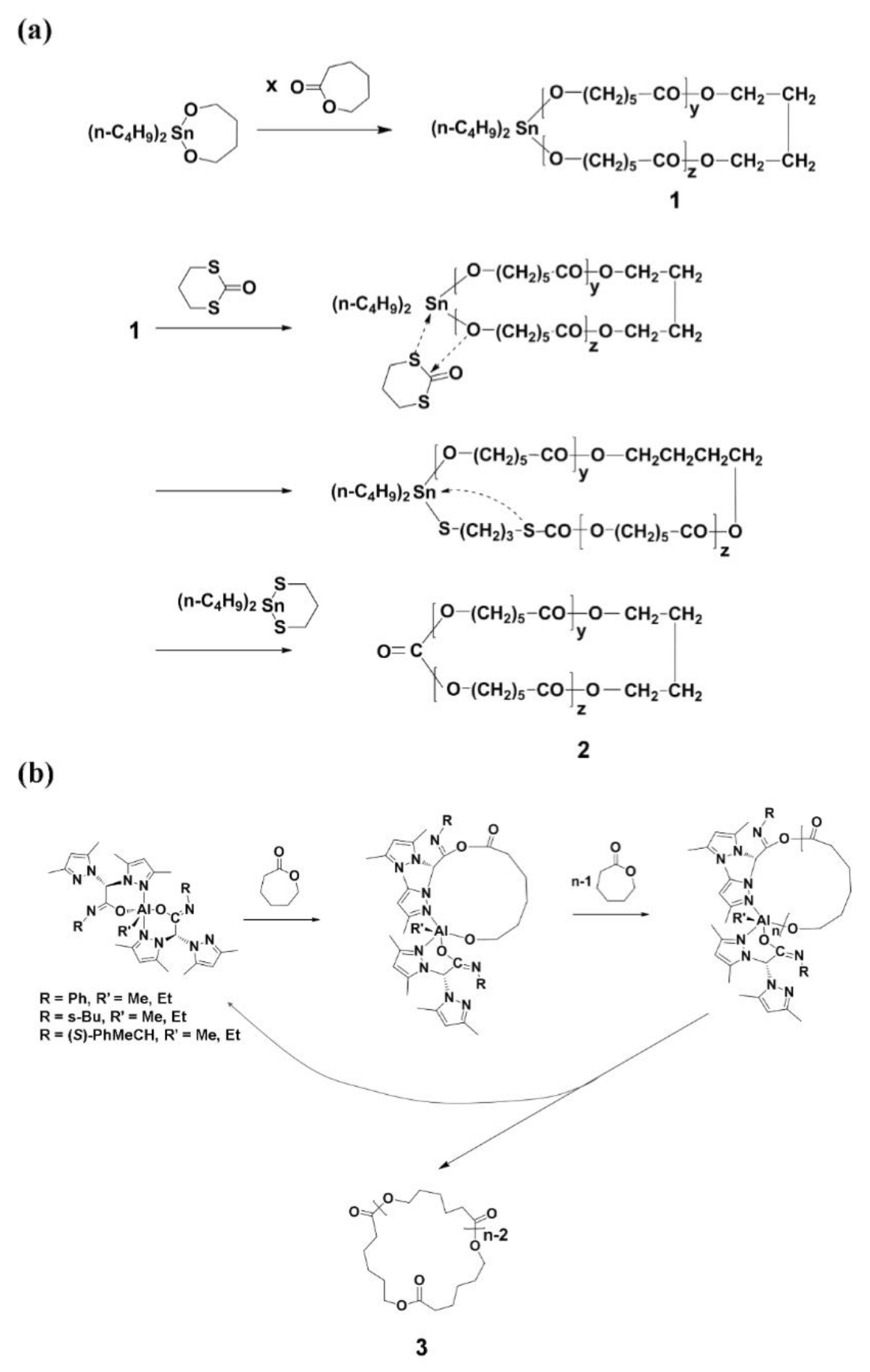
2.1. Ring-Expansion Polymerization
2.2. Intermolecular Cyclization
2.3. Intramolecular Cyclizations
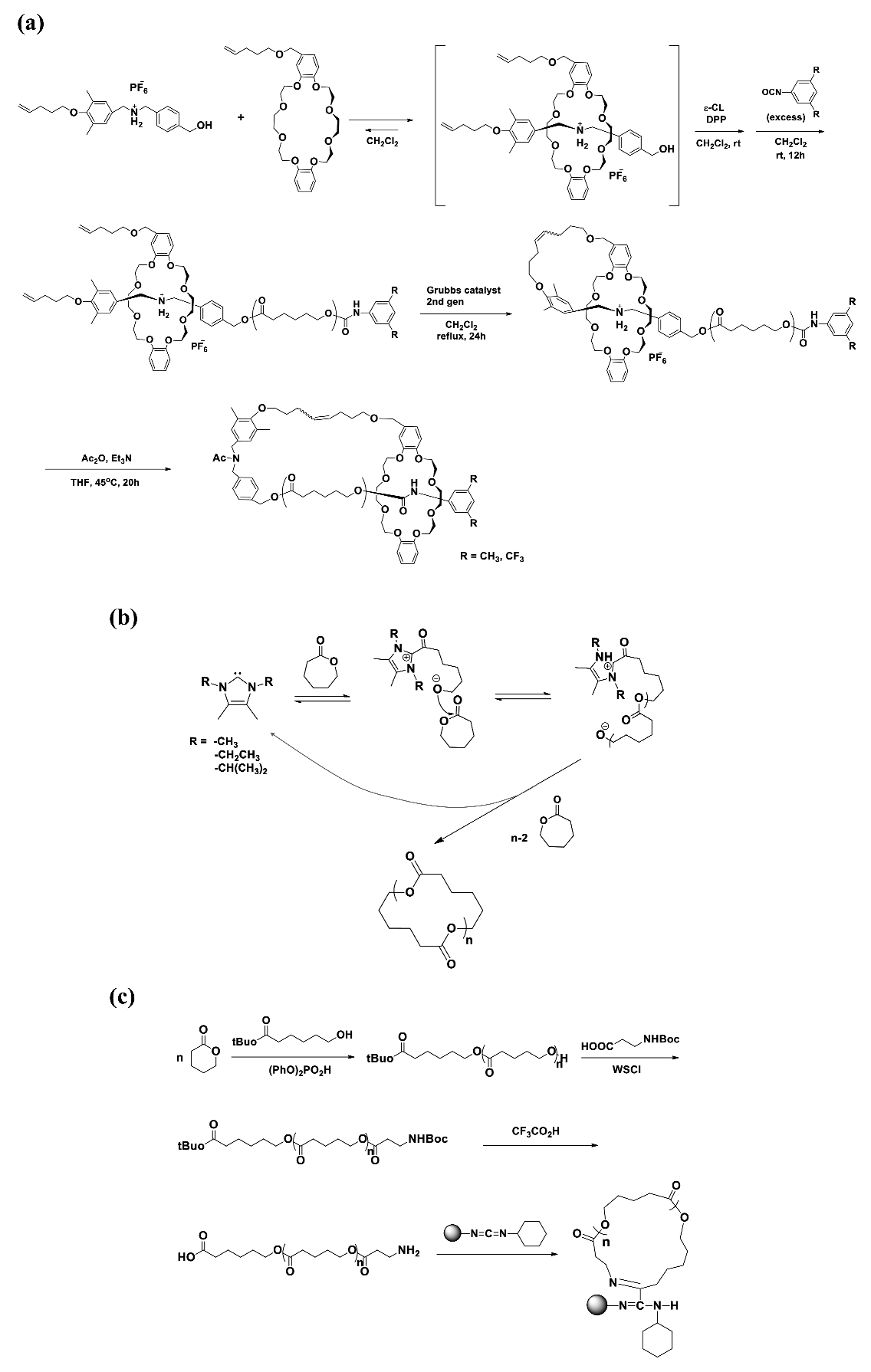
2.3.1. Pseudo[2]rotaxane-Initiated Cyclization
2.3.2. Zwitterionic Polymerization and Cyclization
2.3.3. Cyclic Amidation
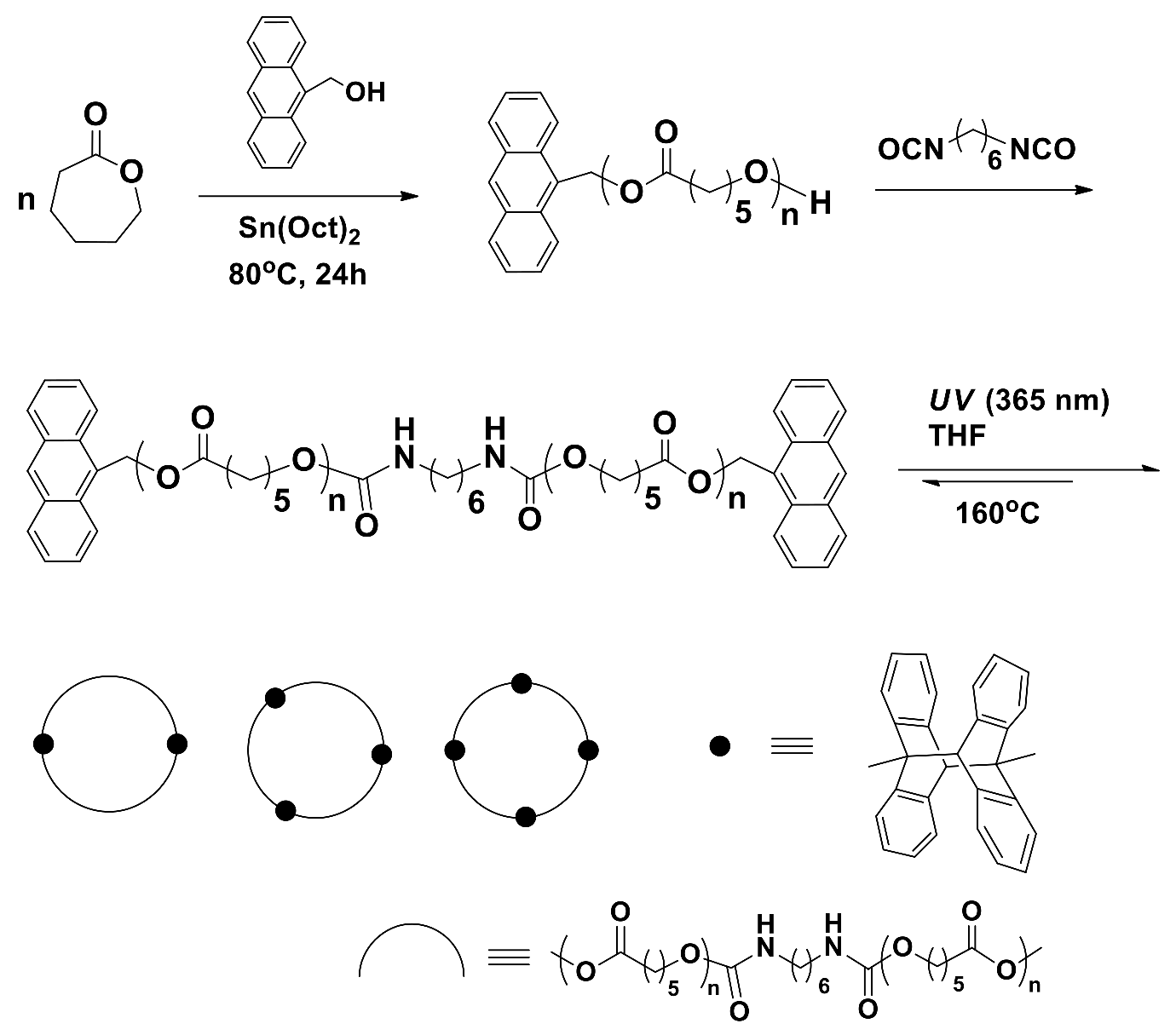
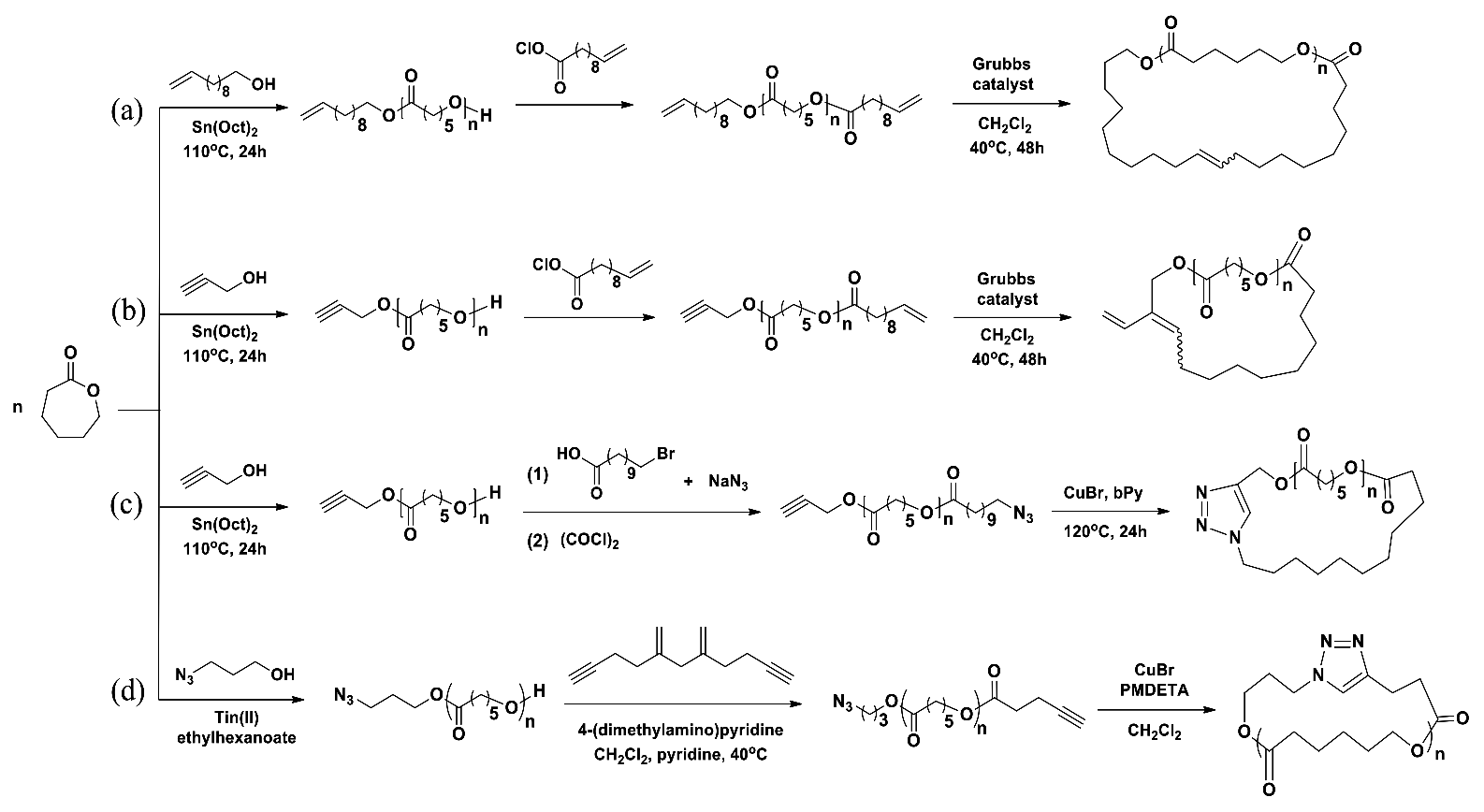
2.3.4. Alkene–Alkene Metathetic Cyclization
2.3.5. Alkyne–Alkene Metathetic Cyclization
2.3.6. Alkyne–Azide Click Cyclization
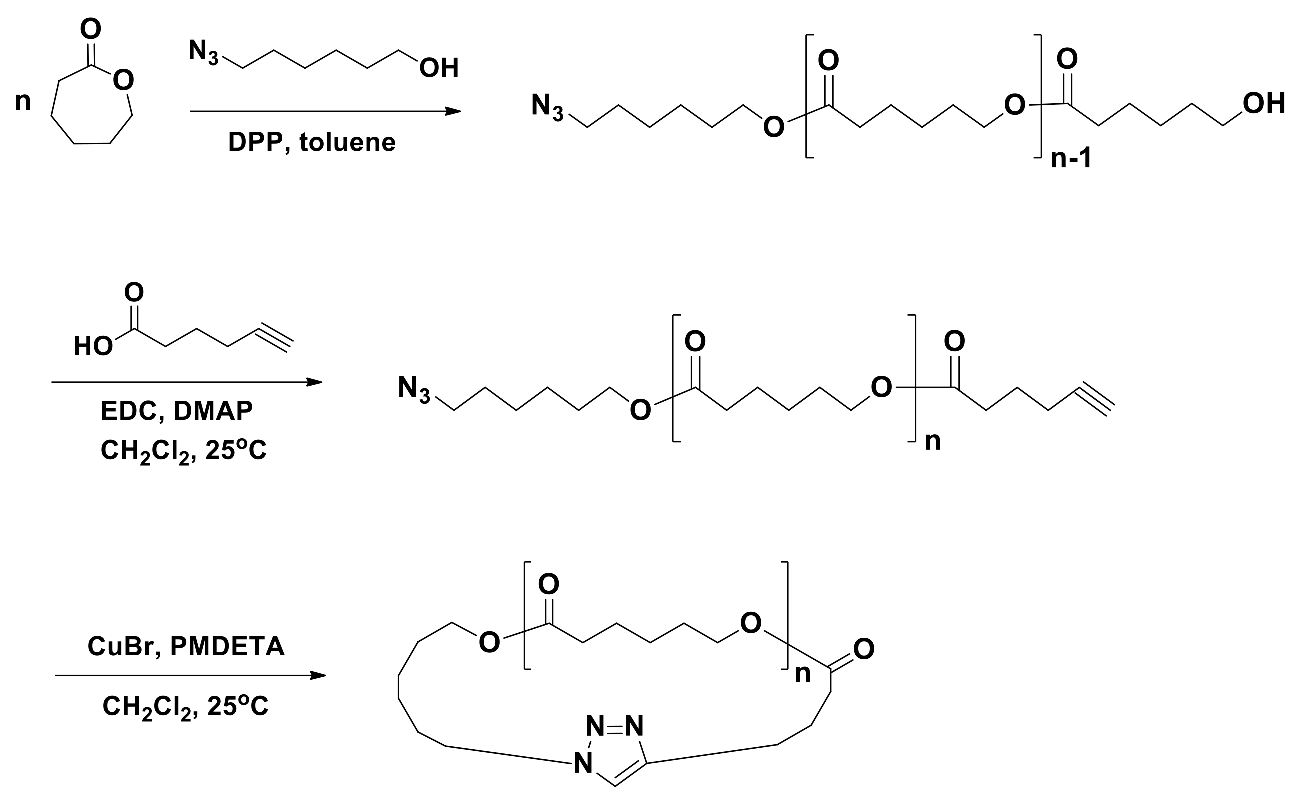
2.3.7. Azide–Alkyne Click Cyclization (I)
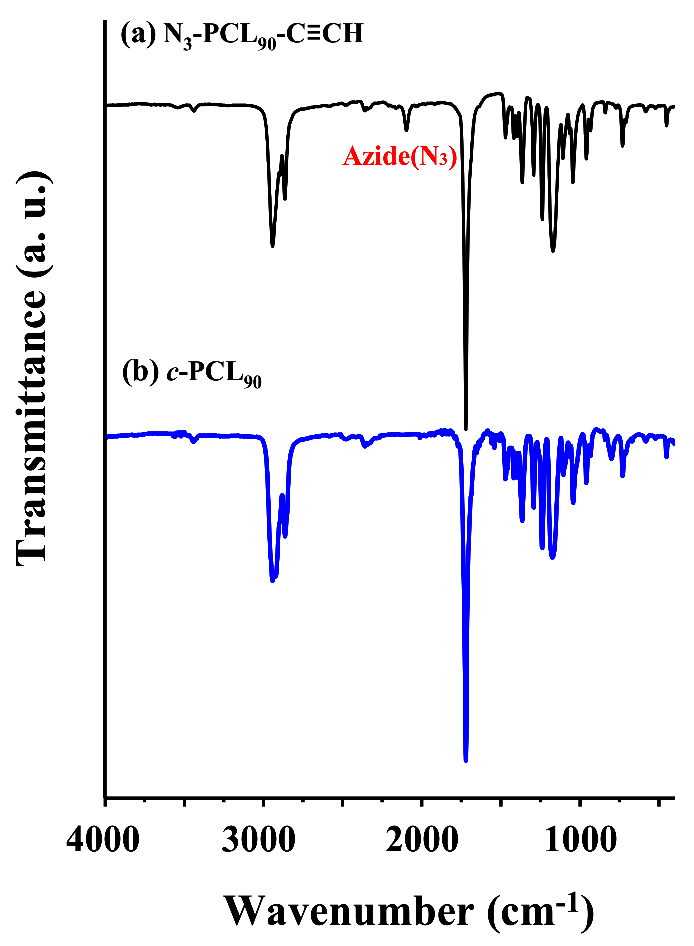
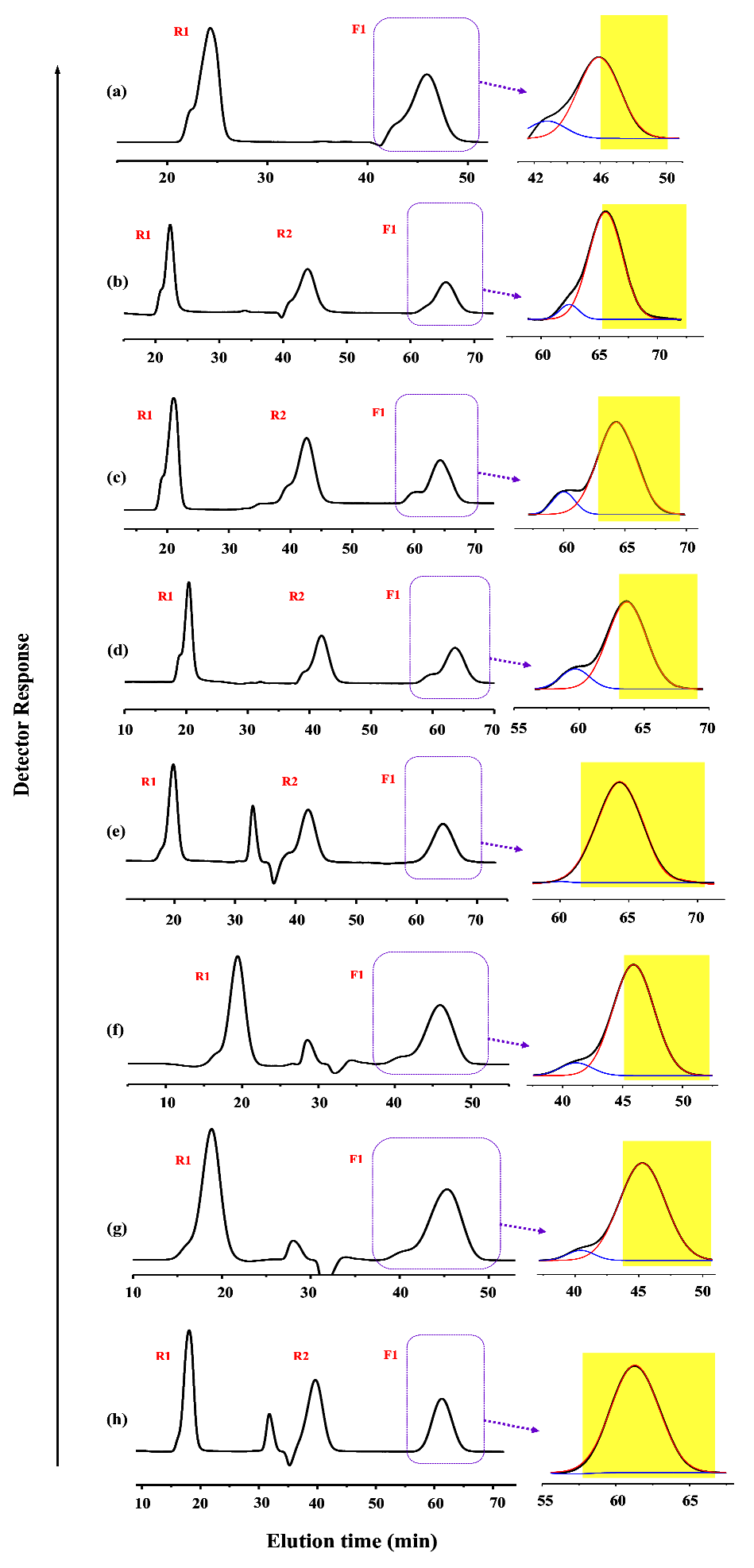
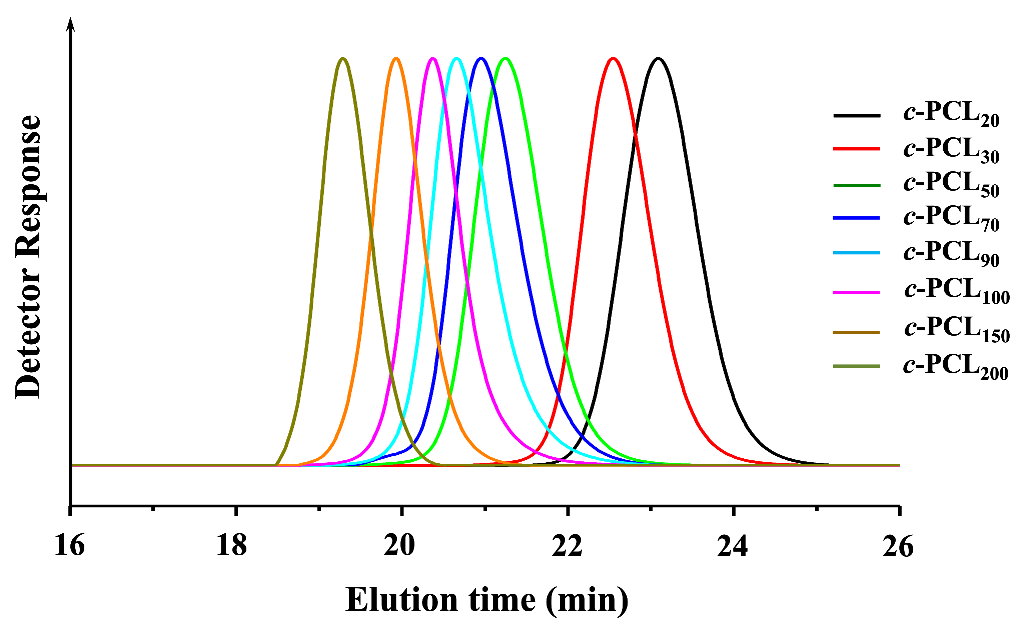
2.3.8. Azide–Alkyne Click Cyclization (II)
3. Chain Structure Characteristics
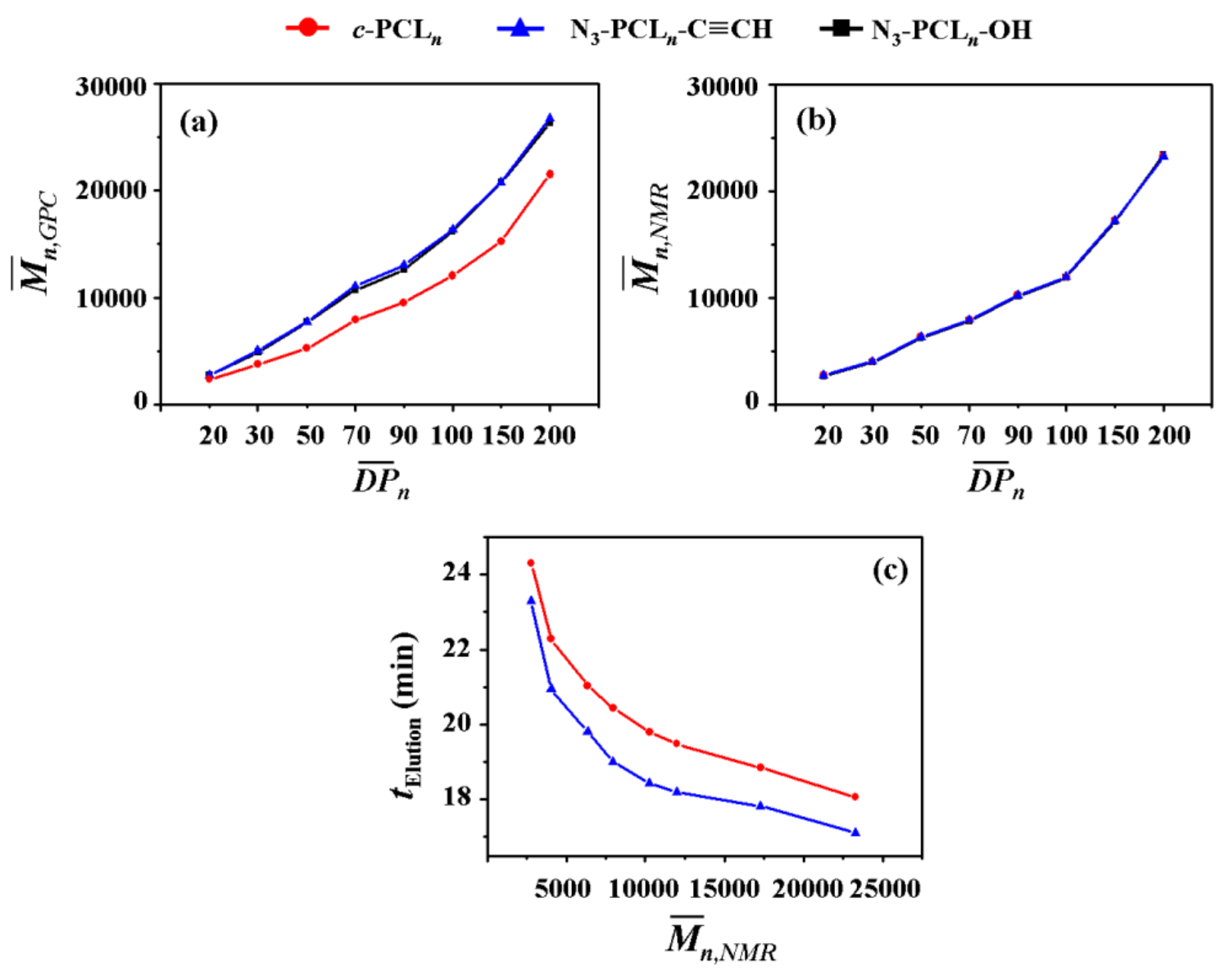
3.1. Hydrodynamic Volume
3.2. Solution Viscosity
3.3. Melt Viscosity and Chain Mobility
4. Properties
4.1. Acid–Catalytic Degradation
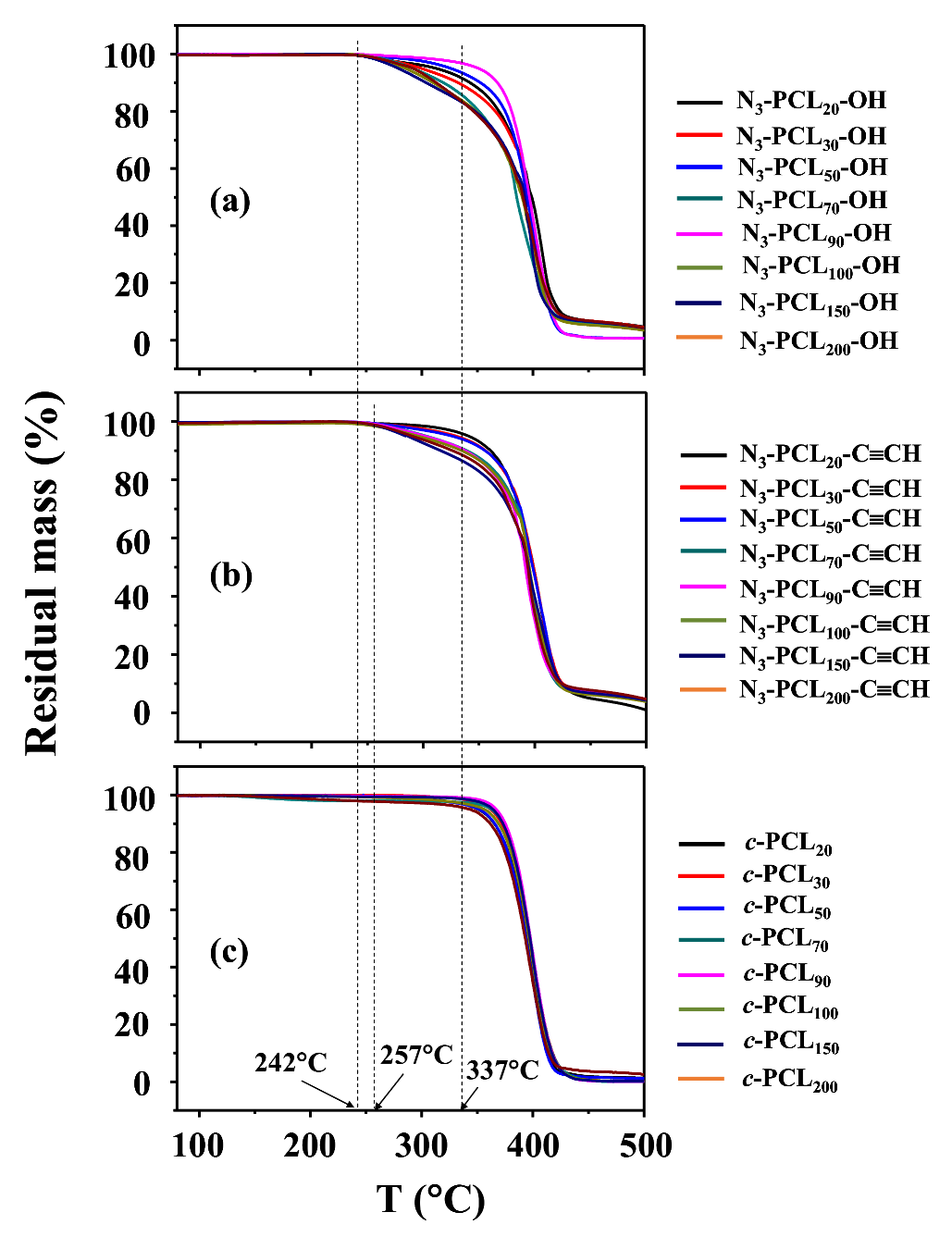
4.2. Thermal Degradation
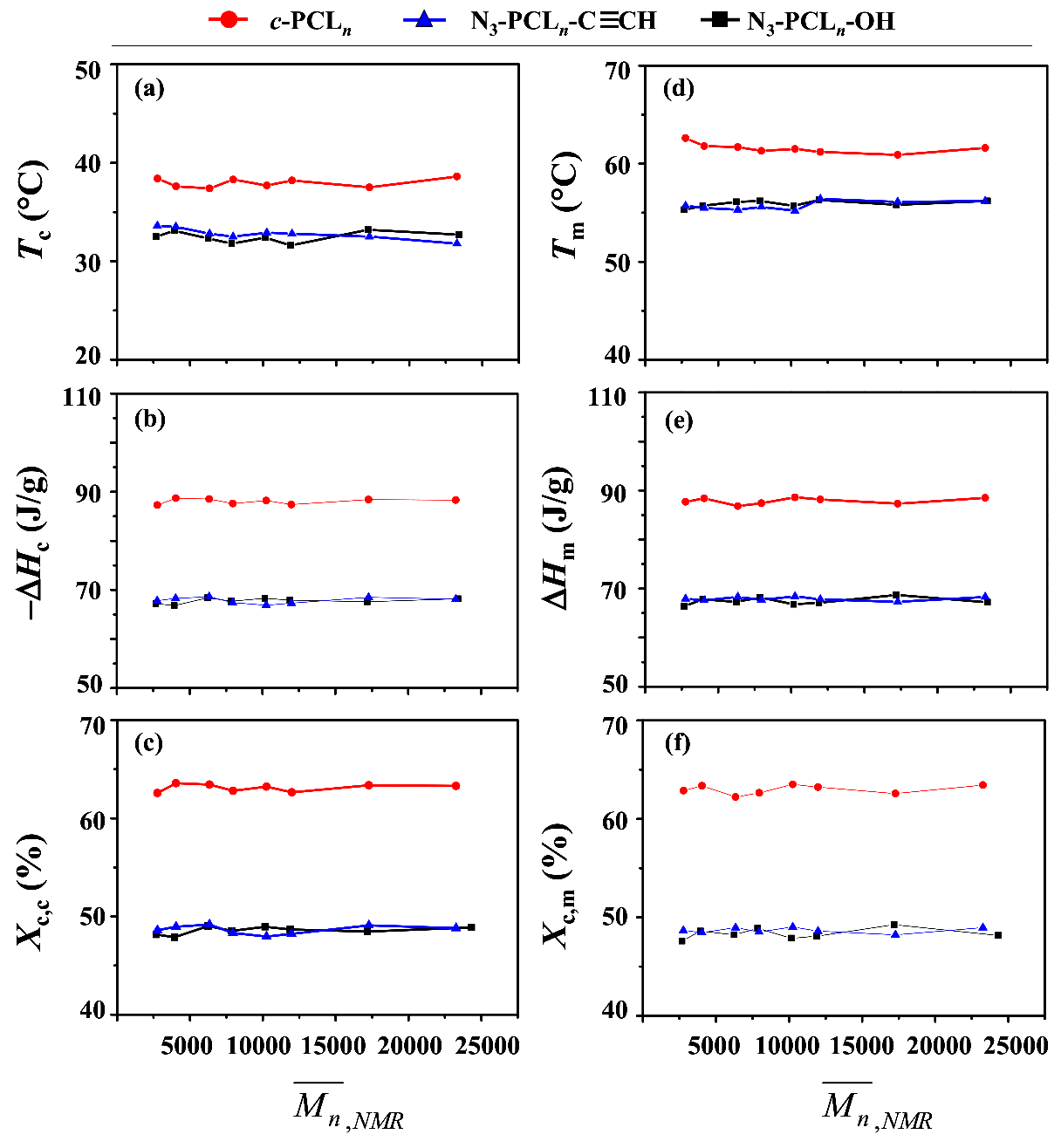
4.3. Phase Transitions (I)
4.4. Phase Transitions (II)
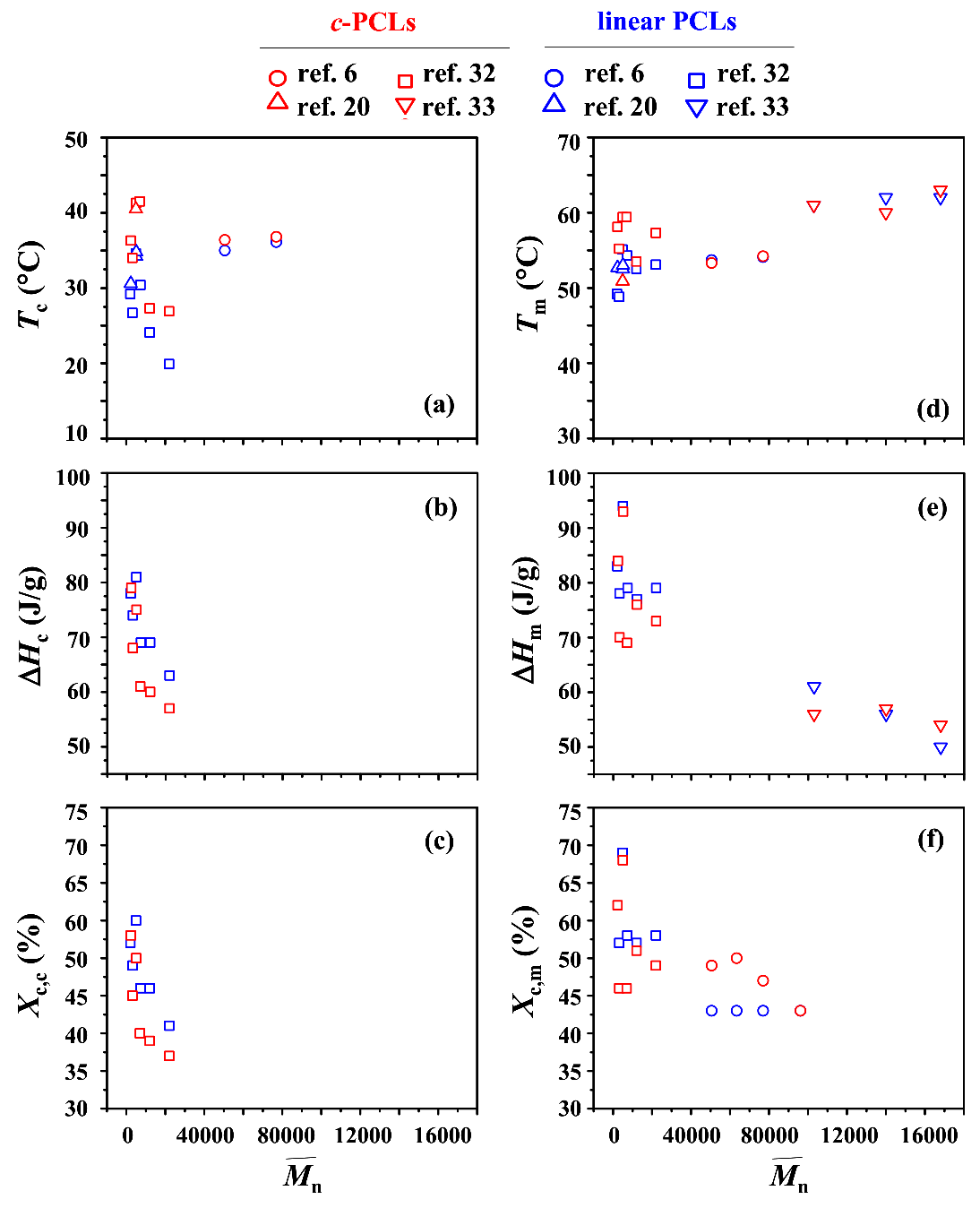
5. Crystallization Characteristics
6. Morphological Characteristics
6.1. Transmission Electron Microscopy (TEM), Electron Diffraction and Atomic Force Microscopy (AFM) Analyses
6.2. Transmission Small-Angle X-ray Scattering (TSAXS) Analysis
7. Summary and Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Cao, P.; Mangadlao, J.D.; de Leon, A.; Su, Z.; Advincula, R.C. Catenated poly(ε-caprolactone) and poly(l-lactide) via ring-expansion strategy. Macromolecules 2015, 48, 3825–3833. [Google Scholar] [CrossRef]
- Abedalwafa, M.; Wang, F.; Wang, L.; Li, C. Biodegradable poly-epsilon-caprolactone (PCL) for tissue engineering applications: A review. Rev. Adv. Mater. Sci. 2013, 34, 123–140. [Google Scholar]
- Azimi, B.; Nourpanah, P.; Rabiee, M.; Arbab, S. Poly(ε-caprolactone) fiber: An overview. J. Eng. Fibers Fabr. 2017, 9, 74–90. [Google Scholar]
