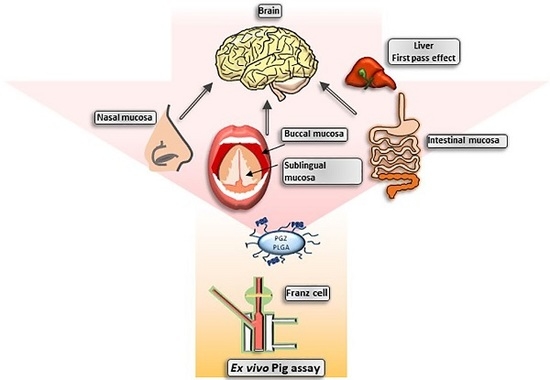
Comparative Study of Ex Vivo Transmucosal Permeation of Pioglitazone Nanoparticles for the Treatment of Alzheimer’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of NPs and Physicochemical Characterization
2.2.2. Tissue Samples
2.2.3. Transmucosal Ex Vivo Permeations
2.2.4. Biopharmaceutical Parameters
2.2.5. Rheological Behavior
2.2.6. Short-Term Stability
2.2.7. Statistical Analysis
3. Results
3.1. Physicochemical Characterization
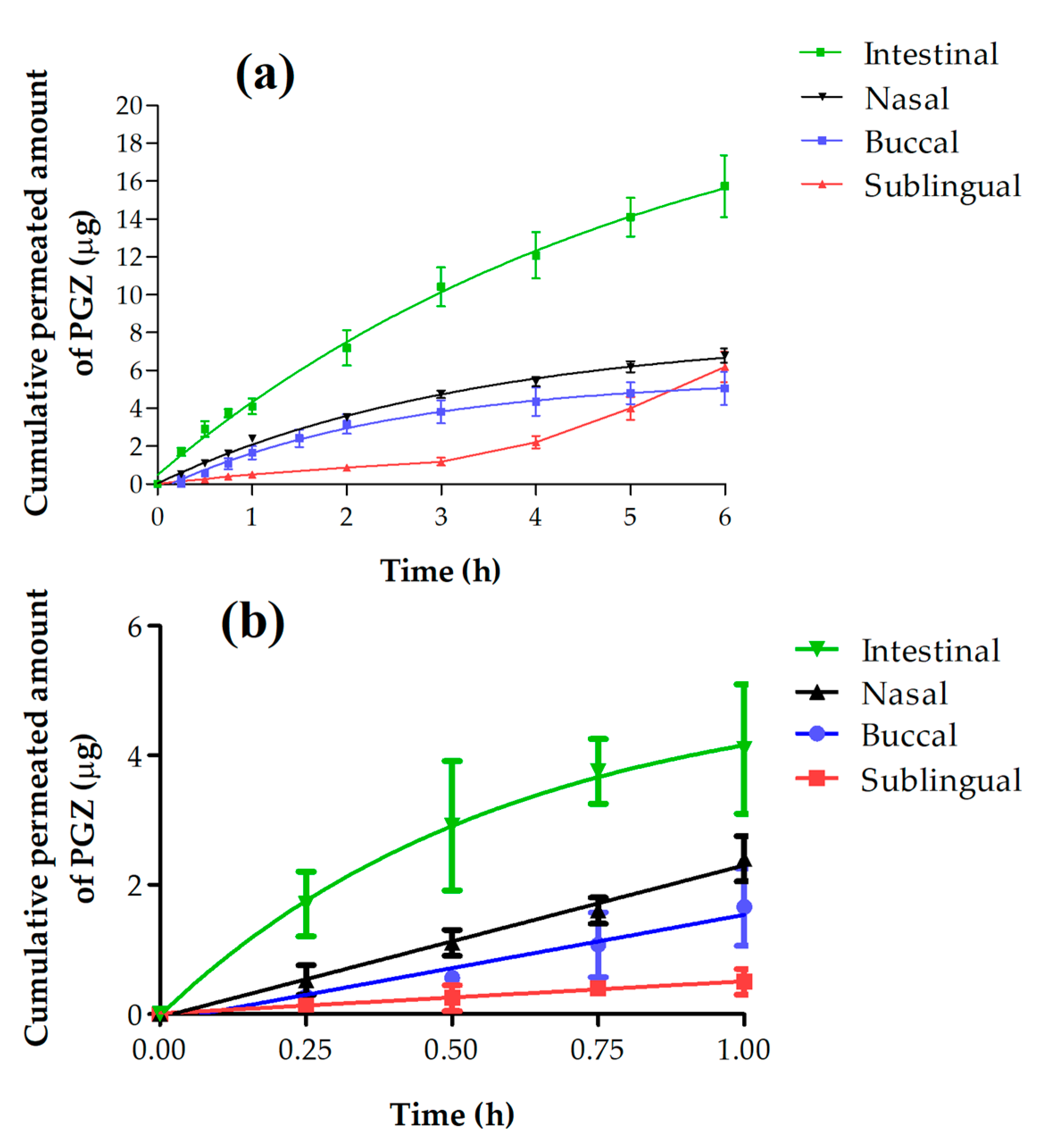
3.2. Ex Vivo Permeation Studies
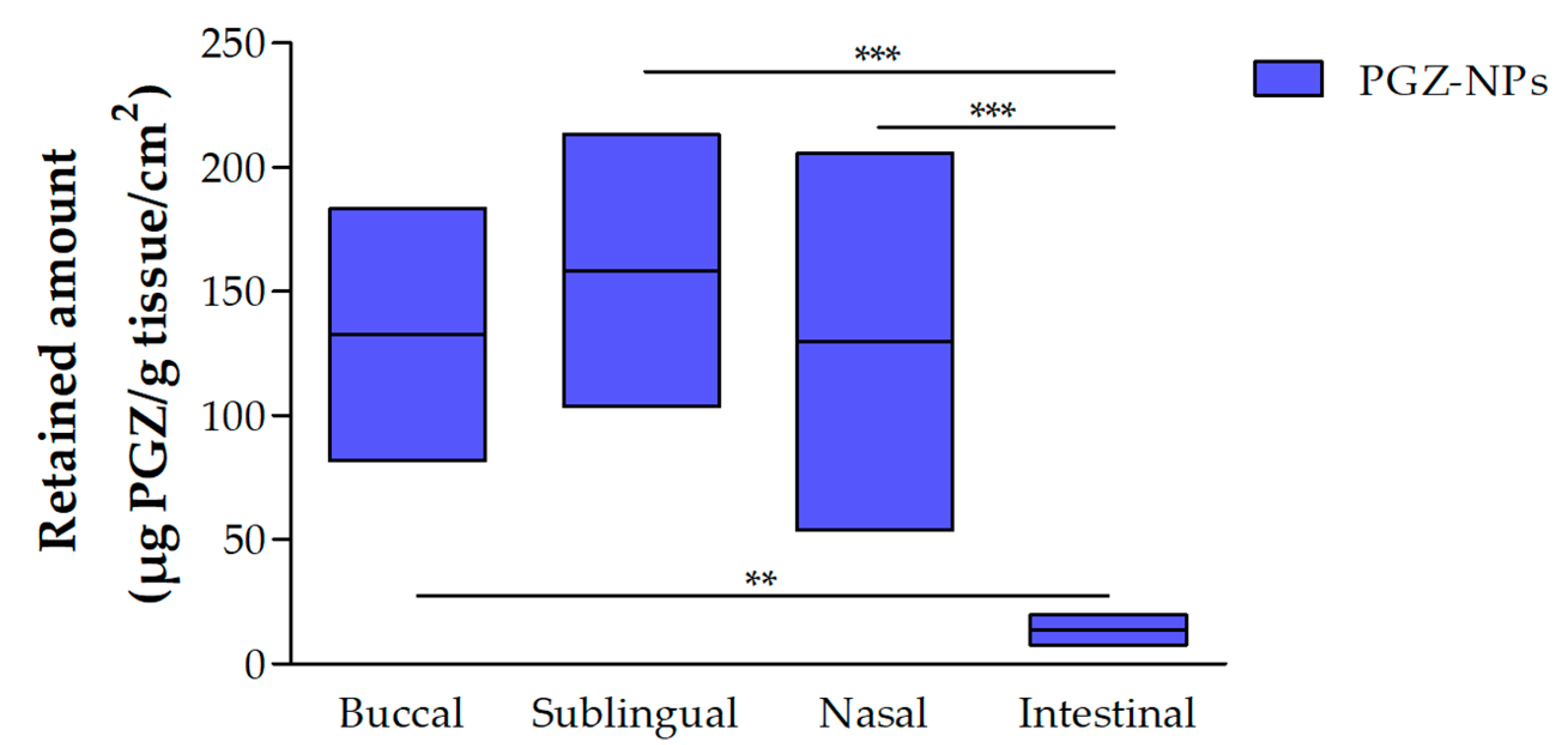
3.2.1. Retained Amount of PGZ
3.2.2. Permeation and Predictions Parameters Data
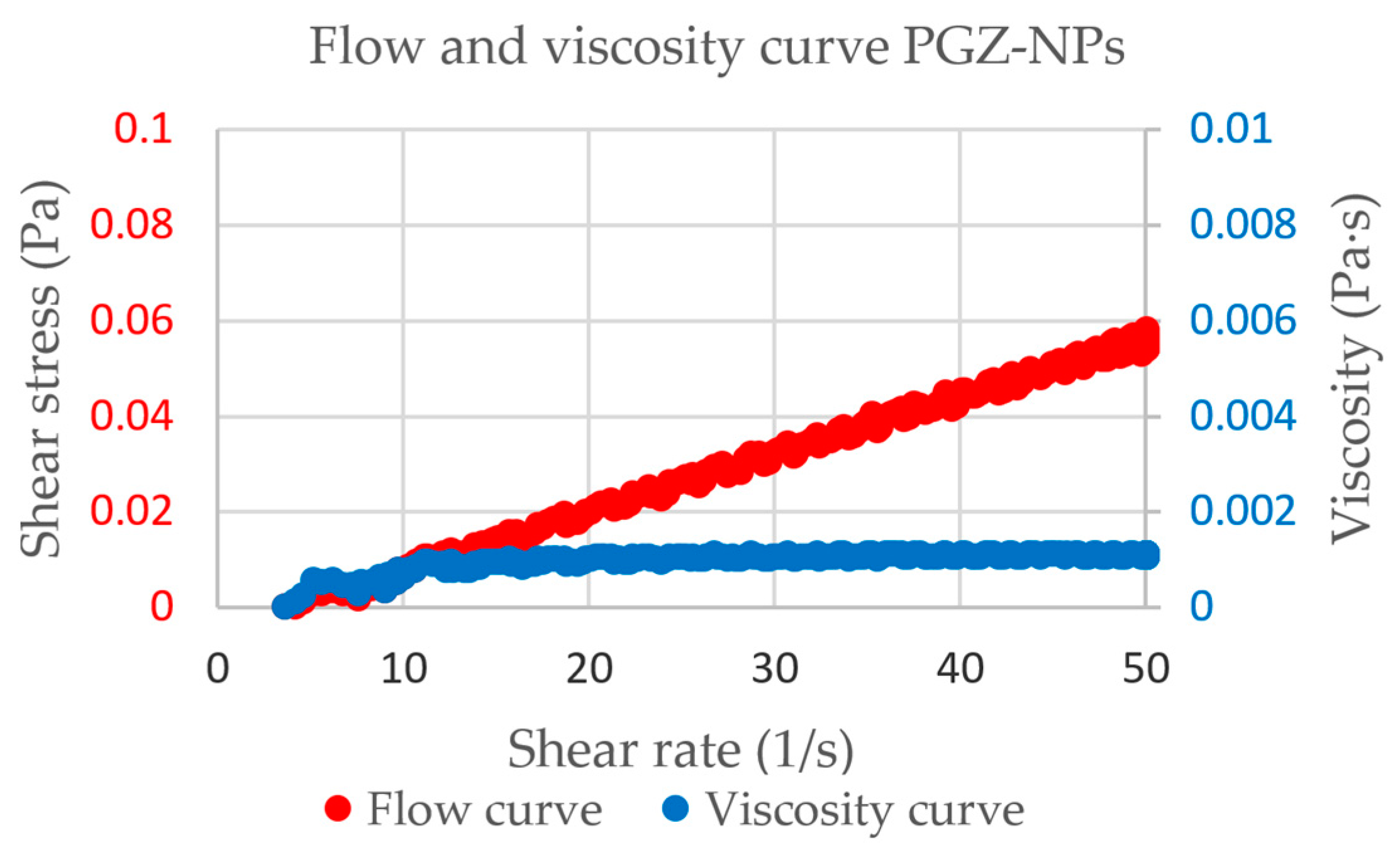
3.3. Rheological Study
3.4. Short-Term Stability
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alzheimer’s Association. 2016 Alzheimer’s disease facts and figures. Alzheimer Dement. J. Alzheimer Assoc. 2016, 12, 459–509. [Google Scholar] [CrossRef]
- El Kadmiri, N.; Said, N.; Slassi, I.; El Moutawakil, B.; Nadifi, S. Biomarkers for Alzheimer Disease: Classical and Novel Candidates’ Review. Neuroscience 2018, 370, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.; Schindler, R.; Schwam, E.; Waldemar, G.; Jones, R.W.; Gauthier, S.; Lopez, O.L.; Cummings, J.; Xu, Y.; Feldman, H.H. Effectiveness of donepezil in reducing clinical worsening in patients with mild-to-moderate alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2009, 28, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Kumar, A.; Keegan, R.M.; Deshmukh, R. Recent advances in the neurobiology and neuropharmacology of Alzheimer’s disease. Biomed. Pharmacother. 2018, 98, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Li, K.; Zhang, L.; Yao, S.; Piao, Z.; Song, L. Influence of the Pro12Ala polymorphism of PPAR-γ on age at onset and sRAGE levels in Alzheimer’s disease. Brain Res. 2009, 1291, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Combarros, O.; Rodriguez-Rodriguez, E.; Mateo, I.; Vazquez-Higuera, J.L.; Infante, J.; Berciano, J.; Sanchez-Juan, P. APOE dependent-association of PPAR-γ genetic variants with Alzheimer’s disease risk. Neurobiol. Aging 2011, 32, 547.e1–547.e6. [Google Scholar] [CrossRef] [PubMed]
