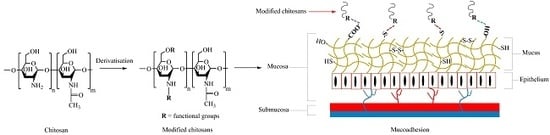
Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems
Abstract
:1. Introduction
2. Chitosan as a Mucoadhesive Material
3. Problems of Chitosan in Mucosal Drug Delivery
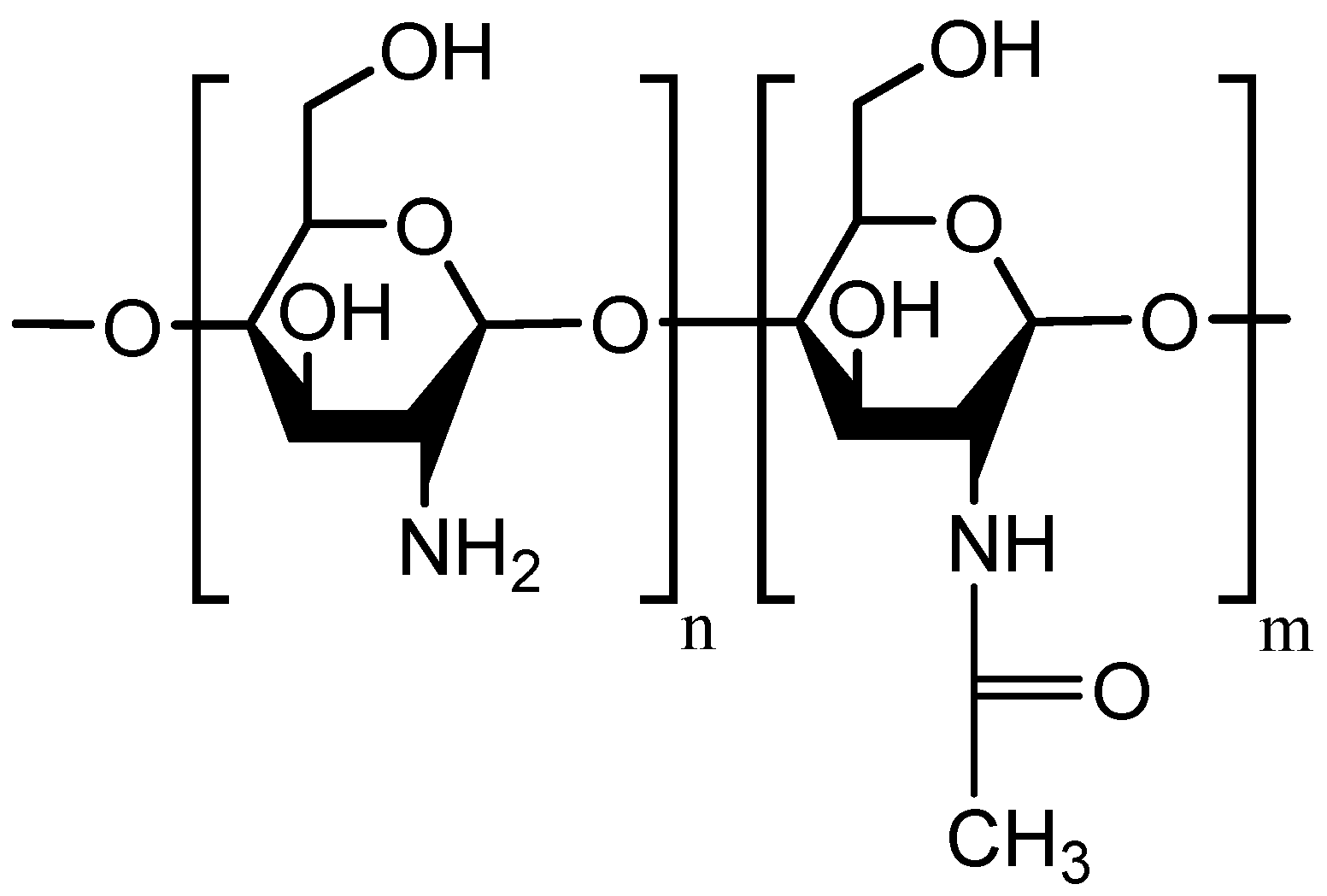
4. Mucoadhesive Chitosan Derivatives
4.1. Trimethyl Chitosan (TMC)
4.2. Carboxymethyl Chitosans
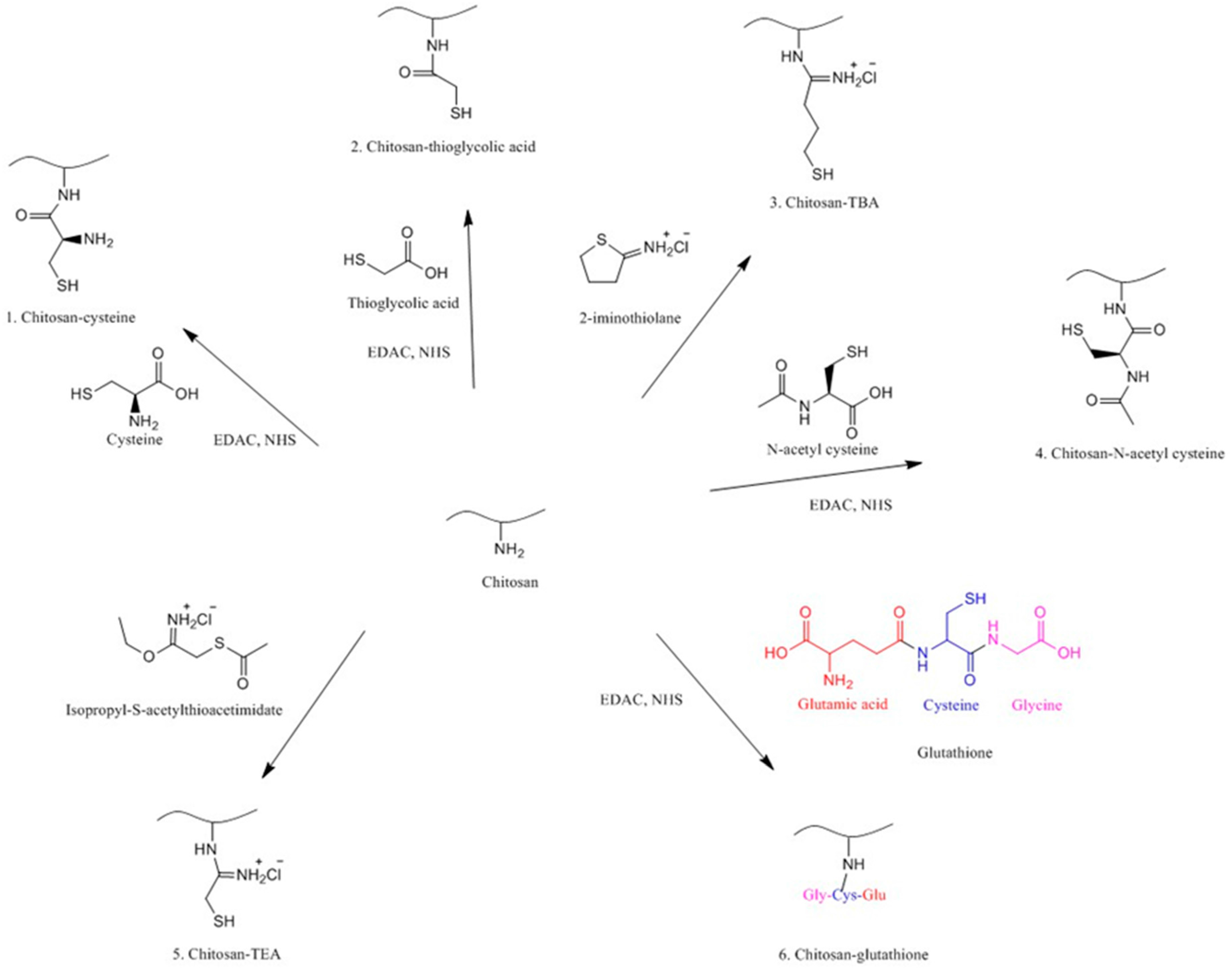
4.3. Thiolated Chitosans
4.3.1. Chitosan-Cysteine
4.3.2. Chitosan-N-Acetyl-Cysteine
4.3.3. Chitosan-Thioglycolic Acid (Chitosan-TGA)
4.3.4. Chitosan-4-Thiobutylamidine
4.3.5. Chitosan-Thioethylamidine
4.3.6. Chitosan-Glutathione
4.3.7. Comparison of Chitosan, Trimethyl Chitosan and Thiolated Chitosan
4.3.8. Pre-Activated (S-Protected) Thiolated Chitosans
4.3.9. Other Thiolated Chitosans
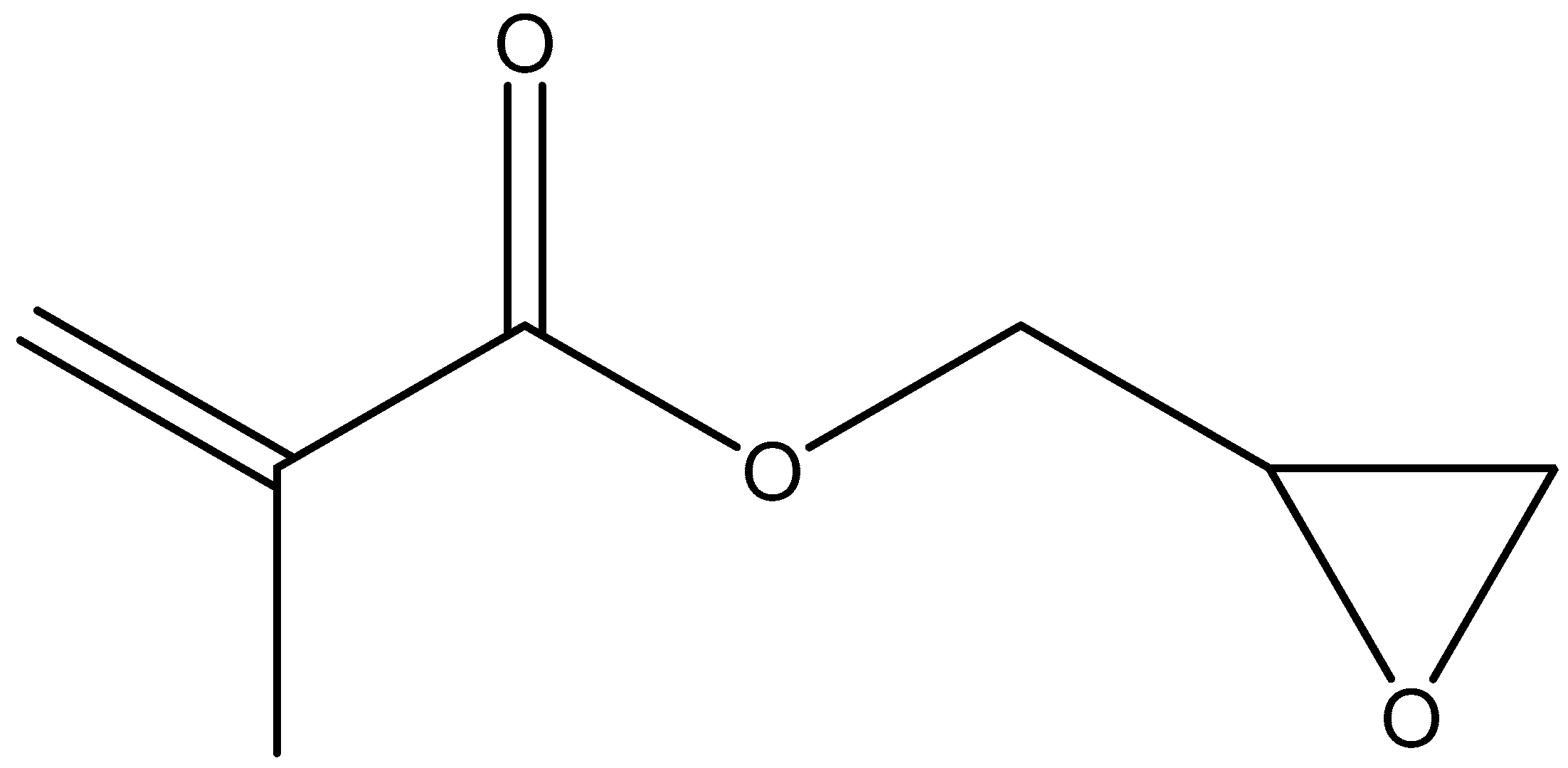
4.4. Acrylated Chitosan
4.5. Half-Acetylated Chitosan
4.6. Glycol Chitosan
4.6.1. Palmitoyl Glycol Chitosan
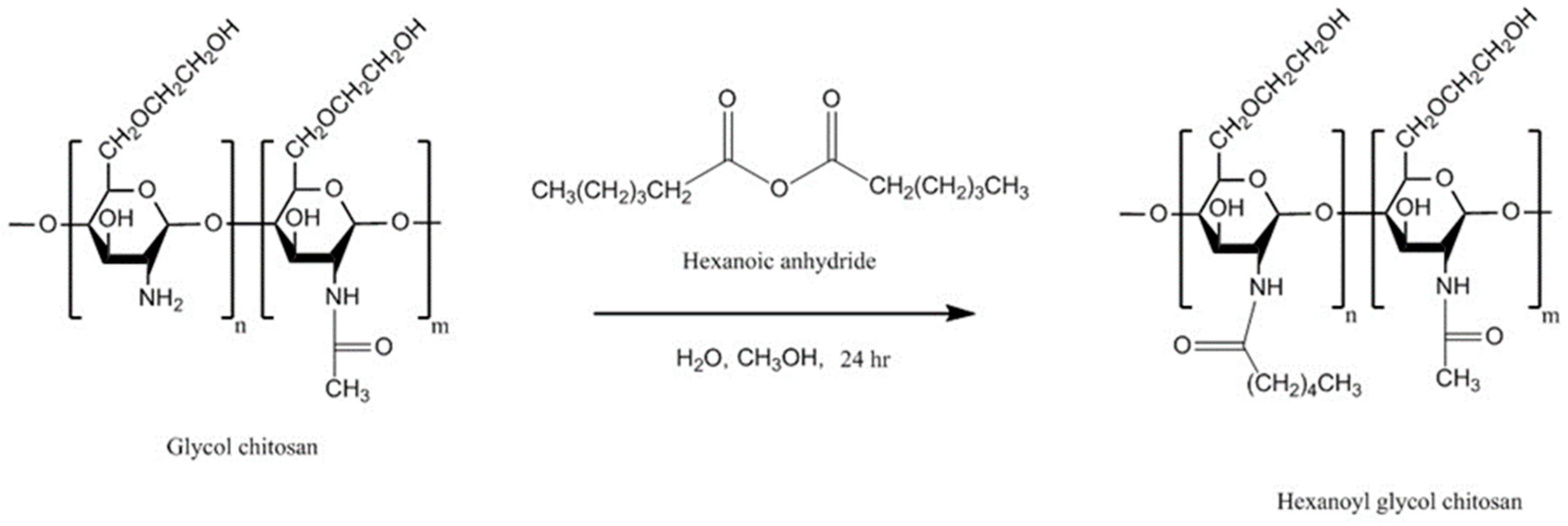
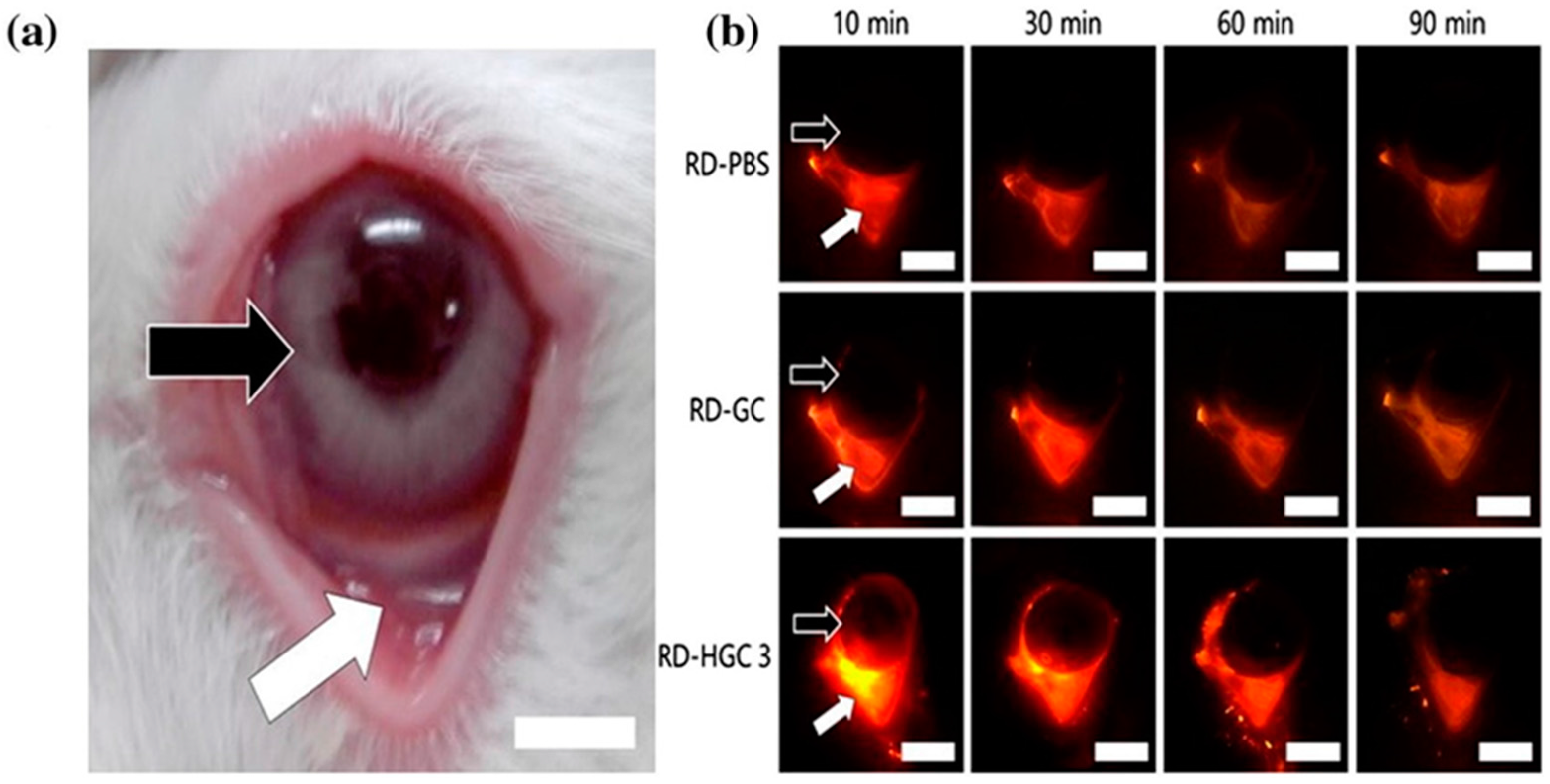
4.6.2. Hexanoyl Glycol Chitosan
4.7. Chitosan Conjugates
4.7.1. Chitosan-Enzyme Inhibitors
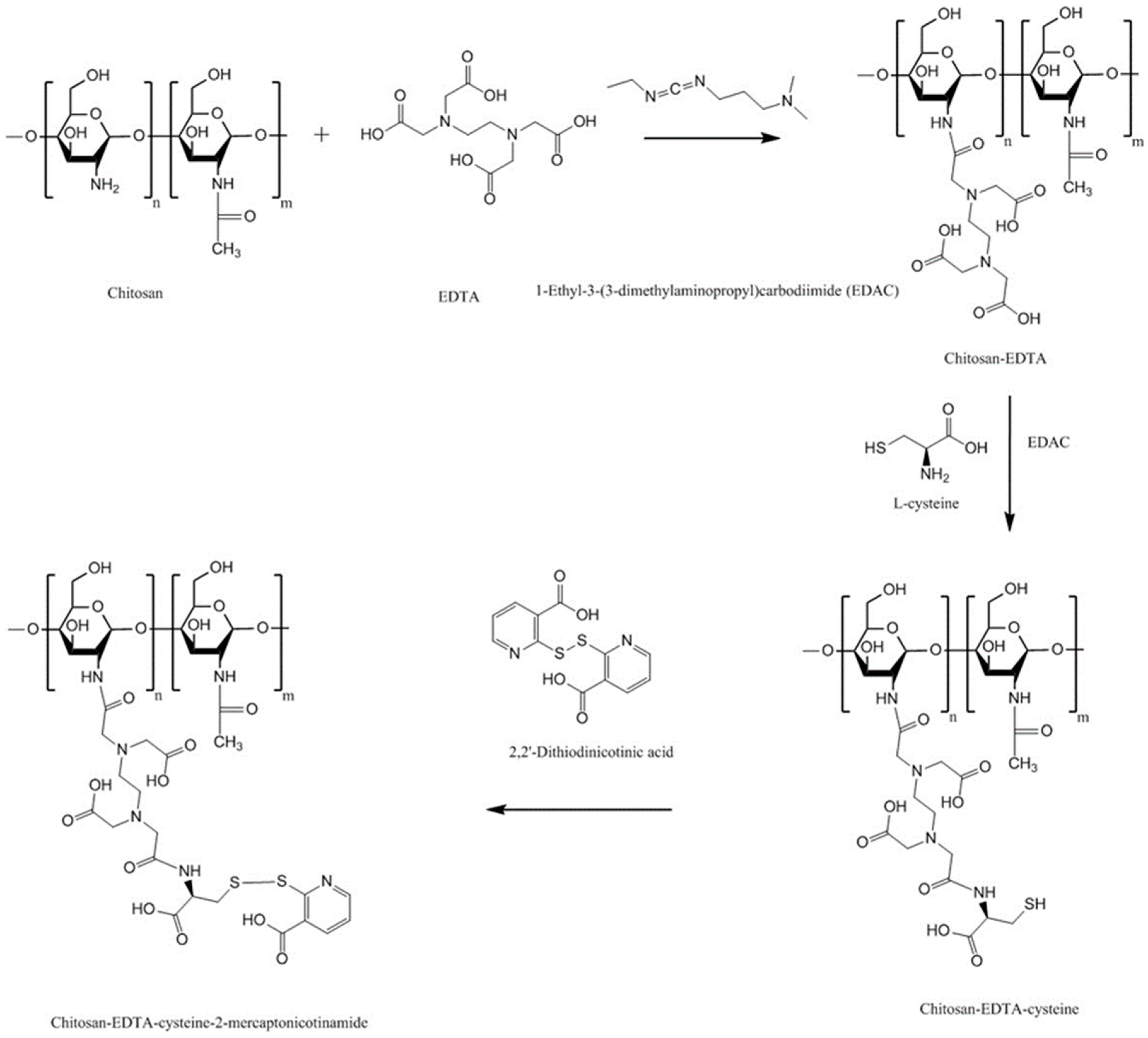
4.7.2. Chitosan-Complexing Agent
4.7.3. Chitosan-EDTA-Enzyme Inhibitors
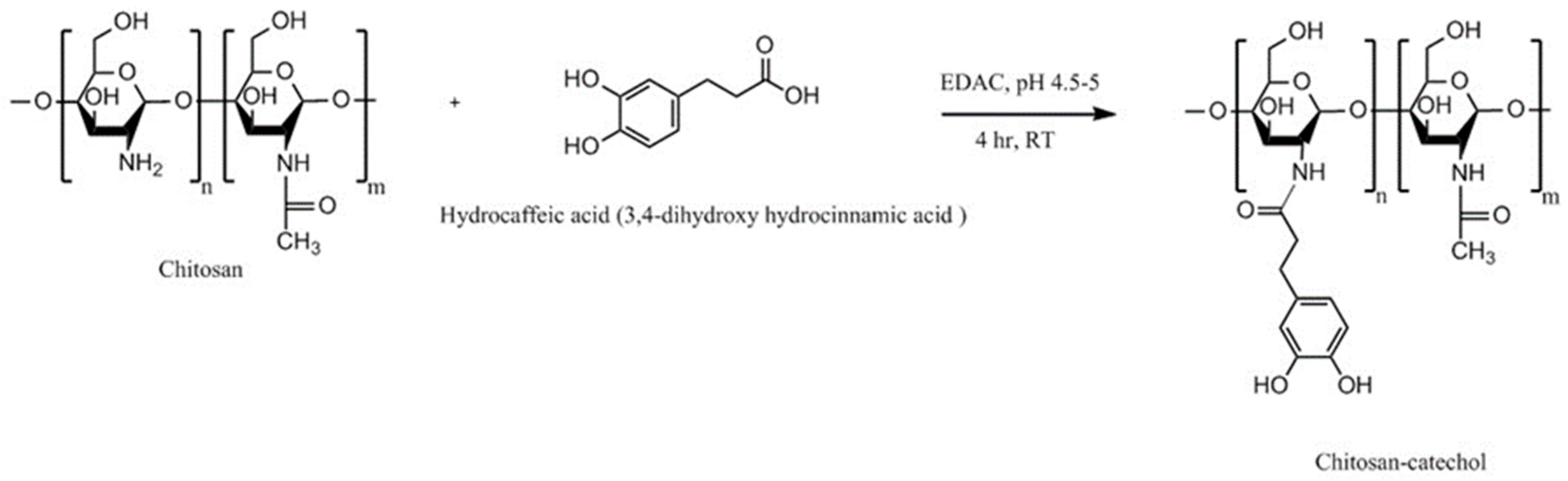
4.8. Chitosan-Catechol (Chi-C)
4.9. Methyl Pyrrolidinone Chitosan
4.10. Cyclodextrin-Chitosan
4.11. Oleoyl-Quaternised Chitosan
5. Comparison of Different Chitosan Derivatives
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Lai, S.K.; Wang, Y.Y.; Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009, 61, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Khutoryanskiy, V.V. Advances in mucoadhesion and mucoadhesive polymers. Macromol. Biosci. 2011, 11, 748–764. [Google Scholar] [CrossRef] [PubMed]
- Leal, J.; Smyth, H.D.C.; Ghosh, D. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int. J. Pharm. 2017, 532, 555–572. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Huang, Y. Nanoscale technology of mucoadhesive interactions. Adv. Drug Deliv. Rev. 2004, 56, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.K.; Wang, Y.Y.; Wirtz, D.; Hanes, J. Micro- and macrorheology of mucus. Adv. Drug Deliv. Rev. 2009, 61, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Boegh, M.; Nielsen, H.M. Mucus as a barrier to drug delivery—Understanding and mimicking the barrier properties. Basic Clin. Pharmacol. Toxicol. 2015, 116, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Bansil, R.; Turner, B.S. Mucin structure, aggregation, physiological functions and biomedical applications. Curr. Opin. Colloid Interface Sci. 2006, 11, 164–170. [Google Scholar] [CrossRef]
- Peppas, N.A.; Buri, P.A. Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues. J. Control. Release 1985, 2, 257–275. [Google Scholar] [CrossRef]
- Serra, L.; Domenech, J.; Peppas, N.A. Engineering design and molecular dynamics of mucoadhesive drug delivery systems as targeting agents. Eur. J. Pharm. Biopharm. 2009, 71, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Date, A.A.; Hanes, J.; Ensign, L.M. Nanoparticles for oral delivery: Design, evaluation and state-of-the-art. J. Control. Release 2016, 240, 504–526. [Google Scholar] [CrossRef] [PubMed]
- Gullberg, E.; Cao, S.; Berg, O.G.; Ilback, C.; Sandegren, L.; Hughes, D.; Andersson, D.I. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog. 2011, 7, e1002158. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Cho, I.H.; Jeong, B.C.; Lee, S.H. Strategies to minimize antibiotic resistance. Int. J. Environ. Res. Public Health 2013, 10, 4274–4305. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A.; Bratengeyer, I.; Valenta, C. Development and in vitro evaluation of a drug delivery system protecting from trypsinic degradation. Int. J. Pharm. 1997, 157, 17–25. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Hornof, M.; Guggi, D. Thiolated chitosans. Eur. J. Pharm. Biopharm. 2004, 57, 9–17. [Google Scholar] [CrossRef]
- Sogias, I.A.; Williams, A.C.; Khutoryanskiy, V.V. Why is chitosan mucoadhesive? Biomacromolecules 2008, 9, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.D.; Patel, H.M.; Surana, S.J.; Vanjari, Y.H.; Belgamwar, V.S.; Pardeshi, C.V. N,N,N-Trimethyl chitosan: An advanced polymer with myriad of opportunities in nanomedicine. Carbohydr. Polym. 2017, 157, 875–902. [Google Scholar] [CrossRef] [PubMed]
- Sogias, I.A.; Khutoryanskiy, V.V.; Williams, A.C. Exploring the factors affecting the solubility of chitosan in water. Macromol. Chem. Phys. 2010, 211, 426–433. [Google Scholar] [CrossRef]
