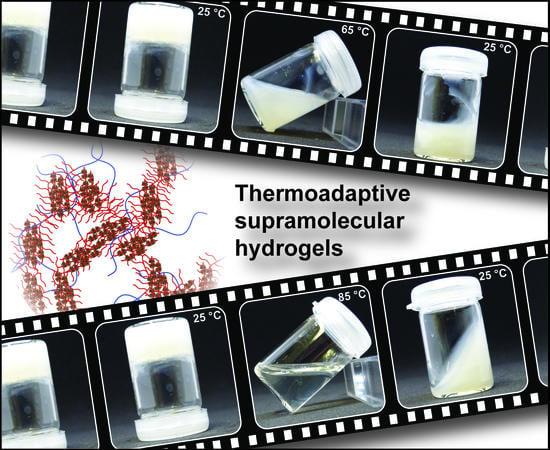
Thermoadaptive Supramolecular α-Cyclodextrin Crystallization-Based Hydrogels via Double Hydrophilic Block Copolymer Templating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of PVP41k-b-POEGMA22k
2.3. Exemplary Formation of Thermoadaptive PVP41k-b-POEGMA22k-Based Supramolecular Hydrogel
2.4. Characterization Methods
3. Results and Discussion
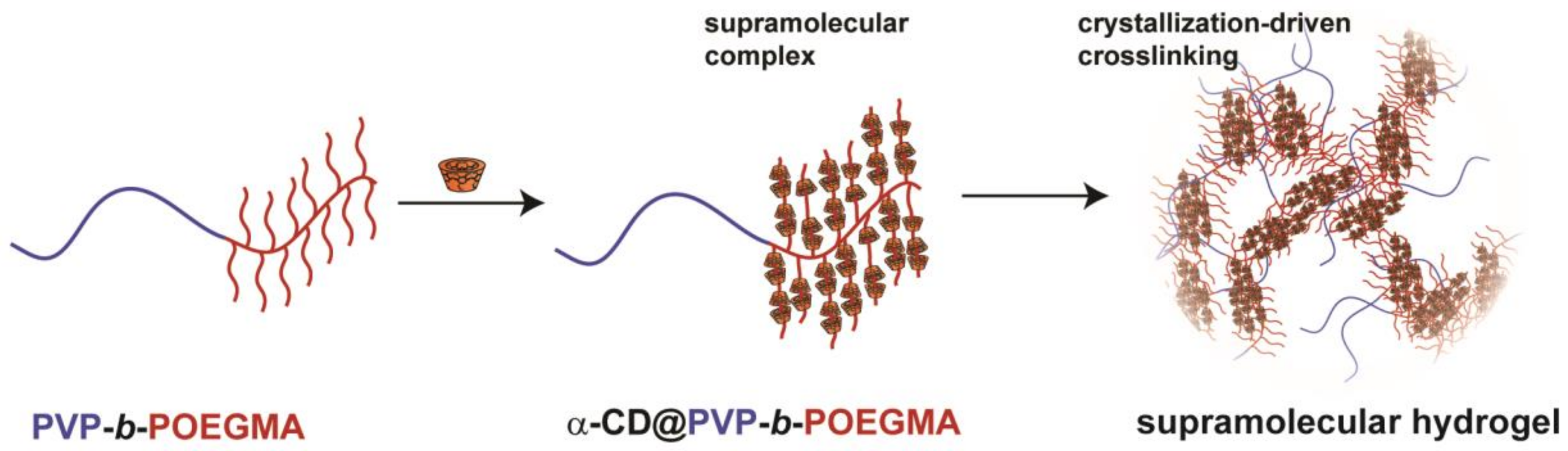
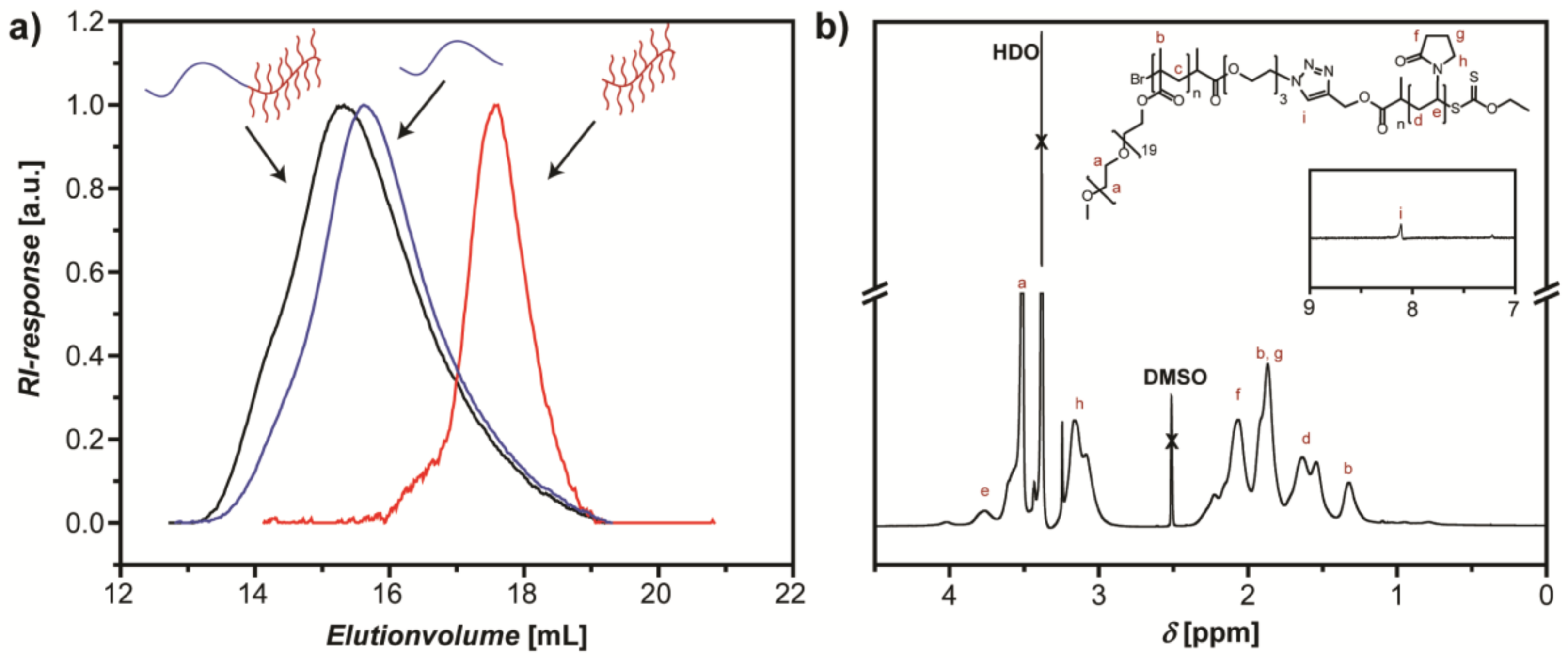
3.1. Synthesis of DHBC PVP-b-POEGMA and Formation of Supramolecular Hydrogels
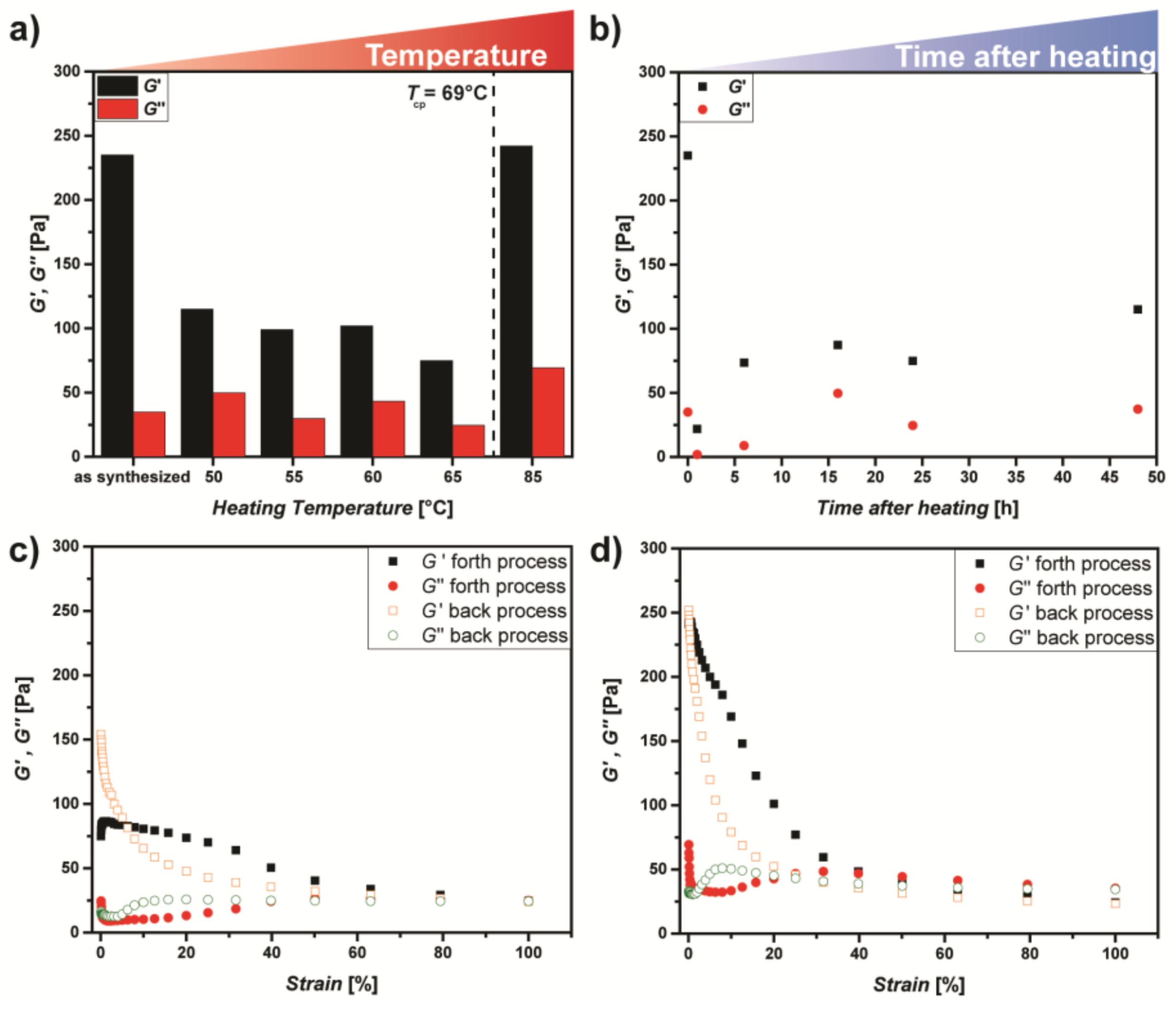
3.2. Thermoadaptive Properties of DHBC-Mediated Supramolecular Hydrogels
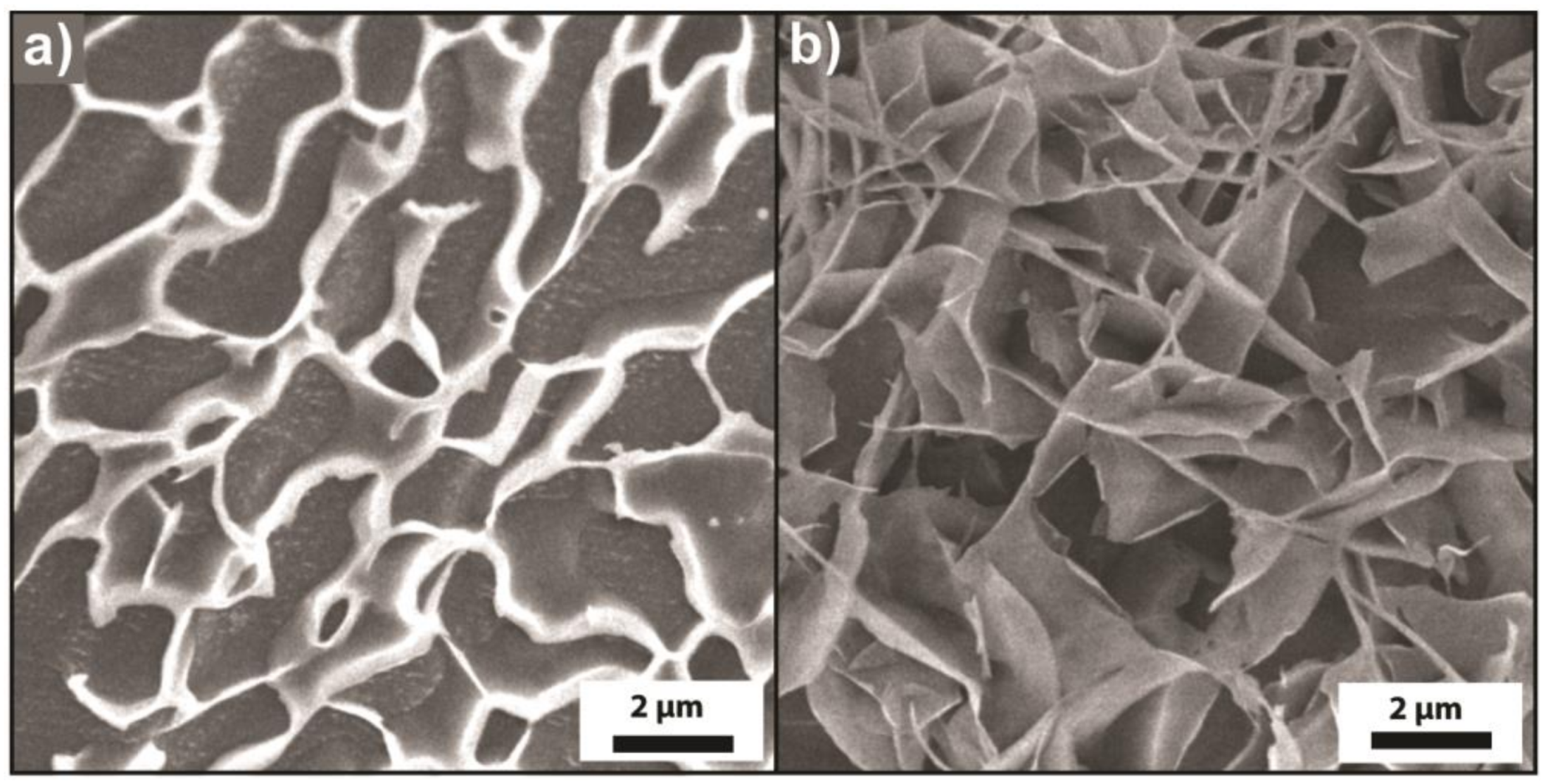
3.3. Microscopic Characterization of Thermoadaptive Hydrogels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bressan, E.; Favero, V.; Gardin, C.; Ferroni, L.; Iacobellis, L.; Favero, L.; Vindigni, V.; Berengo, M.; Sivolella, S.; Zavan, B. Biopolymers for hard and soft engineered tissues: Application in odontoiatric and plastic surgery field. Polymers 2011, 3, 509–526. [Google Scholar] [CrossRef]
- Anseth, K.S.; Bowman, C.N.; Brannon-Peppas, L. Mechanical properties of hydrogels and their experimental determination. Biomaterials 1996, 17, 1647–1657. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3d cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Essawy, H.A.; Ibrahim, H.S. Synthesis and characterization of poly(vinylpyrrolidone-co-methylacrylate) hydrogel for removal and recovery of heavy metal ions from wastewater. React. Funct. Polym. 2004, 61, 421–432. [Google Scholar] [CrossRef]
- Kumru, B.; Shalom, M.; Antonietti, M.; Schmidt, B.V.K.J. Reinforced hydrogels via carbon nitride initiated polymerization. Macromolecules 2017, 50, 1862–1869. [Google Scholar] [CrossRef]