- Radenkovic, M. Pioglitazone and Endothelial Dysfunction: Pleiotropic Effects and Possible Therapeutic Implications. Sci. Pharm. 2014, 82, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Mori, Y.; Nagano, A.; Naiki-Ito, A.; Kato, H.; Nagayasu, Y.; Kobayashi, M.; Kuno, T.; Takahashi, S. Pioglitazone, a Peroxisome Proliferator-Activated Receptor γ Agonist, Suppresses Rat Prostate Carcinogenesis. Int. J. Mol. Sci. 2016, 17, 2071. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Huan, Y.; Liu, S.; Hou, S.; Sun, S.; Li, C.; Liu, Q.; Jiang, Q.; Wang, Y.; Shen, Z. Effect of Chronic Pioglitazone Treatment on Hepatic Gene Expression Profile in Obese C57BL/6J Mice. Int. J. Mol. Sci. 2015, 16, 12213–12229. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Park, H.S.; Lee, J.U.; Bothwell, A.L.; Choi, J.M. Sex-Based Selectivity of PPARγ Regulation in Th1, Th2, and Th17 Differentiation. Int. J. Mol. Sci. 2016, 17, 1347. [Google Scholar] [CrossRef] [PubMed]
- Tobiasova, Z.; Zhang, L.; Yi, T.; Qin, L.; Manes, T.D.; Kulkarni, S.; Lorber, M.I.; Rodriguez, F.C.; Choi, J.M.; Tellides, G.; et al. Peroxisome proliferator-activated receptor-γ agonists prevent in vivo remodeling of human artery induced by alloreactive T cells. Circulation 2011, 124, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Fakhfouri, G.; Ahmadiani, A.; Rahimian, R.; Grolla, A.A.; Moradi, F.; Haeri, A. WIN55212-2 attenuates amyloid-beta-induced neuroinflammation in rats through activation of cannabinoid receptors and PPAR-γ pathway. Neuropharmacology 2012, 63, 653–666. [Google Scholar] [CrossRef] [PubMed]
- El-Zaher, A.A.; Elkady, E.F.; Elwy, H.M.; Saleh, M. Simultaneous spectrophotometric determination of glimepiride and pioglitazone in binary mixture and combined dosage form using chemometric-assisted techniques. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 182, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; Burgdorf, S.; Dani, I.; Saijo, K.; Flossdorf, J.; Hucke, S.; Alferink, J.; Novak, N.; Beyer, M.; Mayer, G.; et al. The nuclear receptor PPARγ selectively inhibits Th17 differentiation in a T cell–intrinsic fashion and suppresses CNS autoimmunity. J. Exp. Med. 2009, 206, 2079–2089. [Google Scholar] [CrossRef] [PubMed]
- Silva-Abreu, M.; Espinoza, L.C.; Rodriguez-Lagunas, M.J.; Fabrega, M.J.; Espina, M.; Garcia, M.L.; Calpena, A.C. Human Skin Permeation Studies with PPARγ Agonist to Improve Its Permeability and Efficacy in Inflammatory Processes. Int. J. Mol. Sci. 2017, 18, 2548. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Sastre, M.; Dumitrescu-Ozimek, L.; Hanke, A.; Dewachter, I.; Kuiperi, C.; O’Banion, K.; Klockgether, T.; Van Leuven, F.; Landreth, G.E. Acute treatment with the PPARγ agonist pioglitazone and ibuprofen reduces glial inflammation and Aβ1–42 levels in APPV717I transgenic mice. Brain 2005, 128, 1442–1453. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Hanyu, H.; Hirao, K.; Kanetaka, H.; Sakurai, H.; Iwamoto, T. Efficacy of PPAR-γ agonist pioglitazone in mild Alzheimer disease. Neurobiol. Aging 2011, 32, 1626–1633. [Google Scholar] [CrossRef] [PubMed]
- Hyma, P.; Abbulu, K. Formulation and characterisation of self-microemulsifying drug delivery system of pioglitazone. Biomed. Prev. Nutr. 2013, 3, 345–350. [Google Scholar] [CrossRef]
- He, W.; Li, Y.; Zhang, R.; Wu, Z.; Yin, L. Gastro-floating bilayer tablets for the sustained release of metformin and immediate release of pioglitazone: Preparation and in vitro/in vivo evaluation. Int. J. Pharm. 2014, 476, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Al-Saleh, A.A.; Akhtar, N.; Al-Mohizea, A.M.; Al-Jenoobi, F.I. Transdermal delivery of antidiabetic drugs: Formulation and delivery strategies. Drug Discov. Today 2015, 20, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Jug, M.; Hafner, A.; Lovric, J.; Kregar, M.L.; Pepic, I.; Vanic, Z.; Cetina-Cizmek, B.; Filipovic-Grcic, J. An overview of in vitro dissolution/release methods for novel mucosal drug delivery systems. J. Pharm. Biomed. Anal. 2018, 147, 350–366. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Santos, B.; Chorilli, M. An overview of polymeric dosage forms in buccal drug delivery: State of art, design of formulations and their in vivo performance evaluation. Mater. Sci. Eng. C 2017. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Santos, B.; Gremiao, M.P.; Chorilli, M. Nanotechnology-based drug delivery systems for the treatment of Alzheimer’s disease. Int. J. Nanomed. 2015, 10, 4981–5003. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A.; das Neves, J.; Sarmento, B. Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: A review. Prog. Polym. Sci. 2014, 39, 2030–2075. [Google Scholar] [CrossRef]
- Lee, G.H.; Lee, S.J.; Jeong, S.W.; Kim, H.C.; Park, G.Y.; Lee, S.G.; Choi, J.H. Antioxidative and antiinflammatory activities of quercetin-loaded silica nanoparticles. Colloids Surf. B Biointerfaces 2016, 143, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Desmet, E.; Van Gele, M.; Lambert, J. Topically applied lipid- and surfactant-based nanoparticles in the treatment of skin disorders. Expert Opin. Drug Deliv. 2016, 14, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Crucho, C.I.C.; Barros, M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Luo, Z.; Zhang, B.; Pang, Z. Biomimetic nanoparticles for inflammation targeting. Acta Pharm. Sin. B 2017, 8, 23–33. [Google Scholar] [CrossRef]
- El-Say, K.M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Mansuri, S.; Kesharwani, P.; Jain, K.; Tekade, R.K.; Jain, N.K. Mucoadhesion: A promising approach in drug delivery system. React. Funct. Polym. 2016, 100, 151–172. [Google Scholar] [CrossRef]
- Labarre, D. The Interactions between Blood and Polymeric Nanoparticles Depend on the Nature and Structure of the Hydrogel Covering the Surface. Polymers 2012, 4, 986–996. [Google Scholar] [CrossRef]
- Shen, Z.; Nieh, M.-P.; Li, Y. Decorating Nanoparticle Surface for Targeted Drug Delivery: Opportunities and Challenges. Polymers 2016, 8, 83. [Google Scholar] [CrossRef]