- Ping, P.; Wang, W.; Chen, X.; Jing, X. Poly(ε-caprolactone) polyurethane and its shape-memory property. Biomacromolecules 2005, 6, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jordan, A.M.; Korley, L.T.J.; Pokorski, J.K. Drawing in poly(ε-caprolactone) fibers: Tuning mechanics, fiber dimensions and surface-modification density. J. Mater. Chem. B 2017, 5, 4499–4506. [Google Scholar] [CrossRef]
- Pérez, R.A.; Córdova, M.E.; Lopez, J.V.; Hoskins, J.N.; Zhang, B.; Grayson, S.M.; Müller, A.J. Nucleation, crystallization, self-nucleation and thermal fractionation of cyclic and linear poly(ε-caprolactone)s. React. Funct. Polym. 2014, 80, 71–82. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Pérez-Camargo, R.A.; Müller, A.J.; Zhang, B.; Grayson, S.M.; Hu, W. Non-monotonic molecular weight dependence of crystallization rates of linear and cyclic poly(epsilon-caprolactone)s in a wide temperature range. Polym. Int. 2016, 65, 1074–1079. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Pérez, R.A.; Müller, A.J.; Zhang, B.; Grayson, S.M.; Hu, W. Comparing crystallization rates between linear and cyclic poly(epsilon-caprolactones) via fast-scan chip-calorimeter. Polymer 2015, 63, 34–40. [Google Scholar] [CrossRef]
- Jia, Z.; Monteiro, M.J. Cyclic polymers: Methods and strategies. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 2085–2097. [Google Scholar] [CrossRef]
- Ogawa, T.; Nakazono, K.; Aoki, D.; Uchida, S.; Takata, T. Effective approaches to cyclic polymer from linear polymer: Synthesis and transformation of macromolecular [1]rotaxane. ACS Macro Lett. 2015, 4, 343–347. [Google Scholar] [CrossRef]
- Ishizone, T.; Han, S.; Hagiwara, M. Synthesis and surface characterization of well-defined amphiphilic block copolymers containing poly[oligo(ethylene glycol)methacrylate] segments. Macromolecules 2006, 39, 962–970. [Google Scholar] [CrossRef]
- Culkin, D.A.; Jeong, W.; Csihony, S.; Gomez, E.D.; Balsara, N.R.; Hedrick, J.L.; Waymouth, R.M. Zwitterionic polymerization of lactide to cyclic poly(lactide) by using N-heterocyclic carbine organocatalysts. Angew. Chem. Int. Ed. 2007, 46, 2627–2630. [Google Scholar] [CrossRef] [PubMed]
- Kricheldorf, H.R.; Eggerstedt, S. Macrocycles 2. Living macrocyclic polymerization of ε-caprolactone with 2,2-dibutyl-2-stanna-1,3-dioxepane as initiator. Macromol. Chem. Phys. 1998, 199, 283–290. [Google Scholar]