- Liu, M.; Ishida, Y.; Ebina, Y.; Sasaki, T.; Hikima, T.; Takata, M.; Aida, T. An anisotropic hydrogel with electrostatic repulsion between cofacially aligned nanosheets. Nature 2014, 517, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Schmidt, B.V.K.J.; Brown, H.R.; Hawker, C.J. One-pot “click” fabrication of slide-ring gels. Macromolecules 2015, 48, 7774–7781. [Google Scholar] [CrossRef]
- Ito, K. Novel cross-linking concept of polymer network: Synthesis, structure, and properties of slide-ring gels with freely movable junctions. Polym. J. 2007, 39, 489–499. [Google Scholar] [CrossRef]
- Nakayama, A.; Kakugo, A.; Gong, J.P.; Osada, Y.; Takai, M.; Erata, T.; Kawano, S. High mechanical strength double-network hydrogel with bacterial cellulose. Adv. Funct. Mater. 2004, 14, 1124–1128. [Google Scholar] [CrossRef]
- Huang, T.; Xu, H.G.; Jiao, K.X.; Zhu, L.P.; Brown, H.R.; Wang, H.L. A novel hydrogel with high mechanical strength: A macromolecular microsphere composite hydrogel. Adv. Mater. 2007, 19, 1622–1626. [Google Scholar] [CrossRef]
- Brown, H.R. A model of the fracture of double network gels. Macromolecules 2007, 40, 3815–3818. [Google Scholar] [CrossRef]
- Wang, Q.; Mynar, J.L.; Yoshida, M.; Lee, E.; Lee, M.; Okuro, K.; Kinbara, K.; Aida, T. High-water-content mouldable hydrogels by mixing clay and a dendritic molecular binder. Nature 2010, 463, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Kumru, B.; Molinari, V.; Shalom, M.; Antonietti, M.; Schmidt, B.V.K.J. Tough high modulus hydrogels derived from carbon-nitride via an ethylene glycol co-solvent route. Soft Matter 2018, 14, 2655–2664. [Google Scholar] [CrossRef] [PubMed]
- Appel, E.A.; del Barrio, J.; Loh, X.J.; Scherman, O.A. Supramolecular polymeric hydrogels. Chem. Soc. Rev. 2012, 41, 6195–6214. [Google Scholar] [CrossRef] [PubMed]