- Ozturk-Atar, K.; Eroglu, H.; Calis, S. Novel advances in targeted drug delivery. J. Drug Target. 2017, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lautenschlager, C.; Schmidt, C.; Fischer, D.; Stallmach, A. Drug delivery strategies in the therapy of inflammatory bowel disease. Adv. Drug Deliv. Rev. 2014, 71, 58–76. [Google Scholar] [CrossRef] [PubMed]
- Fessi, H.; Puisieux, F.; Devissaguet, J.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Clogston, J.; Patri, A. Zeta potential measurement. In Characterization of Nanoparticles Intended for Drug Delivery; McNeil, S.E., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 63–70. [Google Scholar]
- Kapoor, M.; Cloyd, J.C.; Siegel, R.A. A review of intranasal formulations for the treatment of seizure emergencies. J. Control. Release 2016, 237, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Christrup, L.; Lundorff, L.; Werner, M. Novel formulations and routes of administration for opioids in the treatment of breakthrough pain. Therapy 2009, 6, 695–706. [Google Scholar] [CrossRef]
- Wittayalertpanya, S.; Chompootaweep, S.; Thaworn, N. The Pharmacokinetics of Pioglitazone in Thai Healthy Subjects. J. Med. Assoc. Thail. 2006, 89, 2116–2122. [Google Scholar]
- Schramm, G. A Practical Approach to Rheology and Rheometry, 2nd ed.; Gebrueder HAAKE: Karlsruhe, Germany, 1994. [Google Scholar]
- Silva-Abreu, M.; Calpena, A.C.; Espina, M.; Silva, A.M.; Gimeno, A.; Egea, M.A.; Garcia, M.L. Optimization, Biopharmaceutical Profile and Therapeutic Efficacy of Pioglitazone-loaded PLGA-PEG Nanospheres as a Novel Strategy for Ocular Inflammatory Disorders. Pharm. Res. 2018, 35, 11. [Google Scholar] [CrossRef] [PubMed]
- Sozio, P.; Cerasa, L.S.; Marinelli, L.; Di Stefano, A. Transdermal donepezil on the treatment of Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2012, 8, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Ulep, M.G.; Saraon, S.K.; McLea, S. Alzheimer Disease. J. Nurse Pract. 2017. [Google Scholar] [CrossRef]