- Kricheldorf, H.R.; Stricker, A.; Langanke, D. Polylactones, 50. The reactivity of cyclic and noncyclic dibutyltin bisalkoxides as initiators in the polymerization of lactones. Macromol. Chem. Phys. 2001, 202, 2525–2534. [Google Scholar] [CrossRef]
- Kricheldorf, H.R. Biodegradable polymers with variable architectures via ring-expansion Polymerization. J. Polym. Sci. Polym. Chem. 2004, 42, 4723–4742. [Google Scholar] [CrossRef]
- Li, H.; Debuigne, A.; Jérome, R.; Lecomte, P. Synthesis of macrocyclic poly(ε-caprolactone) by intramolecular cross-linking of unsaturated end groups of chains precyclic by the initiation. Angew. Chem. Int. Ed. 2006, 45, 2264–2267. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jérôme, R.; Lecomte, P. Amphiphilic sun-shaped polymers by grafting macrocyclic copolyesters with PEO. Macromolecules 2008, 41, 650–654. [Google Scholar] [CrossRef]
- Castro-Osma, J.A.; Alonso-Moreno, C.; García-Martinez, J.C.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Lara-Sánchez, A.; Otero, A. Ring-opening (ROP) versus ring-extension (REP) polymerization of ε-caprolactone to give linear or cyclic polycaprolatones. Macromolecules 2013, 46, 6388–6394. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Liu, B.; Han, B.; Duan, Z.; Qi, C.; Park, D.; Kim, I. Synthesis of high molecular weight cyclic poly(ε-caprolactone)s of variable ring size based on a light-induced ring-closure approach. Macromol. Rapid Commun. 2015, 36, 1646–1650. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.J.; Jeong, W.; Brown, H.A.; Koo, B.J.; Hedrick, J.L.; Waymouth, R.M. Crystallization of cyclic polymers: Synthesis and crystallization behaviour of high molecular weight cyclic poly(ε-caprolactone)s. Macromolecules 2011, 44, 2773–2779. [Google Scholar] [CrossRef]
- Brown, H.A.; Xiong, S.; Medvedev, G.A.; Chang, Y.A.; Abu-Omar, M.M.; Caruthers, J.M.; Waymouth, R.W. Zwitterionic ring-opening polymerization: Models for kinetics of cyclic poly(caprolactone) synthesis. Macromolecules 2014, 47, 2955–2963. [Google Scholar] [CrossRef]