- Appel, E.A.; Loh, X.J.; Jones, S.T.; Biedermann, F.; Dreiss, C.A.; Scherman, O.A. Ultrahigh-water-content supramolecular hydrogels exhibiting multistimuli responsiveness. J. Am. Chem. Soc. 2012, 134, 11767–11773. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Pitet, L.M.; Wyss, H.M.; Vos, M.; Dankers, P.Y.W.; Meijer, E.W. Tough stimuli-responsive supramolecular hydrogels with hydrogen-bonding network junctions. J. Am. Chem. Soc. 2014, 136, 6969–6977. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.X.; Utech, S.; Gopez, J.D.; Mabesoone, M.F.J.; Hawker, C.J.; Klinger, D. Non-covalent microgel particles containing functional payloads: Coacervation of peg-based triblocks via microfluidics. ACS Appl. Mater. Interfaces 2016, 8, 16914–16921. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.N.; Feldman, K.E.; Lynd, N.A.; Deek, J.; Campos, L.M.; Spruell, J.M.; Hernandez, B.M.; Kramer, E.J.; Hawker, C.J. Tunable, high modulus hydrogels driven by ionic coacervation. Adv. Mater. 2011, 23, 2327–2331. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Li, G.; Liu, X.; Wu, S.; Tong, Z. Redox-responsive gel−sol/sol−gel transition in poly(acrylic acid) aqueous solution containing Fe(III) ions switched by light. J. Am. Chem. Soc. 2008, 130, 16166–16167. [Google Scholar] [CrossRef] [PubMed]
- Lehn, J.-M. Perspectives in chemistry—Aspects of adaptive chemistry and materials. Angew. Chem. Int. Ed. 2015, 54, 3276–3289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, H.; Yang, Z.; Xu, B. Supramolecular hydrogels respond to ligand–receptor interaction. J. Am. Chem. Soc. 2003, 125, 13680–13681. [Google Scholar] [CrossRef] [PubMed]
- Steed, J.W. Supramolecular gel chemistry: Developments over the last decade. Chem. Commun. 2011, 47, 1379–1383. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.V.K.J.; Kugele, D.; von Irmer, J.; Steinkoenig, J.; Mutlu, H.; Rüttiger, C.; Hawker, C.J.; Gallei, M.; Barner-Kowollik, C. Dual-gated supramolecular star polymers in aqueous solution. Macromolecules 2017, 50, 2375–2386. [Google Scholar] [CrossRef]