- Saraiva, C.; Praca, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Jalodia, K.; Kumar, P.; Gautam, H.K. Recent advances in nanoparticle-mediated drug delivery. J. Drug Deliv. Sci. Technol. 2017, 41, 260–268. [Google Scholar] [CrossRef]
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J. Control. Release 2017, 264, 306–332. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J. Drug delivery to the central nervous system by polymeric nanoparticles: What do we know? Adv. Drug Deliv. Rev. 2014, 71, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhao, D.; Li, D.; Wang, X.; Jin, Z.; Zhao, K. Polymer-Based Nanomaterials and Applications for Vaccines and Drugs. Polymers 2018, 10, 31. [Google Scholar] [CrossRef]
- Wen, M.M.; El-Salamouni, N.S.; El-Refaie, W.M.; Hazzah, H.A.; Ali, M.M.; Tosi, G.; Farid, R.M.; Blanco-Prieto, M.J.; Billa, N.; Hanafy, A.S. Nanotechnology-based drug delivery systems for Alzheimer’s disease management: Technical, industrial, and clinical challenges. J. Control. Release 2017, 245, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Watano, S. Direct Permeation of Nanoparticles across Cell Membrane: A Review. KONA Powder Part. J. 2018, 35, 49–65. [Google Scholar] [CrossRef]
- Leroueil, P.R.; Hong, S.; Mecke, A.; Baker, J.R., Jr.; Orr, B.G.; Banaszak Holl, M.M. Nanoparticle interaction with biological membranes: Does nanotechnology present a Janus face? Acc. Chem. Res. 2007, 40, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.R.; Chaurasia, S.; Khan, G.; Chaubey, P.; Kumar, N.; Mishra, B. Cromolyn sodium encapsulated PLGA nanoparticles: An attempt to improve intestinal permeation. Int. J. Biol. Macromol. 2016, 83, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Dunnhaupt, S.; Barthelmes, J.; Hombach, J.; Sakloetsakun, D.; Arkhipova, V.; Bernkop-Schnurch, A. Distribution of thiolated mucoadhesive nanoparticles on intestinal mucosa. Int. J. Pharm. 2011, 408, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Fortuna, A.; Alves, G.; Serralheiro, A.; Sousa, J.; Falcao, A. Intranasal delivery of systemic-acting drugs: Small-molecules and biomacromolecules. Eur. J. Pharm. Biopharm. 