- Hejazi, R.; Amiji, M. Chitosan-based gastrointestinal delivery systems. J. Control. Release 2003, 89, 151–165. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Robinson, J.R.; Longer, M.A.; Veillard, M. Bioadhesive polymers for controlled drug delivery. Ann. N. Y. Acad. Sci. 1987, 507, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Smart, J.D.; Kellaway, I.W.; Worthington, H.E.C. An in vitro investigation of mucosa-adhesive materials for use in controlled drug delivery. J. Pharm. Pharmacol. 1984, 36, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.R.; Mlynek, G.M. Bioadhesive and phase-change polymers for ocular drug delivery. Adv. Drug Deliv. Rev. 1995, 16, 45–50. [Google Scholar] [CrossRef]
- Casettari, L.; Vllasaliu, D.; Castagnino, E.; Stolnik, S.; Howdle, S.; Illum, L. PEGylated chitosan derivatives: Synthesis, characterisations and pharmaceutical applications. Prog. Polym. Sci. 2012, 37, 659–685. [Google Scholar] [CrossRef]
- Watts, P.; Smith, A.; Hinchcliffe, M. ChiSys® as a chitosan-based delivery platform for nasal vaccination. In Mucosal Delivery of Biopharmaceuticals: Biology, Challenges and Strategies; das Neves, J., Sarmento, B., Eds.; Springer: Boston, MA, USA, 2014; pp. 499–516. ISBN 978-1-4614-9524-6. [Google Scholar]
- Bonengel, S.; Bernkop-Schnürch, A. Thiomers-from bench to market. J. Control. Release 2014, 195, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Bonferoni, M.C.; Sandri, G.; Rossi, S.; Ferrari, F.; Caramella, C. Chitosan and its salts for mucosal and transmucosal delivery. Expert Opin. Drug Deliv. 2009, 6, 923–939. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Dash, A.K. A novel in situ gel for sustained drug delivery and targeting. Int. J. Pharm. 2004, 276, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.-T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Illum, L.; Farraj, N.F.; Davis, S.S. Chitosan as a novel nasal delivery system for peptide drugs. Pharm. Res. 1994, 11, 1186–1189. [Google Scholar] [CrossRef] [PubMed]
- Illum, L.; Jabbal-Gill, I.; Hinchcliffe, M.; Fisher, A.N.; Davis, S.S. Chitosan as a novel nasal delivery system for vaccines. Adv. Drug Deliv. Rev. 2001, 51, 81–96. [Google Scholar] [CrossRef]
- Issa, M.M.; Köping-Höggård, M.; Artursson, P. Chitosan and the mucosal delivery of biotechnology drugs. Drug Discov. Today Technol. 2005, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A.; Scholler, S.; Biebel, R.G. Development of controlled drug release systems based on thiolated polymers. J. Control. Release 2000, 66, 39–48. [Google Scholar] [CrossRef]
- Prego, C.; Fabre, M.; Torres, D.; Alonso, M.J. Efficacy and mechanism of action of chitosan nanocapsules for oral peptide delivery. Pharm. Res. 2006, 23, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Sogias, I.A.; Williams, A.C.; Khutoryanskiy, V.V. Chitosan-based mucoadhesive tablets for oral delivery of ibuprofen. Int. J. Pharm. 2012, 436, 602–610. [Google Scholar] [CrossRef] [PubMed]
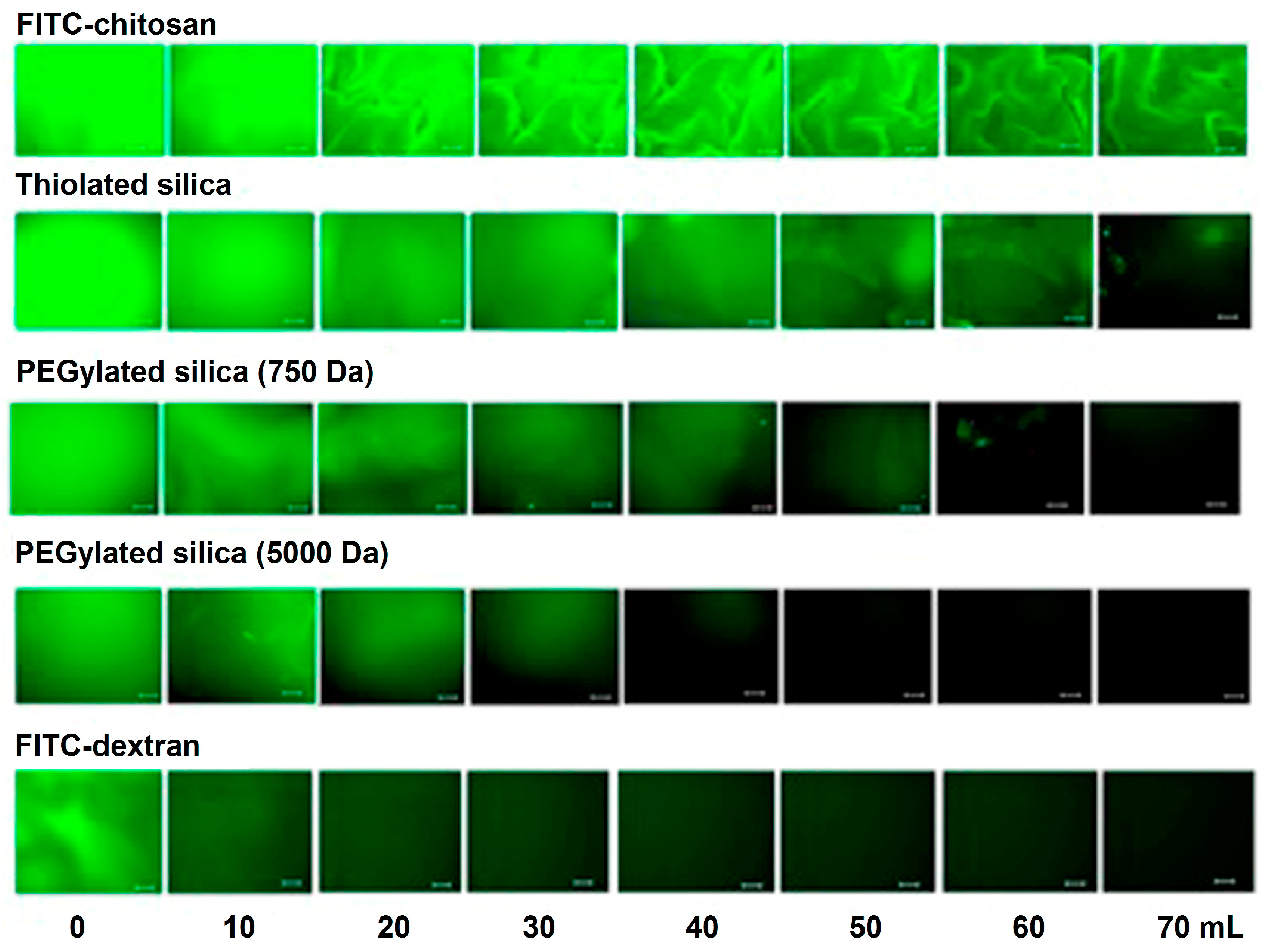
- Irmukhametova, G.S.; Mun, G.A.; Khutoryanskiy, V.V. Thiolated mucoadhesive and PEGylated nonmucoadhesive organosilica nanoparticles from 3-mercaptopropyltrimethoxysilane. Langmuir 2011, 27, 9551–9556. [Google Scholar] [CrossRef] [PubMed]
- Mun, E.A.; Williams, A.C.; Khutoryanskiy, V.V. Adhesion of thiolated silica nanoparticles to urinary bladder mucosa: Effects of PEGylation, thiol content and particle size. Int. J. Pharm. 2016, 512, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Tonglairoum, P.; Brannigan, R.P.; Opanasopit, P.; Khutoryanskiy, V.V. Maleimide-bearing nanogels as novel mucoadhesive materials for drug delivery. J. Mater. Chem. B 2016, 4, 6581–6587. [Google Scholar] [CrossRef]
- Kaldybekov, D.B.; Tonglairoum, P.; Opanasopit, P.; Khutoryanskiy, V.V. Mucoadhesive maleimide-functionalised liposomes for drug delivery to urinary bladder. Eur. J. Pharm. Sci. 2018, 111, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Behrens, I.; Pena, A.I.; Alonso, M.J.; Kissel, T. Comparative uptake studies of bioadhesive and non-bioadhesive nanoparticles in human intestinal cell lines and rats: The effect of mucus on particle adsorption and transport. Pharm. Res. 2002, 19, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-S.; Kim, C.-S.; Lee, K.-M. The intracellular uptake ability of chitosan-coated Poly (d,l-lactide-co-glycolide) nanoparticles. Arch. Pharm. Res. 2008, 31, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Thongborisute, J.; Takeuchi, H.; Yamamoto, H.; Kawashima, Y. Visualization of the penetrative and mucoadhesive properties of chitosan and chitosan-coated liposomes through the rat intestine. J. Liposome Res. 2006, 16, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Atuma, C.; Strugala, V.; Allen, A.; Holm, L. The adherent gastrointestinal mucus gel layer: Thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G922–G929. [Google Scholar] [CrossRef] [PubMed]