- Shin, E.J.; Brown, H.A.; Gonzalez, S.; Jeong, W.; Hedrick, J.L.; Waymouth, R.M. Zwitterionic copolymerization: Synthesis of cyclic gradient copolymers. Angew. Chem. Int. Ed. 2011, 50, 6388–6391. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Ando, D.; Uno, T.; Itoh, T.; Kubo, M. Preparation and intramolecular cyclization of α-carboxyl, ω-Amino heterodifunctional poly(ε-caprolactone) and poly(δ-valerolactone) with silica-supported condensation agent. Aust. J. Basic Appl. Sci. 2014, 8, 820–822. [Google Scholar]
- Makiguchi, K.; Satoh, T.; Kakuchi, T. Diphenyl phosphate as an efficient cationic organocatalyst for controlled/living ring-opening polymerization of δ-valerolactone and ε-caprolactone. Macromolecules 2011, 44, 1999–2005. [Google Scholar] [CrossRef]
- Kubo, M.; Hayashi, T.; Kobayashi, H.; Tsuboi, K.; Itoh, T. Synthesis of α-carboxyl, ω-amino heterodifunctional polystyrene and its intramolecular cyclization. Macromolecules 1997, 30, 2805–2807. [Google Scholar] [CrossRef]
- Wood, B.R.; Hodge, P.; Semlyen, J.A. Cyclic polyesters: 1. Preparation by a new synthetic method, using polymer-supported reagents. Polymer 1993, 34, 3052–3058. [Google Scholar] [CrossRef]
- Xie, M.; Shi, J.; Ding, L.; Li, J.; Han, H.; Zhang, Y. Cyclic poly(ε-caprolactone) synthesized by combination of ring-opening polymerization with ring-closure metathesis, ring closing enyne metathesis, or “click” reaction. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 3022–3033. [Google Scholar] [CrossRef]
- Hoskins, J.N.; Grayson, S.M. Synthesis and degradation behavior of cyclic poly(ε-caprolactone). Macromolecules 2009, 42, 6406–6413. [Google Scholar] [CrossRef]
- Rao, J.; Zhang, J.; Liu, S. Facile preparation of well-defined AB2 Y-shaped miktoarm star polypeptide copolymer via the combination of ring-opening polymerization and click chemistry. Biomacromolecules 2008, 9, 2586–2593. [Google Scholar] [CrossRef] [PubMed]
- Urbani, C.N.; Bell, C.A; Lonsdale, D.; Whittaker, M.R.; Monteiro, M.J. Reactive Alkyne and azide beads to increase yields of novel polymeric stars and dendrimers via the “click” reaction. Macromolecules 2007, 40, 7056–7059. [Google Scholar] [CrossRef]
- Schäler, K.; Ostas, E.; Schröter, K.; Thurn-Albrecht, T.; Binder, W.H.; Saalwächter, K. Influence of chain topology on polymer dynamics and crystallization. Investigation of linear and cyclic poly(ε-caprolactone)s by 1H solid-state NMR methods. Macromolecules 2011, 44, 2743–2754. [Google Scholar] [CrossRef]