- Schmidt, B.V.K.J.; Barner-Kowollik, C. Dynamic macromolecular material design—The versatility of cyclodextrin-based host–guest chemistry. Angew. Chem. Int. Ed. 2017, 56, 8350–8369. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Redox-responsive self-healing materials formed from host-guest polymers. Nat. Commun. 2011, 2, 511. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Kobayashi, R.; Takashima, Y.; Hashidzume, A.; Yamaguchi, H. Macroscopic self-assembly through molecular recognition. Nat. Chem. 2010, 3, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Li, T.; Zhao, Q.; Yang, X.; Wu, J.; Luo, Y.; Xie, T. Supramolecular lego assembly towards three-dimensional multi-responsive hydrogels. Adv. Mater. 2014, 26, 5665–5669. [Google Scholar] [CrossRef] [PubMed]
- Kakuta, T.; Takashima, Y.; Nakahata, M.; Otsubo, M.; Yamaguchi, H.; Harada, A. Preorganized hydrogel: Self-healing properties of supramolecular hydrogels formed by polymerization of host–guest-monomers that contain cyclodextrins and hydrophobic guest groups. Adv. Mater. 2013, 25, 2849–2853. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Zhu, M.; Sun, Y.; Xu, J.; Feng, Q.; Lin, S.; Wu, T.; Xu, J.; Tian, F.; Xia, J.; et al. Robust biopolymeric supramolecular “host-guest macromer” hydrogels reinforced by in situ formed multivalent nanoclusters for cartilage regeneration. Macromolecules 2016, 49, 866–875. [Google Scholar] [CrossRef]
- Takashima, Y.; Hatanaka, S.; Otsubo, M.; Nakahata, M.; Kakuta, T.; Hashidzume, A.; Yamaguchi, H.; Harada, A. Expansion–contraction of photoresponsive artificial muscle regulated by host–guest interactions. Nat. Commun. 2012, 3, 1270. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Zhou, Z.; Ni, X.; Leong, K.W. Formation of supramolecular hydrogels induced by inclusion complexation between pluronics and α-cyclodextrin. Macromolecules 2001, 34, 7236–7237. [Google Scholar] [CrossRef]