- Varum, F.J.O.; Veiga, F.; Sousa, J.S.; Basit, A.W. An investigation into the role of mucus thickness on mucoadhesion in the gastrointestinal tract of pig. Eur. J. Pharm. Sci. 2010, 40, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Varum, F.J.O.; Veiga, F.; Sousa, J.S.; Basit, A.W. Mucus thickness in the gastrointestinal tract of laboratory animals. J. Pharm. Pharmacol. 2012, 64, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Deacona, M.P.; Davis, S.S.; White, R.J.; Nordman, H.; Carlstedt, I.; Errington, N.; Rowe, A.J.; Harding, S.E. Are chitosan–mucin interactions specific to different regions of the stomach? Velocity ultracentrifugation offers a clue. Carbohydr. Polym. 1999, 38, 235–238. [Google Scholar] [CrossRef]
- Caramella, C.; Ferrari, F.; Bonferoni, M.C.; Rossi, S.; Sandri, G. Chitosan and its derivatives as drug penetration enhancers. J. Drug Deliv. Sci. Technol. 2010, 20, 5–13. [Google Scholar] [CrossRef]
- Jeong, Y.-I.; Kim, D.-G.; Jang, M.-K.; Nah, J.-W. Preparation and spectroscopic characterization of methoxy poly(ethylene glycol)-grafted water-soluble chitosan. Carbohydr. Res. 2008, 343, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A. Chitosan and its derivatives: Potential excipients for peroral peptide delivery systems. Int. J. Pharm. 2000, 194, 1–13. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Tanfani, F. The N-permethylation of chitosan and the preparation of N-trimethyl chitosan iodide. Carbohydr. Polym. 1985, 5, 297–307. [Google Scholar] [CrossRef]
- Verheul, R.J.; Amidi, M.; van der Wal, S.; van Riet, E.; Jiskoot, W.; Hennink, W.E. Synthesis, characterization and in vitro biological properties of O-methyl free N,N,N-trimethylated chitosan. Biomaterials 2008, 29, 3642–3649. [Google Scholar] [CrossRef] [PubMed]
- Sieval, A.B.; Thanou, M.; Kotzé, A.F.; Verhoef, J.C.; Brussee, J.; Junginger, H.E. Preparation and NMR characterization of highly substituted N-trimethyl chitosan chloride. Carbohydr. Polym. 1998, 36, 157–165. [Google Scholar] [CrossRef]
- De Britto, D.; Assis, O.B.G. A novel method for obtaining a quaternary salt of chitosan. Carbohydr. Polym. 2007, 69, 305–310. [Google Scholar] [CrossRef]
- Benediktsdóttir, B.E.; Gaware, V.S.; Rúnarsson, Ö.V.; Jónsdóttir, S.; Jensen, K.J.; Másson, M. Synthesis of N,N,N-trimethyl chitosan homopolymer and highly substituted N-alkyl-N,N-dimethyl chitosan derivatives with the aid of di-tert-butyldimethylsilyl chitosan. Carbohydr. Polym. 2011, 86, 1451–1460. [Google Scholar] [CrossRef]
- Wu, M.; Long, Z.; Xiao, H.; Dong, C. Recent research progress on preparation and application of N,N,N-trimethyl chitosan. Carbohydr. Res. 2016, 434, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Dung, P.L.; Milas, M.; Rinaudo, M.; Desbrières, J. Water soluble derivatives obtained by controlled chemical modifications of chitosan. Carbohydr. Polym. 1994, 24, 209–214. [Google Scholar] [CrossRef]
- Jintapattanakit, A.; Junyaprasert, V.B.; Kissel, T. The role of mucoadhesion of trimethyl chitosan and PEGylated trimethyl chitosan nanocomplexes in insulin uptake. J. Pharm. Sci. 2009, 98, 4818–4830. [Google Scholar] [CrossRef] [PubMed]
- Casettari, L.; Vllasaliu, D.; Mantovani, G.; Howdle, S.M.; Stolnik, S.; Illum, L. Effect of PEGylation on the toxicity and permeability enhancement of chitosan. Biomacromolecules 2010, 11, 2854–2865. [Google Scholar] [CrossRef] [PubMed]
- Hauptstein, S.; Boengel, S.; Griessinger, J.; Bernkop-Schnürch, A. Synthesis and characterization of pH tolerant and mucoadhesive (thiol–polyethylene glycol) chitosan graft polymer for drug delivery. J. Pharm. Sci. 2014, 103, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Sayin, B.; Somavarapu, S.; Li, X.W.; Sesardic, D.; Şenel, S.; Alpar, O.H. TMC-MCC (N-trimethyl chitosan-mono-N-carboxymethyl chitosan) nanocomplexes for mucosal delivery of vaccines. Eur. J. Pharm. Sci. 2009, 38, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Sajomsang, W.; Ruktanonchai, U.R.; Gonil, P.; Nuchuchua, O. Mucoadhesive property and biocompatibility of methylated N-aryl chitosan derivatives. Carbohydr. Polym. 2009, 78, 945–952. [Google Scholar] [CrossRef]
- Keely, S.; Rullay, A.; Wilson, C.; Carmichael, A.; Carrington, S.; Corfield, A.; Haddleton, D.M.; Brayden, D.J. In vitro and ex vivo intestinal tissue models to measure mucoadhesion of poly(methacrylate) and N-trimethylated chitosan polymers. Pharm. Res. 2005, 22, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.N.E.; Pearson, J.P.; Huton, D.A.; Allen, A.; Detmar, P.W. Interaction of polyacrylates with porcine pepsin and the gastric mucus barrier: A mechanism for mucosal protection. Clin. Sci. 1994, 87, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Jonker, C.; Hamman, J.H.; Kotzé, A.F. Intestinal paracellular permeation enhancement with quaternised chitosan: In situ and in vitro evaluation. Int. J. Pharm. 2002, 238, 205–213. [Google Scholar] [CrossRef]