- Su, H.; Chen, H.; Díaz, A.; Casas, M.T.; Ouiggalí, J.; Hoskins, J.N.; Grayson, S.N.; Pérez, R.A.; Müller, A.J. New insight on the crystallization and melting of cyclic PCL chains on the basis of a modified Thomson-Gibbs equation. Polymer 2013, 54, 846–859. [Google Scholar] [CrossRef]
- López, J.V.; Pérez-Camargo, R.A.; Zhang, B.; Grayson, S.M.; Müller, A.J. The influence of small amounts of linear polycaprolactone chains on the crystallization cyclic analogue molecules. RSC Adv. 2016, 6, 48049–48063. [Google Scholar] [CrossRef]
- Pérez, R.A.; López, J.V.; Hoskins, J.N.; Zhang, B.; Grayson, S.M. Nucleation and antinucleation effects of functionalized carbon nanotubes on cyclic and linear poly(ε-caprolactones). Macromolecules 2014, 47, 3553–3566. [Google Scholar] [CrossRef]
- Córdova, M.E.; Lorenzo, A.T.; Müller, A.J.; Hoskins, J.N.; Grayson, S.M. A comparative study on the crystallization behavior of analogous linear and cyclic poly(ε-caprolactone). Macromolecules 2011, 44, 1742–1746. [Google Scholar] [CrossRef]
- Li, W.; Prud’homme, R.E. Orientation and miscibility of poly(ε-caprolactone)/poly(styrene-co-acrylonitrile) mixtures. Polymer 1994, 35, 3260–3267. [Google Scholar] [CrossRef]
- Ramkumar, D.H.S.; Bhattacharya, M. Steady shear and dynamic properties of biodegradable polyesters. Polym. Eng. Sci. 1998, 38, 1426–1435. [Google Scholar] [CrossRef]
- Gimenez, J.; Cassagnau, P.; Michel, A. Bulk polymerization of ε-caprolactone: Rheological predicative laws. J. Rheol. 2000, 44, 527–547. [Google Scholar] [CrossRef]
- Acierno, S.; Di Maio, E.; Iannace, S.; Grizzuti, N. Structure development during crystallization of polycaprolactone. Rheol. Acta 2006, 45, 387–392. [Google Scholar] [CrossRef]
- Izuka, A.; Winter, H.H.; Hashimoto, T. Molecular weight dependence of viscoelasticity of polycaprolactone critical gels. Macromolecules 1992, 25, 2422–2428. [Google Scholar] [CrossRef]
- Herrera, D.; Zamora, J.C.; Bello, A.; Grimau, M.; Laredo, E.; Müller, A.J.; Lodge, T.P. Miscibility and crystallization in polycarbonate/poly(ε-caprolactone) blends: Application of the self-concentration model. Macromolecules 2005, 38, 5109–5117. [Google Scholar] [CrossRef]