- Li, J.; Harada, A.; Kamachi, M. Sol–gel transition during inclusion complex formation between α-cyclodextrin and high molecular weight poly(ethylene glycol)s in aqueous solution. Polym. J. 1994, 26, 1019–1026. [Google Scholar] [CrossRef]
- Li, J.; Ni, X.; Leong, K.W. Injectable drug-delivery systems based on supramolecular hydrogels formed by poly(ethylene oxide)s and α-cyclodextrin. J. Biomed. Mater. Res. Part A 2003, 65A, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Williamson, G.S.; Yang, H. Branched polyrotaxane hydrogels consisting of alpha-cyclodextrin and low-molecular-weight four-arm polyethylene glycol and the utility of their thixotropic property for controlled drug release. Colloids Surf. B 2018, 165, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Huh, K.M.; Ooya, T.; Lee, W.K.; Sasaki, S.; Kwon, I.C.; Jeong, S.Y.; Yui, N. Supramolecular-structured hydrogels showing a reversible phase transition by inclusion complexation between poly(ethylene glycol) grafted dextran and α-cyclodextrin. Macromolecules 2001, 34, 8657–8662. [Google Scholar] [CrossRef]
- Kai, D.; Low, Z.W.; Liow, S.S.; Abdul Karim, A.; Ye, H.; Jin, G.; Li, K.; Loh, X.J. Development of lignin supramolecular hydrogels with mechanically responsive and self-healing properties. ACS Sustain. Chem. Eng. 2015, 3, 2160–2169. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Ni, X.; Wang, X.; Li, H.; Leong, K.W. Self-assembled supramolecular hydrogels formed by biodegradable peo–phb–peo triblock copolymers and α-cyclodextrin for controlled drug delivery. Biomaterials 2006, 27, 4132–4140. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, S.M.; Schlaad, H.; Antonietti, M. Aqueous self-assembly of purely hydrophilic block copolymers into giant vesicles. Angew. Chem. Int. Ed. 2015, 54, 9715–9718. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.V.K.J. Double hydrophilic block copolymer self-assembly in aqueous solution. Macromol. Chem. Phys. 2018, 219, 1700494. [Google Scholar] [CrossRef]