- Smith, J.; Wood, E.; Dornish, M. Effect of chitosan on epithelial cell tight junctions. Pharm. Res. 2004, 21, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Hamman, J.H.; Schultz, C.M.; Kotzé, A.F. N-trimethyl chitosan chloride: Optimum degree of quaternization for drug absorption enhancement across epithelial cells. Drug Dev. Ind. Pharm. 2003, 29, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, J.; Zhu, X.; Shan, W.; Li, L.; Zhong, J.; Zhang, Z.; Huang, Y. Efficient mucus permeation and tight junction opening by dissociable “mucus-inert” agent coated trimethyl chitosan nanoparticles for oral insulin delivery. J. Control. Release 2016, 222, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Nazar, H.; Fatouros, D.G.; van der Merwe, S.M.; Bouropoulos, N.; Avgouropoulos, G.; Tsibouklis, J.; Roldo, M. Thermosensitive hydrogels for nasal drug delivery: The formulation and characterization of systems based on N-trimethyl chitosan chloride. Eur. J. Pharm. Biopharm. 2011, 77, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Van der Merwe, S.M.; Verhoef, J.C.; Verheijden, J.H.M.; Kotzé, A.F.; Junginger, H.E. Trimethylated chitosan as polymeric absorption enhancer for improved peroral delivery of peptide drugs. Eur. J. Pharm. Biopharm. 2004, 58, 225–235. [Google Scholar] [CrossRef] [PubMed]
- DeSesso, J.M.; Jacobson, C.F. Anatomical and physiological parameters affecting gastrointestinal absorption in humans and rats. Food Chem. Toxicol. 2001, 39, 209–228. [Google Scholar] [CrossRef]
- Kotzé, A.F.; de Leeuw, B.J.; Lueßen, H.L.; de Boer, A.G.; Verhoef, J.C.; Junginger, H.E. Chitosans for enhanced delivery of therapeutic peptides across intestinal epithelia: In vitro evaluation in Caco-2 cell monolayers. Int. J. Pharm. 1997, 159, 243–253. [Google Scholar] [CrossRef]
- Deli, M.A. Potential use of tight junction modulators to reversibly open membranous barriers and improve drug delivery. Biochim. Biophys. Acta Biomembr. 2009, 1788, 892–910. [Google Scholar] [CrossRef] [PubMed]
- Thanou, M.; Nihot, M.T.; Jansen, M.; Verhoef, J.C.; Junginger, H.E. Mono-N-carboxymethyl chitosan (MCC), a polyampholytic chitosan derivative, enhances the intestinal absorption of low molecular weight heparin across intestinal epithelia in vitro and in vivo. J. Pharm. Sci. 2001, 90, 38–46. [Google Scholar] [CrossRef]
- Upadhyaya, L.; Singh, J.; Agarwal, V.; Tewari, R.P. The implications of recent advances in carboxymethyl chitosan based targeted drug delivery and tissue engineering applications. J. Control. Release 2014, 186, 54–87. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M.; Nair, S.V.; Tokura, S.; Tamura, H.; Selvamurugan, N. Novel carboxymethyl derivatives of chitin and chitosan materials and their biomedical applications. Prog. Mater. Sci. 2010, 55, 675–709. [Google Scholar] [CrossRef]
- Chen, X.-G.; Park, H.-J. Chemical characteristics of O-carboxymethyl chitosans related to the preparation conditions. Carbohydr. Polym. 2003, 53, 355–359. [Google Scholar] [CrossRef]
- Vikhoreva, G.A.; Gal’braikh, L.S. Rheological properties of solutions of chitosan and carboxymethylchitin. Fibre Chem. 1997, 29, 287–291. [Google Scholar] [CrossRef]
- An, N.T.; Dung, P.L.; Thien, D.T.; Dong, N.T.; Nhi, T.T.Y. An improved method for synthesizing N,N′-dicarboxymethylchitosan. Carbohydr. Polym. 2008, 73, 261–264. [Google Scholar] [CrossRef]
- An, N.T.; Thien, D.T.; Dong, N.T.; Dung, P.L. Water-soluble N-carboxymethylchitosan derivatives: Preparation, characteristics and its application. Carbohydr. Polym. 2009, 75, 489–497. [Google Scholar] [CrossRef]
- Upadhyaya, L.; Singh, J.; Agarwal, V.; Tewari, R.P. Biomedical applications of carboxymethyl chitosans. Carbohydr. Polym. 2013, 91, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.-C.; Luo, D.-K. Preparation of carboxymethyl chitosan in aqueous solution under microwave irradiation. Carbohydr. Res. 2005, 340, 1351–1356. [Google Scholar] [CrossRef] [PubMed]
- Di Colo, G.; Zambito, Y.; Burgalassi, S.; Nardini, I.; Saettone, M.F. Effect of chitosan and N-carboxymethylchitosan on intraocular penetration of topically applied ofloxacin. Int. J. Pharm. 2004, 273, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, M.; Gong, S. Novel thiolated carboxymethyl chitosan-g-β-cyclodextrin as mucoadhesive hydrophobic drug delivery carriers. Carbohydr. Polym. 2008, 73, 117–125. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Brandt, U.M.; Clausen, A.E. Synthesis and in vitro evaluation of chitosan-cysteine conjugates. Sci. Pharm. 1999, 67, 196–208. [Google Scholar]