- Persenaire, O.; Alexandre, M.; Degée, P.; Dubois, P. Mechanisms and kinetics of thermal degradation of poly(ε-caprolactone). Biomacromolecules 2001, 2, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jéröme, R.; Lecomte, P. Synthesis of tadpole-shaped copolyesters based on living macrocyclic poly(ε-caprolactone). Polymer 2006, 47, 8406–8413. [Google Scholar] [CrossRef]
- Kaji, H.; Horii, F. One- and two-dimensional solid-state 13C NMR analyses of the solid structure and molecular motion of poly(ε-caprolactone) isothermally crystallized from the melt. Macromolecules 1997, 30, 5791–5798. [Google Scholar] [CrossRef]
- Crescenzi, V.; Manzini, G.; Calzolar, G.; Borri, C. Thermodynamics of fusion of poly-β-propiolactone and poly-ε-caprolactone. Comparative analysis of the melting of aliphatic polylactone and polyester chains. Eur. Polym. J. 1972, 8, 449–463. [Google Scholar] [CrossRef]
- Khambatta, F.B.; Warner, F.; Russell, T.; Stein, R.S. Small-angle X-ray and light scattering studies of the morphology of blends of poly(ε-caprolactone) with poly(vinyl chloride). J. Polym. Sci. Part B Polym. Phys. 1976, 14, 1391–1424. [Google Scholar] [CrossRef]
- Schick, C. Differential scanning calorimetry (DSC) of semicrystalline polymers. Anal. Bioanal. Chem. 2009, 395, 1589–1611. [Google Scholar] [CrossRef] [PubMed]
- Wurm, A.; Merzlyakov, M.; Schick, C. Isothermal crystallization of PCL studied by temperature modulated dynamic mechanical and TMDSC analysis. J. Therm. Anal. Calorim. 1999, 56, 1155–1161. [Google Scholar] [CrossRef]
- Strobl, G. The Physics of Polymers: Concepts for Understanding Their Structures and Behavior, 3rd ed.; Springer: Berlin, Germany, 2007. [Google Scholar]
- Heck, B.; Hugel, T.; Lijima, M.; Sadiku, E.; Strobl, G. Steps in the transition of an entangled polymer melt to the partially crystalline state. New J. Phys. 1999, 1, 17. [Google Scholar]
- Pitt, C.G.; Chasalow, F.I.; Hibionada, Y.M. Aliphatic polyesters, I. The degradation of poly(ε-caprolactone) in vivo. J. Appl. Polym. Sci. 1981, 26, 3779–3787. [Google Scholar] [CrossRef]
| Polymer | [M]/[I] b | Reaction Time (h) | Yield c (%) | Mn,theo d (g/mol) | e (g/mol) | f (g/mol) | PDI g |
|---|---|---|---|---|---|---|---|