- Casse, O.; Shkilnyy, A.; Linders, J.; Mayer, C.; Häussinger, D.; Völkel, A.; Thünemann, A.F.; Dimova, R.; Cölfen, H.; Meier, W.; et al. Solution behavior of double-hydrophilic block copolymers in dilute aqueous solution. Macromolecules 2012, 45, 4772–4777. [Google Scholar] [CrossRef]
- Willersinn, J.; Bogomolova, A.; Brunet Cabré, M.; Schmidt, B.V.K.J. Vesicles of double hydrophilic pullulan and poly(acrylamide) block copolymers: A combination of synthetic- and bio-derived blocks. Polym. Chem. 2017, 8, 1244–1254. [Google Scholar] [CrossRef]
- Park, H.; Walta, S.; Rosencrantz, R.R.; Korner, A.; Schulte, C.; Elling, L.; Richtering, W.; Boker, A. Micelles from self-assembled double-hydrophilic phema-glycopolymer-diblock copolymers as multivalent scaffolds for lectin binding. Polym. Chem. 2016, 7, 878–886. [Google Scholar] [CrossRef]
- Willersinn, J.; Schmidt, B.V.K.J. Self-assembly of double hydrophilic poly(2-ethyl-2-oxazoline)-b-poly(n-vinylpyrrolidone) block copolymers in aqueous solution. Polymers 2017, 9, 293. [Google Scholar] [CrossRef]
- Al Nakeeb, N.; Willersinn, J.; Schmidt, B.V.K.J. Self-assembly behavior and biocompatible crosslinking of double hydrophilic linear-brush block copolymers. Biomacromolecules 2017, 18, 3695–3705. [Google Scholar] [CrossRef] [PubMed]
- Hartlieb, M.; Pretzel, D.; Wagner, M.; Hoeppener, S.; Bellstedt, P.; Gorlach, M.; Englert, C.; Kempe, K.; Schubert, U.S. Core cross-linked nanogels based on the self-assembly of double hydrophilic poly(2-oxazoline) block copolymers. J. Mater. Chem. B 2015, 3, 1748–1759. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Amano, Y.; Nakasako, S.; Ohta, S.; Ito, T. Biocompatible star block copolymer hydrogel cross-linked with calcium ions. ACS Biomater. Sci. Eng. 2015, 1, 914–918. [Google Scholar] [CrossRef]