- Kast, C.E.; Frick, W.; Losert, U.; Bernkop-Schnürch, A. Chitosan-thioglycolic acid conjugate: A new scaffold material for tissue engineering? Int. J. Pharm. 2003, 256, 183–189. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Hornof, M.; Zoidl, T. Thiolated polymers—Thiomers: Synthesis and in vitro evaluation of chitosan–2-iminothiolane conjugates. Int. J. Pharm. 2003, 260, 229–237. [Google Scholar] [CrossRef]
- Schmitz, T.; Grabovac, V.; Palmberger, T.F.; Hoffer, M.H.; Bernkop-Schnürch, A. Synthesis and characterization of a chitosan-N-acetyl cysteine conjugate. Int. J. Pharm. 2008, 347, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Kafedjiiski, K.; Krauland, A.H.; Hoffer, M.H.; Bernkop-Schnürch, A. Synthesis and in vitro evaluation of a novel thiolated chitosan. Biomaterials 2005, 26, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Kafedjiiski, K.; Föger, F.; Werle, M.; Bernkop-Schnürch, A. Synthesis and in vitro evaluation of a novel chitosan-glutathione conjugate. Pharm. Res. 2005, 22, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A.; Schwarz, V.; Steininger, S. Polymers with thiol groups: A new generation of mucoadhesive polymers. Pharm. Res. 1999, 16, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shu, Y.; Hao, T.; Wang, Y.; Qian, Y.; Duan, C.; Sun, H.; Lin, Q.; Wang, C. A chitosan-glutathione based injectable hydrogel for suppression of oxidative stress damage in cardiomyocytes. Biomaterials 2013, 34, 9071–9081. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Ding, J.; He, C.; Cui, L.; Tang, C.; Yin, C. Drug permeability and mucoadhesion properties of thiolated trimethyl chitosan nanoparticles in oral insulin delivery. Biomaterials 2009, 30, 5691–5700. [Google Scholar] [CrossRef] [PubMed]
- Sakloetsakun, D.; Hombach, J.M.R.; Bernkop-Schnürch, A. In situ gelling properties of chitosan-thioglycolic acid conjugate in the presence of oxidizing agents. Biomaterials 2009, 30, 6151–6157. [Google Scholar] [CrossRef] [PubMed]
- Martien, R.; Loretz, B.; Thaler, M.; Majzoob, S.; Bernkop-Schnürch, A. Chitosan-thioglycolic acid conjugate: An alternative carrier for oral nonviral gene delivery? J. Biomed. Mater. Res. Part A 2007, 82A, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Barthelmes, J.; Perera, G.; Hombach, J.; Dünnhaupt, S.; Bernkop-Schnürch, A. Development of a mucoadhesive nanoparticulate drug delivery system for a targeted drug release in the bladder. Int. J. Pharm. 2011, 416, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Soler, R.; Bruschini, H.; Martins, J.R.; Dreyfuss, J.L.; Camara, N.O.; Alves, M.T.; Leite, K.R.; Truzzi, J.C.; Nader, H.B.; Srougi, M.; et al. Urinary glycosaminoglycans as biomarker for urothelial injury: Is it possible to discriminate damage from recovery? Urology 2008, 72, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Grabovac, V.; Guggi, D.; Bernkop-Schnürch, A. Comparison of the mucoadhesive properties of various polymers. Adv. Drug Deliv. Rev. 2005, 57, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Langoth, N.; Kahlbacher, H.; Schöffmann, G.; Schmerold, I.; Schuh, M.; Franz, S.; Kurka, P.; Bernkop-Schnürch, A. Thiolated chitosans: Design and in vivo evaluation of a mucoadhesive buccal peptide drug delivery system. Pharm. Res. 2006, 23, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Dünnhaupt, S.; Barthelmes, J.; Hombach, J.; Sakloetsakun, D.; Arkhipova, V.; Bernkop-Schnürch, A. Distribution of thiolated mucoadhesive nanoparticles on intestinal mucosa. Int. J. Pharm. 2011, 408, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Guggi, D.; Kast, C.E.; Bernkop-Schnürch, A. In vivo evaluation of an oral salmon calcitonin-delivery system based on a thiolated chitosan carrier matrix. Pharm. Res. 2003, 20, 1989–1994. [Google Scholar] [CrossRef] [PubMed]
- Krauland, A.H.; Leitner, V.M.; Grabovac, V.; Bernkop-Schnürch, A. In vivo evaluation of a nasal insulin delivery system based on thiolated chitosan. J. Pharm. Sci. 2006, 95, 2463–2472. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.H.; Lee, J.-S.; Kim, G.-H.; Lee, H.G. Preparation, characteristics, and stability of glutathione-loaded nanoparticles. J. Agric. Food Chem. 2011, 59, 11264–11269. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, F.A.; Atyabi, F.; Dinarvand, R. Preparation and in vitro evaluation of mucoadhesion and permeation enhancement of thiolated chitosan-pHEMA core-shell nanoparticles. Nanomedicine 2009, 5, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Xu, Y.; Shen, J.; Ping, Q.; Su, Z.; You, W. Chitosan-glutathione conjugate-coated poly(butyl cyanoacrylate) nanoparticles: Promising carriers for oral thymopentin delivery. Carbohydr. Polym. 2011, 86, 51–57. [Google Scholar] [CrossRef]