2014, 88, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Liu, M.; Khan, M.W.; Zhai, G. Progress in brain targeting drug delivery system by nasal route. J. Control. Release 2017, 268, 364–389. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Touitou, E.; Illum, L. Nasal drug delivery. Drug Deliv. Transl. Res. 2013, 3, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, C.V.; Belgamwar, V.S. Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood-brain barrier: An excellent platform for brain targeting. Expert Opin. Drug Deliv. 2013, 10, 957–972. [Google Scholar] [CrossRef] [PubMed]
- Mogoşanu, G.D.; Grumezescu, A.M.; Bejenaru, C.; Bejenaru, L.E. Polymeric protective agents for nanoparticles in drug delivery and targeting. Int. J. Pharm. 2016, 510, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Abdelhalim, M.A. The rheological properties of different GNPs. Lipids Health Dis. 2012, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Campos, F.; Clares Naveros, B.; Lopez Serrano, O.; Alonso Merino, C.; Calpena Campmany, A.C. Evaluation of novel nystatin nanoemulsion for skin candidosis infections. Mycoses 2013, 56, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Abrego, G.; Alvarado, H.L.; Egea, M.A.; Gonzalez-Mira, E.; Calpena, A.C.; Garcia, M.L. Design of nanosuspensions and freeze-dried PLGA nanoparticles as a novel approach for ophthalmic delivery of pranoprofen. J. Pharm. Sci. 2014, 103, 3153–3164. [Google Scholar] [CrossRef] [PubMed]
- Ramos Yacasi, G.R.; Garcia Lopez, M.L.; Espina Garcia, M.; Parra Coca, A.; Calpena Campmany, A.C. Influence of freeze-drying and γ-irradiation in preclinical studies of flurbiprofen polymeric nanoparticles for ocular delivery using d-(+)-trehalose and polyethylene glycol. Int. J. Nanomed. 2016, 11, 4093–4106. [Google Scholar] [CrossRef] [PubMed]

| Flow Curve—Models: | |
|---|---|
| Newton | |
| Bingham | |
| Ostwald-de-Waele | |
| Herschel-Bulkley | |
| Casson | |
| Cross | |
| Permeation and Prediction Parameters | Buccal a | Sublingual b | Nasal c |