| N3-PCL20-OH | 20 | 2.0 | 96.5 | 2440 | 2680 | 2700 | 1.10 |
| N3-PCL30-OH | 30 | 5.0 | 97.3 | 3600 | 3940 | 4900 | 1.06 |
| N3-PCL50-OH | 50 | 8.0 | 97.2 | 5900 | 6250 | 7700 | 1.09 |
| N3-PCL70-OH | 70 | 10.0 | 96.5 | 8200 | 7850 | 10,700 | 1.09 |
| N3-PCL90-OH | 90 | 14.0 | 96.8 | 10,500 | 10,100 | 12,600 | 1.08 |
| N3-PCL100-OH | 100 | 20.0 | 96.4 | 11,700 | 11,900 | 16,200 | 1.06 |
| N3-PCL150-OH | 150 | 29.0 | 97.3 | 17,400 | 17,200 | 20,800 | 1.07 |
| N3-PCL200-OH | 200 | 42.0 | 97.1 | 23,100 | 24,300 | 26,400 | 1.07 |
| Polymer | Yield a (%) | Mn,theo b (g/mol) | c (g/mol) | d (g/mol) | PDI e |
|---|---|---|---|---|---|
| N3-PCL20-C≡CH | 96.8 | 2530 | 2770 | 2700 | 1.10 |
| c-PCL20 | 37.7 | 2540 | 2790 | 2300 | 1.07 |
| N3-PCL30-C≡CH | 97.7 | 3690 | 4000 | 5000 | 1.09 |
| c-PCL30 | 38.1 | 3700 | 4100 | 3700 | 1.09 |
| N3-PCL50-C≡CH | 97.2 | 5990 | 6340 | 7700 | 1.06 |
| c-PCL50 | 37.9 | 6000 | 6350 | 5200 | 1.07 |
| N3-PCL70-C≡CH | 97.5 | 8300 | 7950 | 11,100 | 1.08 |
| c-PCL70 | 39.5 | 8300 | 8000 | 7900 | 1.10 |
| N3-PCL90-C≡CH | 98.1 | 10,600 | 10,200 | 13,000 | 1.10 |
| c-PCL90 | 38.9 | 10,700 | 10,200 | 9500 | 1.08 |
| N3-PCL100-C≡CH | 96.8 | 11,700 | 11,900 | 16,400 | 1.10 |
| c-PCL100 | 40.2 | 11,800 | 12,000 | 12,100 | 1.06 |
| N3-PCL150-C≡CH | 97.5 | 17,500 | 17,200 | 20,800 | 1.11 |
| c-PCL150 | 40.5 | 17,500 | 17,300 | 15,300 | 1.13 |
| N3-PCL200-C≡CH | 97.6 | 23,200 | 23,200 | 26,800 | 1.13 |
| c-PCL200 | 41.1 | 23,300 | 23,200 | 21,500 | 1.11 |
| Precursor Reactant | Reaction Mixture a | ||
|---|---|---|---|
| c-PCL b (%) | Cyclic Byproducts c (%) | Linear Polymers d (%) | |
| N3-PCL20-C≡CH | 37.7 | 8.7 | 53.6 |
| N3-PCL30-C≡CH | 38.1 | 3.8 | 58.1 |
| N3-PCL50-C≡CH | 37.9 | 6.3 | 55.8 |
| N3-PCL70-C≡CH | 39.5 | 8.3 | 52.2 |
| N3-PCL90-C≡CH | 38.9 | 0.2 | 60.9 |
| N3-PCL100-C≡CH | 40.2 | 4.0 | 55.8 |
| N3-PCL150-C≡CH | 40.5 | 2.6 | 56.9 |
| N3-PCL200-C≡CH | 41.1 | 0.4 | 58.5 |
| Polymer a | b (g/mol) | PDI c | Tc d (°C) | ΔHc e (J/g) | Xc,m f (%) | Tm g (°C) | ΔHm h (J/g) | Xc,m i (%) |
|---|---|---|---|---|---|---|---|---|
| c-PCL20 | 2790 | 1.07 | 38.4 | −87.3 | 62.6 | 62.6 | 87.7 | 62.9 |
| N3-PCL20-C≡CH | 2770 | 1.10 | 33.6 | −67.8 | 48.6 | 55.7 | 67.9 | 48.7 |