- Mei, D.; Mao, S.; Sun, W.; Wang, Y.; Kissel, T. Effect of chitosan structure properties and molecular weight on the intranasal absorption of tetramethylpyrazine phosphate in rats. Eur. J. Pharm. Biopharm. 2008, 70, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Shuai, X.; Unger, F.; Wittmar, M.; Xie, X.; Kissel, T. Synthesis, characterization and cytotoxicity of poly(ethylene glycol)-graft-trimethyl chitosan block copolymers. Biomaterials 2005, 26, 6343–6356. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A.; Steininger, S. Synthesis and characterisation of mucoadhesive thiolated polymers. Int. J. Pharm. 2000, 194, 239–247. [Google Scholar] [CrossRef]
- Krauland, A.H.; Guggi, D.; Bernkop-Schnürch, A. Oral insulin delivery: The potential of thiolated chitosan-insulin tablets on non-diabetic rats. J. Control. Release 2004, 95, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Föger, F.; Schmitz, T.; Bernkop-Schnürch, A. In vivo evaluation of an oral delivery system for P-gp substrates based on thiolated chitosan. Biomaterials 2006, 27, 4250–4255. [Google Scholar] [CrossRef] [PubMed]
- Dünnhaupt, S.; Barthelmes, J.; Rahmat, D.; Leithner, K.; Thurner, C.C.; Friedl, H.; Bernkop-Schnürch, A. S-protected thiolated chitosan for oral delivery of hydrophilic macromolecules: Evaluation of permeation enhancing and efflux pump inhibitory properties. Mol. Pharm. 2012, 9, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Dünnhaupt, S.; Barthelmes, J.; Thurner, C.C.; Waldner, C.; Sakloetsakun, D.; Bernkop-Schnürch, A. S-protected thiolated chitosan: Synthesis and in vitro characterization. Carbohydr. Polym. 2012, 90, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Dünnhaupt, S.; Barthelmes, J.; Iqbal, J.; Perera, G.; Thurner, C.C.; Friedl, H.; Bernkop-Schnürch, A. In vivo evaluation of an oral drug delivery system for peptides based on S-protected thiolated chitosan. J. Control. Release 2012, 160, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, S.; Mortazavian, E.; Mohammadi, Z.; Samadi, F.Y.; Samadikhah, H.; Taheritarigh, S.; Tehrani, N.R.; Rafiee-Tehrani, M. Thiolated methylated dimethylaminobenzyl chitosan: A novel chitosan derivative as a potential delivery vehicle. Int. J. Biol. Macromol. 2017, 95, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Davidovich-Pinhas, M.; Bianco-Peled, H. Novel mucoadhesive system based on sulfhydryl-acrylate interactions. J. Mater. Sci. Mater. Med. 2010, 21, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Davidovich-Pinhas, M.; Bianco-Peled, H. Alginate-PEGAc: A new mucoadhesive polymer. Acta Biomater. 2011, 7, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Eshel-Green, T.; Bianco-Peled, H. Mucoadhesive acrylated block copolymers micelles for the delivery of hydrophobic drugs. Colloids Surf. B 2016, 139, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Štorha, A.; Mun, E.A.; Khutoryanskiy, V.V. Synthesis of thiolated and acrylated nanoparticles using thiol-ene click chemistry: Towards novel mucoadhesive materials for drug delivery. RSC Adv. 2013, 3, 12275–12279. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, X.; Han, J.; Song, G.; Nie, J. Photo-polymeriable chitosan derivative prepared by Michael reaction of chitosan and polyethylene glycol diacrylate (PEGDA). Int. J. Biol. Macromol. 2009, 45, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Shitrit, Y.; Bianco-Peled, H. Acrylated chitosan for mucoadhesive drug delivery systems. Int. J. Pharm. 2017, 517, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Albarkah, Y.A.; Green, R.J.; Khutoryanskiy, V.V. Probing the mucoadhesive interactions between porcine gastric mucin and some water-soluble polymers. Macromol. Biosci. 2015, 15, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Smart, J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Li, H.; Xiao, Q.; Liu, Y.; Zhu, J.; Du, Y. Water-solubility of chitosan and its antimicrobial activity. Carbohydr. Polym. 2006, 63, 367–374. [Google Scholar] [CrossRef]
- Palazzo, C.; Trapani, G.; Ponchel, G.; Trapani, A.; Vauthier, C. Mucoadhesive properties of low molecular weight chitosan- or glycol chitosan- and corresponding thiomer-coated poly(isobutylcyanoacrylate) core-shell nanoparticles. Eur. J. Pharm. Biopharm. 2017, 117, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Trapani, A.; Sitterberg, J.; Bakowsky, U.; Kissel, T. The potential of glycol chitosan nanoparticles as carrier for low water soluble drugs. Int. J. Pharm. 2009, 375, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Uchegbu, I.F.; Carlos, M.; McKay, C.; Hou, X.; Schätzlein, A.G. Chitosan amphiphiles provide new drug delivery opportunities. Polym. Int. 2014, 63, 1145–1153. [Google Scholar] [CrossRef]