- Hwang, J.; Heil, T.; Antonietti, M.; Schmidt, B.V.K.J. Morphogenesis of metal–organic mesocrystals mediated by double hydrophilic block copolymers. J. Am. Chem. Soc. 2018, 140, 2947–2956. [Google Scholar] [CrossRef] [PubMed]
- Cölfen, H.; Antonietti, M. Mesocrystals: Inorganic superstructures made by highly parallel crystallization and controlled alignment. Angew. Chem. Int. Ed. 2005, 44, 5576–5591. [Google Scholar] [CrossRef] [PubMed]
- Quemener, D.; Davis, T.P.; Barner-Kowollik, C.; Stenzel, M.H. Raft and click chemistry: A versatile approach to well-defined block copolymers. Chem. Commun. 2006, 5051–5053. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.; Save, M.; Arathoon, B.; Charleux, B. Preparation of a xanthate-terminated dextran by click chemistry: Application to the synthesis of polysaccharide-coated nanoparticles via surfactant-free ab initio emulsion polymerization of vinyl acetate. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 2845–2857. [Google Scholar] [CrossRef]
- Huh, K.M.; Cho, Y.W.; Chung, H.; Kwon, I.C.; Jeong, S.Y.; Ooya, T.; Lee, W.K.; Sasaki, S.; Yui, N. Supramolecular hydrogel formation based on inclusion complexation between poly(ethylene glycol)-modified chitosan and α-cyclodextrin. Macromol. Biosci. 2004, 4, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Yamagami, M.; Kamitakahara, H.; Yoshinaga, A.; Takano, T. Thermo-reversible supramolecular hydrogels of trehalose-type diblock methylcellulose analogues. Carbohydr. Polym. 2018, 183, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.V.K.J.; Hetzer, M.; Ritter, H.; Barner-Kowollik, C. Modulation of the thermoresponsive behavior of poly(N,N-diethylacrylamide) via cyclodextrin host/guest interactions. Macromol. Rapid Commun. 2013, 34, 1306–1311. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, V.R.; Hoogenboom, R. Solution polymeric optical temperature sensors with long-term memory function powered by supramolecular chemistry. Chem. Eur. J. 2015, 21, 1302–1311. [Google Scholar] [CrossRef] [PubMed]