| N3-PCL20-OH | 2680 | 1.10 | 32.5 | −67.2 | 48.2 | 55.3 | 66.4 | 47.6 |
| c-PCL30 | 4100 | 1.09 | 37.6 | −88.7 | 63.6 | 61.8 | 88.4 | 63.4 |
| N3-PCL30-C≡CH | 4000 | 1.09 | 33.5 | −68.3 | 49.0 | 55.5 | 67.6 | 48.5 |
| N3-PCL30-OH | 3940 | 1.06 | 33.1 | −66.8 | 47.9 | 55.7 | 67.8 | 48.6 |
| c-PCL50 | 6350 | 1.07 | 37.4 | −88.5 | 63.4 | 61.7 | 86.8 | 62.2 |
| N3-PCL50-C≡CH | 6340 | 1.06 | 32.8 | −68.6 | 49.2 | 55.3 | 68.3 | 49.0 |
| N3-PCL50-OH | 6250 | 1.09 | 32.3 | −68.4 | 49.0 | 56.1 | 67.3 | 48.2 |
| c-PCL70 | 8000 | 1.10 | 38.3 | −87.6 | 62.8 | 61.3 | 87.4 | 62.7 |
| N3-PCL70-C≡CH | 7950 | 1.08 | 32.5 | −67.4 | 48.3 | 55.6 | 67.7 | 48.5 |
| N3-PCL70-OH | 7850 | 1.09 | 31.8 | −67.7 | 48.5 | 56.2 | 68.2 | 48.9 |
| c-PCL90 | 10,200 | 1.08 | 37.7 | −88.2 | 63.2 | 61.5 | 88.6 | 63.5 |
| N3-PCL90-C≡CH | 10,200 | 1.10 | 32.9 | −66.9 | 48.0 | 55.2 | 68.4 | 49.0 |
| N3-PCL90-OH | 10,100 | 1.08 | 32.4 | −68.3 | 49.0 | 55.7 | 66.8 | 47.9 |
| c-PCL100 | 12,000 | 1.06 | 38.2 | −87.4 | 62.7 | 61.2 | 88.2 | 63.2 |
| N3-PCL100-C≡CH | 11,900 | 1.10 | 32.8 | −67.3 | 48.2 | 56.4 | 67.8 | 48.6 |
| N3-PCL100-OH | 11,900 | 1.06 | 31.6 | −67.9 | 48.7 | 56.3 | 67.1 | 48.1 |
| c-PCL150 | 17,300 | 1.13 | 37.5 | −88.4 | 63.4 | 60.9 | 87.3 | 62.6 |
| N3-PCL150-C≡CH | 17,200 | 1.11 | 32.5 | −68.5 | 49.1 | 56.1 | 67.3 | 48.2 |
| N3-PCL150-OH | 17,200 | 1.07 | 33.2 | −67.6 | 48.5 | 55.8 | 68.7 | 49.3 |
| c-PCL200 | 23,200 | 1.11 | 38.6 | −88.3 | 63.3 | 61.6 | 88.5 | 63.4 |
| N3-PCL200-C≡CH | 23,200 | 1.13 | 31.8 | −68.1 | 48.8 | 56.2 | 68.3 | 49.0 |
| N3-PCL200-OH | 24,300 | 1.07 | 32.7 | −68.2 | 48.9 | 56.2 | 67.2 | 48.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, L.; Ryu, W.; Kim, H.; Ree, M. Precise Synthesis, Properties, and Structures of Cyclic Poly(ε-caprolactone)s. Polymers 2018, 10, 577. https://doi.org/10.3390/polym10060577
Xiang L, Ryu W, Kim H, Ree M. Precise Synthesis, Properties, and Structures of Cyclic Poly(ε-caprolactone)s. Polymers. 2018; 10(6):577. https://doi.org/10.3390/polym10060577
Chicago/Turabian StyleXiang, Li, Wonyeong Ryu, Heesoo Kim, and Moonhor Ree. 2018. "Precise Synthesis, Properties, and Structures of Cyclic Poly(ε-caprolactone)s" Polymers 10, no. 6: 577. https://doi.org/10.3390/polym10060577