- Uchegbu, I.F.; Andreas, G.S.; Laurence, T.; Alexander, I.G.; Julieann, S.; Soryia, S.; Erasto, M. Polymeric chitosan-based vesicles for drug delivery. J. Pharm. Pharmacol. 1998, 50, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Siew, A.; Le, H.; Thiovolet, M.; Gellert, P.; Schatzlein, A.; Uchegbu, I. Enhanced oral absorption of hydrophobic and hydrophilic drugs using quaternary ammonium palmitoyl glycol chitosan nanoparticles. Mol. Pharm. 2012, 9, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Khutoryanskiy, V.V.; Stewart, A.; Rahman, S.; Papahadjopoulos-Sternberg, B.; Dufes, C.; McCarthy, D.; Wilson, C.G.; Lyons, R.; Carter, K.C.; et al. Carbohydrate-based micelle clusters which enhance hydrophobic drug bioavailability by up to 1 order of magnitude. Biomacromolecules 2006, 7, 3452–3459. [Google Scholar] [CrossRef] [PubMed]
- Bonferoni, M.C.; Sandri, G.; Ferrari, F.; Rossi, S.; Larghi, V.; Zambito, Y.; Caramella, C. Comparison of different in vitro and ex vivo methods to evaluate mucoadhesion of glycol-palmitoyl chitosan micelles. J. Drug Deliv. Sci. Technol. 2010, 20, 419–424. [Google Scholar] [CrossRef]
- Martin, L.; Wilson, C.G.; Koosha, F.; Tetley, L.; Gray, A.I.; Senel, S.; Uchegbu, I.F. The release of model macromolecules may be controlled by the hydrophobicity of palmitoyl glycol chitosan hydrogels. J. Control. Release 2002, 80, 87–100. [Google Scholar] [CrossRef]
- Cho, I.S.; Park, C.G.; Huh, B.K.; Cho, M.O.; Khatun, Z.; Li, Z.; Kang, S.-W.; Choy, Y.B.; Huh, K.M. Thermosensitive hexanoyl glycol chitosan-based ocular delivery system for glaucoma therapy. Acta Biomater. 2016, 39, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.S.; Cho, M.O.; Li, Z.; Nurunnabi, M.; Park, S.Y.; Kang, S.-W.; Huh, K.M. Synthesis and characterization of a new photo-crosslinkable glycol chitosan thermogel for biomedical applications. Carbohydr. Polym. 2016, 144, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Lueβen, H.L.; de Boer, A.G.; Verhoef, J.C.; Junginger, H.E. Novel peroral dosage forms with protease inhibitory activities. II. Design of fast dissolving poly(acrylate) and controlled drug-releasing capsule formulations with trypsin inhibiting properties. Int. J. Pharm. 1996, 138, 13–23. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Kast, C.E. Chemically modified chitosans as enzyme inhibitors. Adv. Drug Deliv. Rev. 2001, 52, 127–137. [Google Scholar] [CrossRef]
- Watanabe, S.-I.; Takeuchi, T.; Chey, W.Y. Mediation of trypsin inhibitor-induced pancreatic hypersecretion by secretin and cholecystokinin in rats. Gastroenterology 1992, 102, 621–628. [Google Scholar] [CrossRef]
- Song, Y.; Huang, Z.; Song, Y.; Tian, Q.; Liu, X.; She, Z.; Jiao, J.; Lu, E.; Deng, Y. The application of EDTA in drug delivery systems: Doxorubicin liposomes loaded via NH4EDTA gradient. Int. J. Nanomed. 2014, 9, 3611–3621. [Google Scholar] [CrossRef]
- Grießinger, J.A.; Hauptstein, S.; Laffleur, F.; Netsomboon, K.; Bernkop-Schnürch, A. Evaluation of the impact of multivalent metal ions on the permeation behavior of Dolutegravir sodium. Drug Dev. Ind. Pharm. 2016, 42, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Netsomboon, K.; Suchaoin, W.; Laffleur, F.; Prüfert, F.; Bernkop-Schnürch, A. Multifunctional adhesive polymers: Preactivated thiolated chitosan-EDTA conjugates. Eur. J. Pharm. Biopharm. 2017, 111, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A.; Scerbe-Saiko, A. Synthesis and in vitro evaluation of chitosan-EDTA-protease-inhibitor conjugates which might be useful in oral delivery of peptides and proteins. Pharm. Res. 1998, 15, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A.; Krajicek, M.E. Mucoadhesive polymers as platforms for peroral peptide delivery and absorption: Synthesis and evaluation of different chitosan–EDTA conjugates. J. Control. Release 1998, 50, 215–223. [Google Scholar] [CrossRef]
- Kim, K.; Kim, K.; Ryu, J.H.; Lee, H. Chitosan-catechol: A polymer with long-lasting mucoadhesive properties. Biomaterials 2015, 52, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Lee, Y.; Kong, W.H.; Kim, T.G.; Park, T.G.; Lee, H. Catechol-functionalized chitosan/pluronic hydrogels for tissue adhesives and hemostatic materials. Biomacromolecules 2011, 12, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Ryu, J.H.; Lee, D.Y.; Lee, H. Bio-inspired catechol conjugation converts water-insoluble chitosan into a highly water-soluble, adhesive chitosan derivative for hydrogels and LbL assembly. Biomater. Sci. 2013, 1, 783–790. [Google Scholar] [CrossRef]
- Ryu, J.H.; Hong, S.; Lee, H. Bio-inspired adhesive catechol-conjugated chitosan for biomedical applications: A mini review. Acta Biomater. 2015, 27, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Scherer, N.F.; Messersmith, P.B. Single-molecule mechanics of mussel adhesion. Proc. Natl. Acad. Sci. USA 2006, 103, 12999–13003. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.M.; Smart, J.D.; Nevell, T.G.; Ewen, R.J.; Eaton, P.J.; Tsibouklis, J. Mucin/poly(acrylic acid) interactions: A spectroscopic investigation of mucoadhesion. Biomacromolecules 2003, 4, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.G.; Smart, J.D.; Tsibouklis, J.; Dettmar, P.W.; Hampson, F.; Davis, J.A.; Kelly, G.; Wilber, W.R. An investigation of mucus/polymer rheological synergism using synthesised and characterised poly(acrylic acid)s. Int. J. Pharm. 2001, 217, 87–100. [Google Scholar] [CrossRef]