|---|---|---|---|
| Jss (µg/(min/cm2)) × 102 | 4.28 | 5.19 | 5.19 |
| (2.83–5.72) | (4.91–5.50) | (4.91–5.50) | |
| Kp (cm/min) × 105 | 4.28 | 5.19 | 5.20 |
| (2.83–5.72) | (4.91–5.50) | (4.92–5.50) | |
| Tl (min) | 41.21 | 175.60 a | 3.00 a,b |
| (27.27–55.15) | (174.30–179.50) | (1.08–5.00) | |
| P1 (cm) × 104 | 93.74 | 547.37 a | 8.85 a,b |
| (93.73–93.74) | (514.35–592.73) | (3.37–16.51) | |
| P2 (min−1) | 0.004 | 0.0009 | 0.05 a,b |
| (0.003–0.006) | (0.0009–0.0009) | (0.03–0.15) | |
| Mean Transit Time, MTT (day) | 5.80 | 4.54 | 4.17 a |
| (3.84–7.77) | (4.31–4.77) | (3.95–4.41) | |
| Css (µg/mL) | 0.02 | 0.02 | 0.20 a,b |
| (0.01–0.03) | (0.02–0.02) | (0.19–0.21) |
| Rheologic Parameters | PGZ-NPs |
|---|---|
| Viscosity (mPa·s) at 50 s−1 and 25 °C | 1.110 ± 2.362 × 10−2 |
| Flow behavior (best fitting model) | Newtonian Newton * (r = 0.9993) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Abreu, M.; Espinoza, L.C.; Halbaut, L.; Espina, M.; García, M.L.; Calpena, A.C. Comparative Study of Ex Vivo Transmucosal Permeation of Pioglitazone Nanoparticles for the Treatment of Alzheimer’s Disease. Polymers 2018, 10, 316. https://doi.org/10.3390/polym10030316
Silva-Abreu M, Espinoza LC, Halbaut L, Espina M, García ML, Calpena AC. Comparative Study of Ex Vivo Transmucosal Permeation of Pioglitazone Nanoparticles for the Treatment of Alzheimer’s Disease. Polymers. 2018; 10(3):316. https://doi.org/10.3390/polym10030316
Chicago/Turabian StyleSilva-Abreu, Marcelle, Lupe Carolina Espinoza, Lyda Halbaut, Marta Espina, María Luisa García, and Ana Cristina Calpena. 2018. "Comparative Study of Ex Vivo Transmucosal Permeation of Pioglitazone Nanoparticles for the Treatment of Alzheimer’s Disease" Polymers 10, no. 3: 316. https://doi.org/10.3390/polym10030316
APA StyleSilva-Abreu, M., Espinoza, L. C., Halbaut, L., Espina, M., García, M. L., & Calpena, A. C. (2018). Comparative Study of Ex Vivo Transmucosal Permeation of Pioglitazone Nanoparticles for the Treatment of Alzheimer’s Disease. Polymers, 10(3), 316. https://doi.org/10.3390/polym10030316