| Polymer | Polymer Content [wt.%] | α-CD Content [wt.%] | Guest/α-CD Ratio | State 1 | G’ [Pa] 2 | G’’ [Pa] 2 | Tsp [°C] 1 | Tcp [°C] 1 | Thermo-Adaptive 1 |
|---|---|---|---|---|---|---|---|---|---|
| PVP41k-b-POEGMA22k | 0.9 | 10.7 | 0.33 | sol | 26 | 28 | - | 64 | - |
| PVP41k-b-POEGMA22k | 1.7 | 10.6 | 0.62 | weak gel | 50 | 36 | - | 65 | - |
| PVP41k-b-POEGMA22k | 2.2 | 10.5 | 0.81 | gel | 60 | 22 | 27 | 68 | x |
| PVP41k-b-POEGMA22k | 3.3 | 10.4 | 1.22 | gel | 235 | 35 | 45 | 69 | x |
| PVP41k-b-POEGMA22k | 6.5 | 10.0 | 2.51 | gel | 842 | 170 | 59 | 71 | - |
| PVP41k-b-POEGMA22k | 3.3 | 5.2 | 2.45 | flowing gel | 57 | 32 | - | 54 | - |
| PVP41k-b-POEGMA22k | 3.3 | 8.0 | 1.59 | gel | 83 | 54 | 31 | 60 | x |
| PVP41k + POEGMA22k | 1.7 + 1.7 | 10.4 | 1.26 | gel | 1110 | 253 | 45 | 71 | x |
| POEGMA22k | 1.7 | 10.4 | 1.80 | gel | 3040 | 964 | 55 | 72 | - |
| PVP41k | 1.7 | 10.4 | - | solution | - | - | - | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Kumru, B.; Al Nakeeb, N.; Willersinn, J.; Schmidt, B.V.K.J. Thermoadaptive Supramolecular α-Cyclodextrin Crystallization-Based Hydrogels via Double Hydrophilic Block Copolymer Templating. Polymers 2018, 10, 576. https://doi.org/10.3390/polym10060576
Li T, Kumru B, Al Nakeeb N, Willersinn J, Schmidt BVKJ. Thermoadaptive Supramolecular α-Cyclodextrin Crystallization-Based Hydrogels via Double Hydrophilic Block Copolymer Templating. Polymers. 2018; 10(6):576. https://doi.org/10.3390/polym10060576
Chicago/Turabian StyleLi, Tingting, Baris Kumru, Noah Al Nakeeb, Jochen Willersinn, and Bernhard V. K. J. Schmidt. 2018. "Thermoadaptive Supramolecular α-Cyclodextrin Crystallization-Based Hydrogels via Double Hydrophilic Block Copolymer Templating" Polymers 10, no. 6: 576. https://doi.org/10.3390/polym10060576