- Mortazavi, S.A.; Carpenter, B.G.; Smart, J.D. A comparative study on the role played by mucus glycoproteins in the rheological behaviour of the mucoadhesive/mucosal interface. Int. J. Pharm. 1993, 94, 195–201. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Ilari, P.; Tomasetti, M. Preparation and characteristic properties of 5-methyl pyrrolidinone chitosan. Carbohydr. Polym. 1993, 20, 99–105. [Google Scholar] [CrossRef]
- Muzzarelli, R. Methyl Pyrrolidinone Chitosan, Production Process and Uses Thereof. WO1992009635A1, 11 June 1992. [Google Scholar]
- Rinaudo, M.; Desbrières, J.; Le Dung, P.; Thuy Binh, P.; Dong, N.T. NMR investigation of chitosan derivatives formed by the reaction of chitosan with levulinic acid. Carbohydr. Polym. 2001, 46, 339–348. [Google Scholar] [CrossRef]
- Kurita, Y.; Isogai, A. Reductive N-alkylation of chitosan with acetone and levulinic acid in aqueous media. Int. J. Biol. Macromol. 2010, 47, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Muzzarelli, C.; Caramella, C. Assessment of chitosan derivatives as buccal and vaginal penetration enhancers. Eur. J. Pharm. Sci. 2004, 21, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-K.; Hong, M.-S.; Kim, Y.-B.; Han, S.-K. Effect of penetration enhancers (pyrrolidone derivatives) on multilamellar liposomes of stratum corneum lipid: A study by UV spectroscopy and differential scanning calorimetry. Int. J. Pharm. 1993, 95, 43–50. [Google Scholar] [CrossRef]
- Sasaki, H.; Kojima, M.; Mori, Y.; Nakamura, J.; Shibasaki, J. Enhancing effect of pyrrolidone derivatives on transdermal penetration of 5-fluorouracil, triamcinolone acetonide, indomethacin, and flurbiprofen. J. Pharm. Sci. 1991, 80, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Auzély-Velty, R.; Rinaudo, M. Chitosan derivatives bearing pendant cyclodextrin cavities: Synthesis and inclusion performance. Macromolecules 2001, 34, 3574–3580. [Google Scholar] [CrossRef]
- Venter, J.P.; Kotzé, A.F.; Auzély-Velty, R.; Rinaudo, M. Synthesis and evaluation of the mucoadhesivity of a CD-chitosan derivative. Int. J. Pharm. 2006, 313, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Chaleawlert-umpon, S.; Nuchuchua, O.; Saesoo, S.; Gonil, P.; Ruktanonchai, U.R.; Sajomsang, W.; Pimpha, N. Effect of citrate spacer on mucoadhesive properties of a novel water-soluble cationic β-cyclodextrin-conjugated chitosan. Carbohydr. Polym. 2011, 84, 186–194. [Google Scholar] [CrossRef]
- Yostawonkul, J.; Surassmo, S.; Iempridee, T.; Pimtong, W.; Suktham, K.; Sajomsang, W.; Gonil, P.; Ruktanonchai, U.R. Surface modification of nanostructure lipid carrier (NLC) by oleoyl-quaternized-chitosan as a mucoadhesive nanocarrier. Colloids Surf. B Biointerfaces 2017, 149, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Thanou, M.; Florea, B.I.; Langemeÿer, M.W.E.; Verhoef, J.C.; Junginger, H.E. N-trimethylated chitosan chloride (TMC) improves the intestinal permeation of the peptide drug buserelin in vitro (Caco-2 Cells) and in vivo (rats). Pharm. Res. 2000, 17, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, C.V.; Belgamwar, V.S. Controlled synthesis of N,N,N-trimethyl chitosan for modulated bioadhesion and nasal membrane permeability. Int. J. Biol. Macromol. 2016, 82, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Kast, C.E.; Valenta, C.; Leopold, M.; Bernkop-Schnürch, A. Design and in vitro evaluation of a novel bioadhesive vaginal drug delivery system for clotrimazole. J. Control. Release 2002, 81, 347–354. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Guggi, D.; Pinter, Y. Thiolated chitosans: Development and in vitro evaluation of a mucoadhesive, permeation enhancing oral drug delivery system. J. Control. Release 2004, 94, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Strandman, S.; Zhu, J.X.X.; Barralet, J.; Cerruti, M. Genipin-crosslinked catechol-chitosan mucoadhesive hydrogels for buccal drug delivery. Biomaterials 2015, 37, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tam, M.; Samaei, S.; Lerouge, S.; Barralet, J.; Stevenson, M.M.; Cerruti, M. Mucoadhesive chitosan hydrogels as rectal drug delivery vessels to treat ulcerative colitis. Acta Biomater. 2017, 48, 247–257. [Google Scholar] [CrossRef] [PubMed]

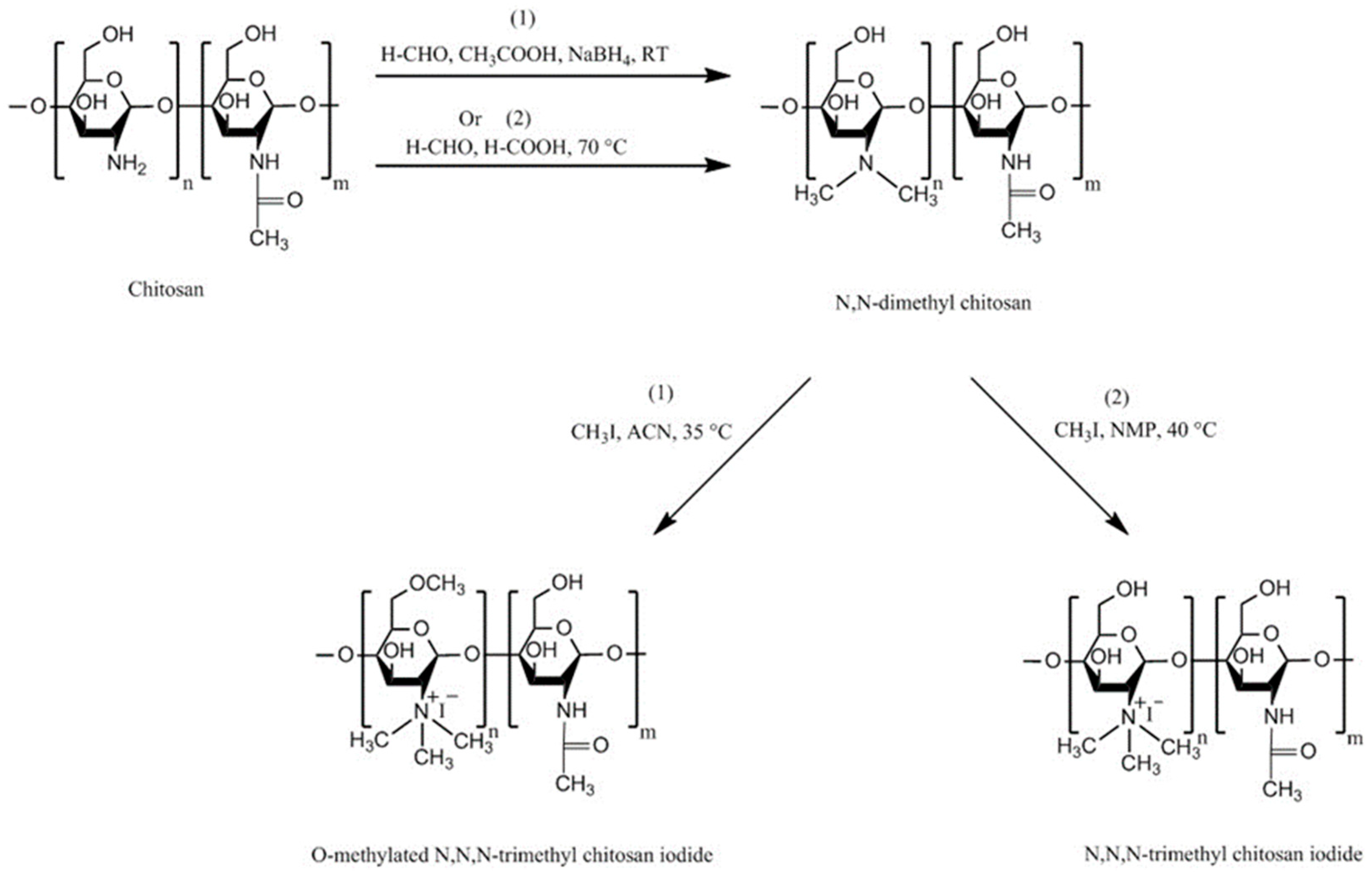
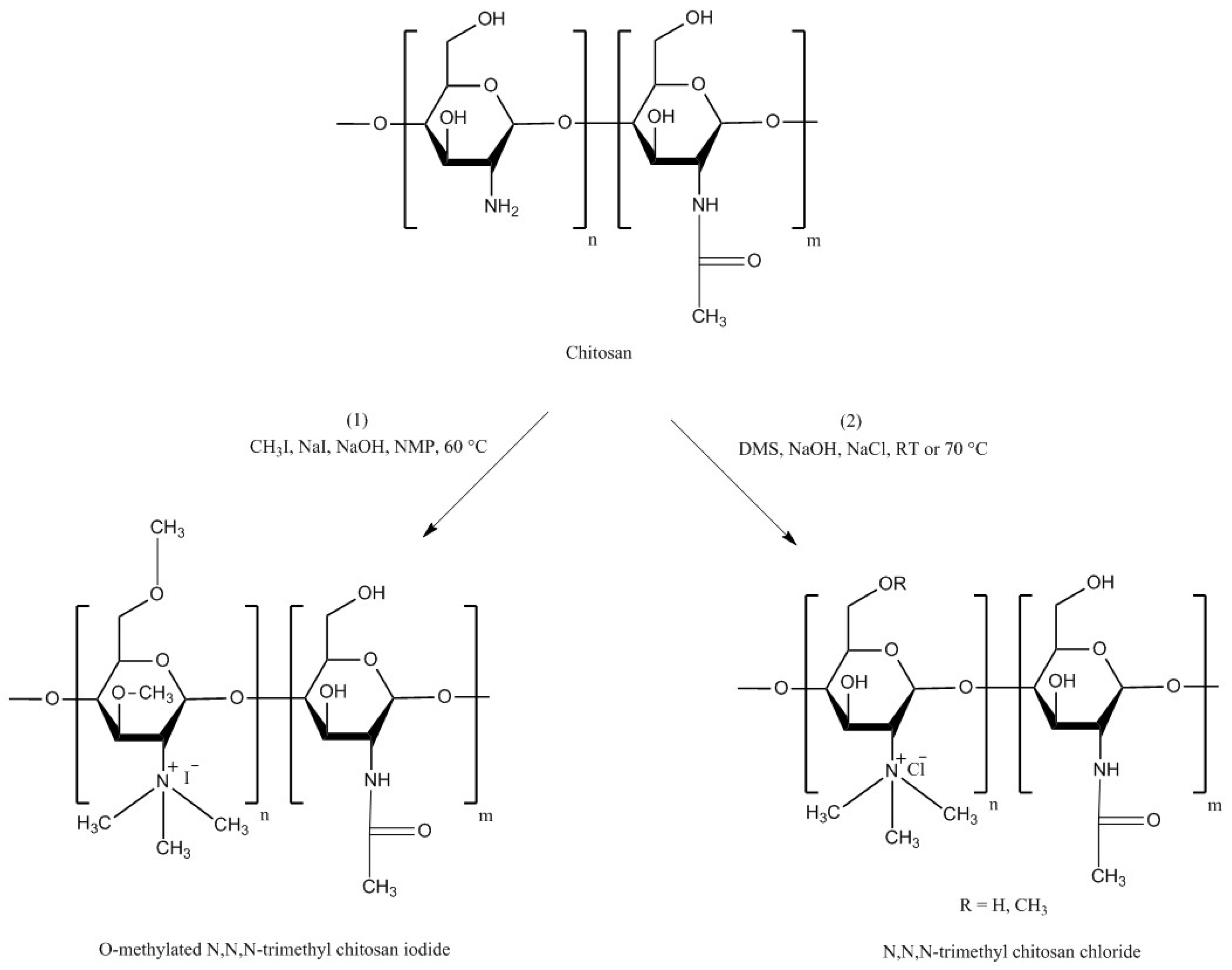
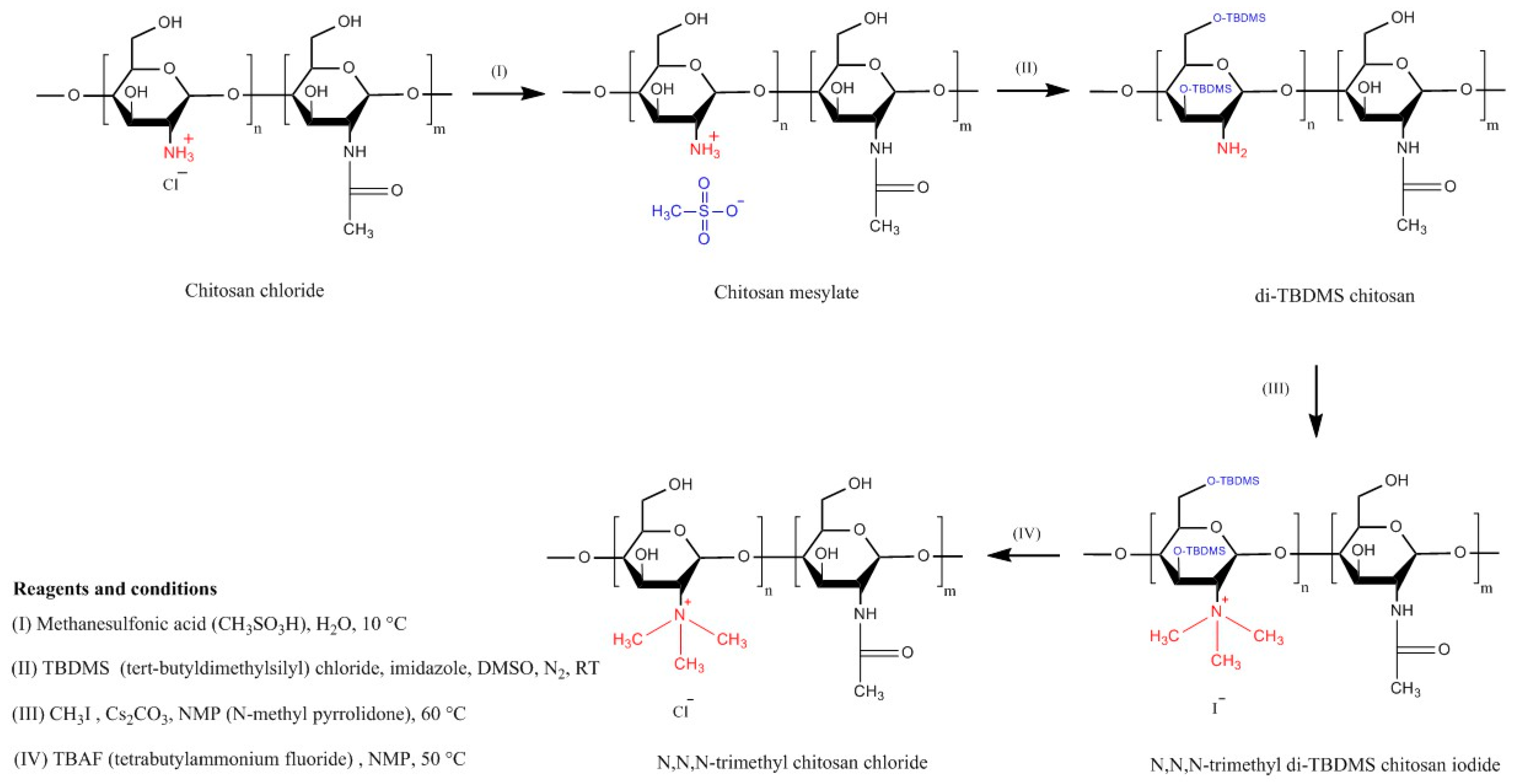
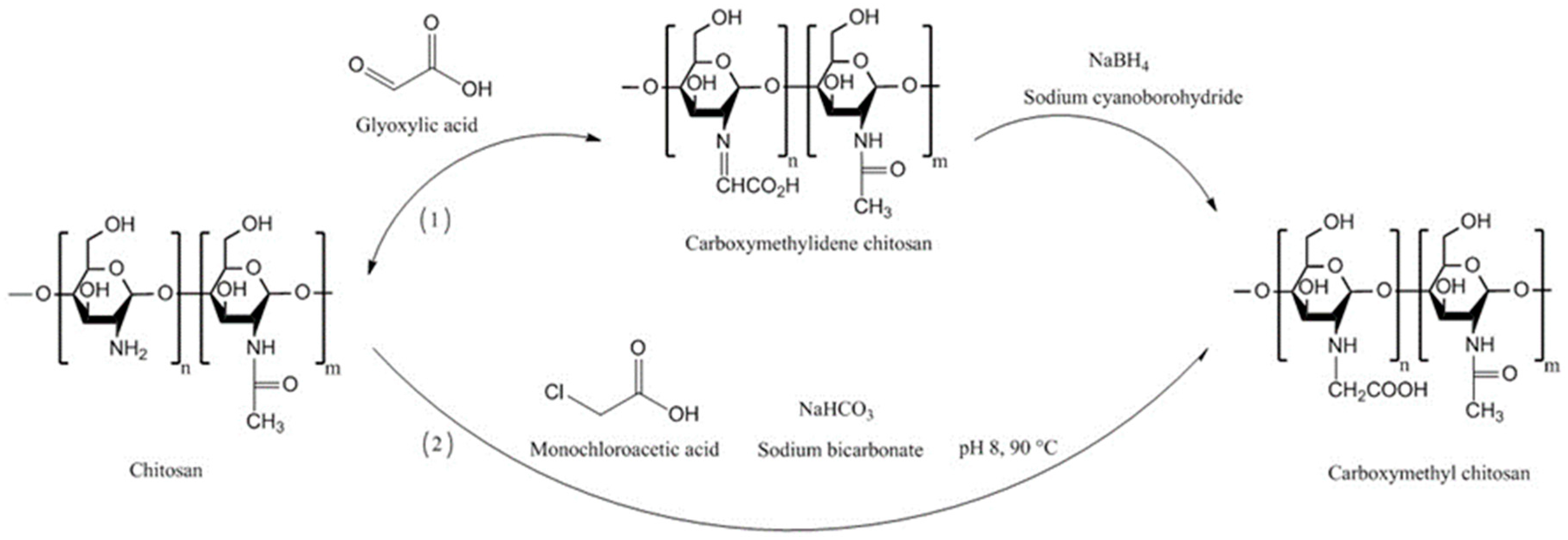
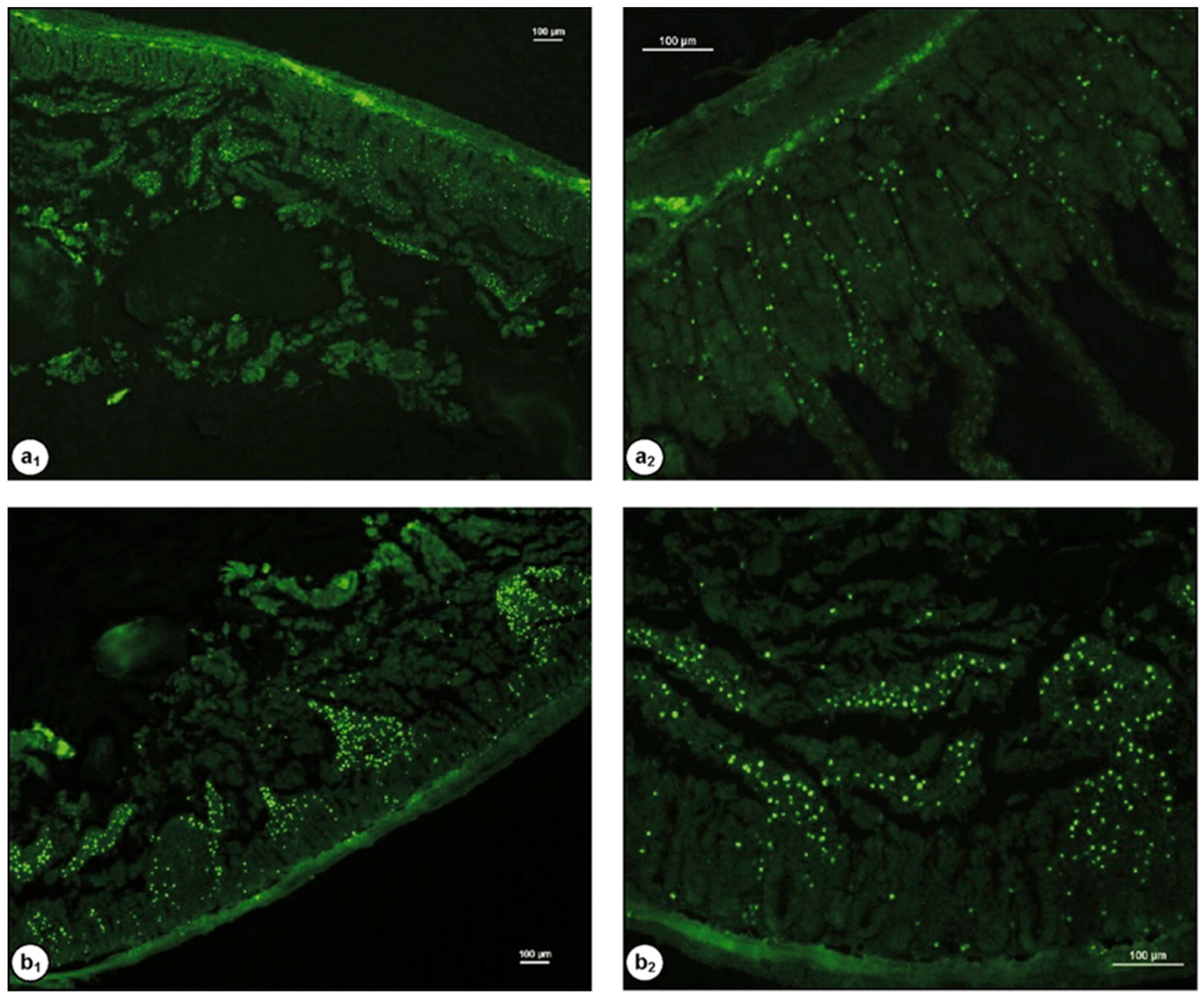
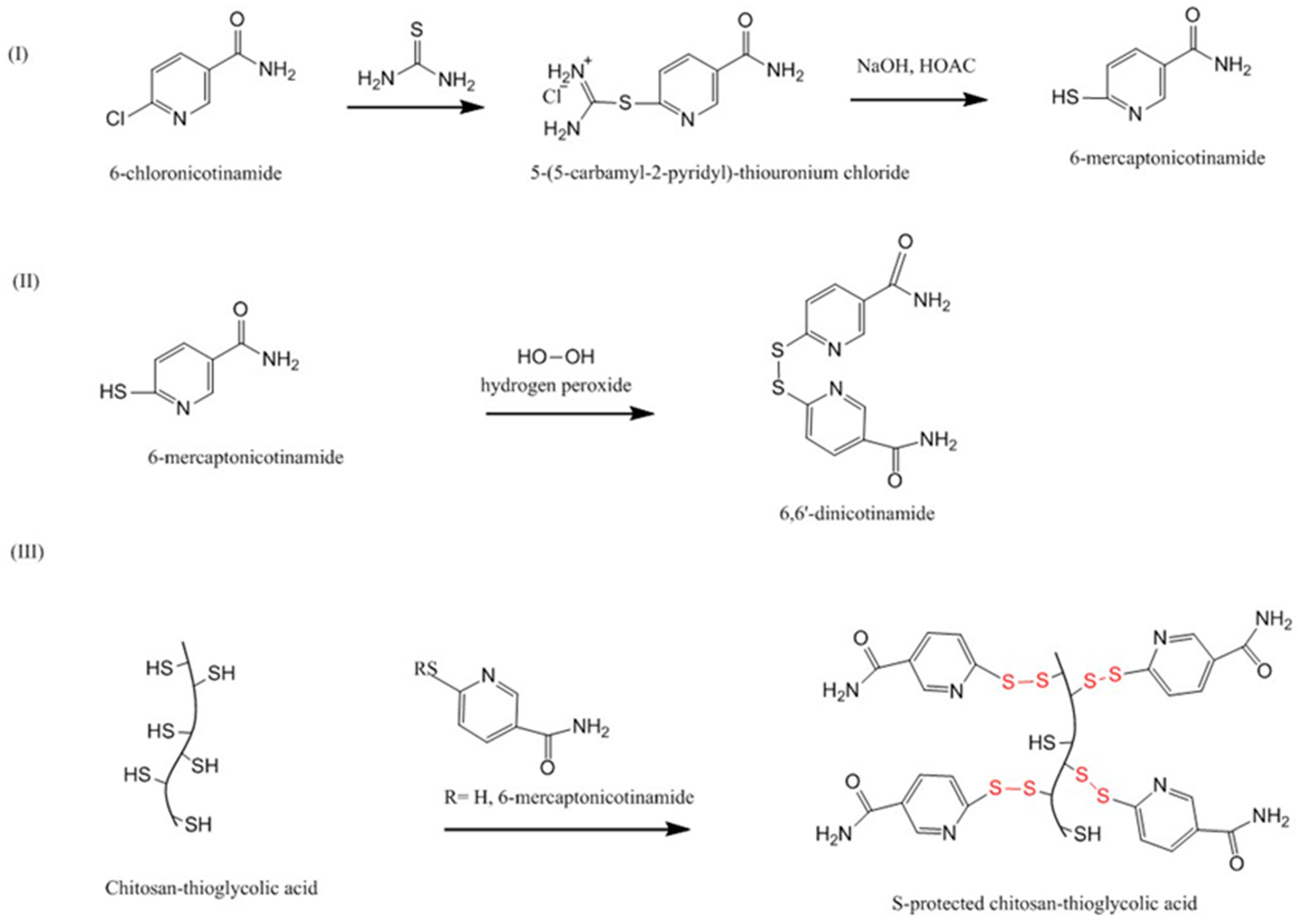
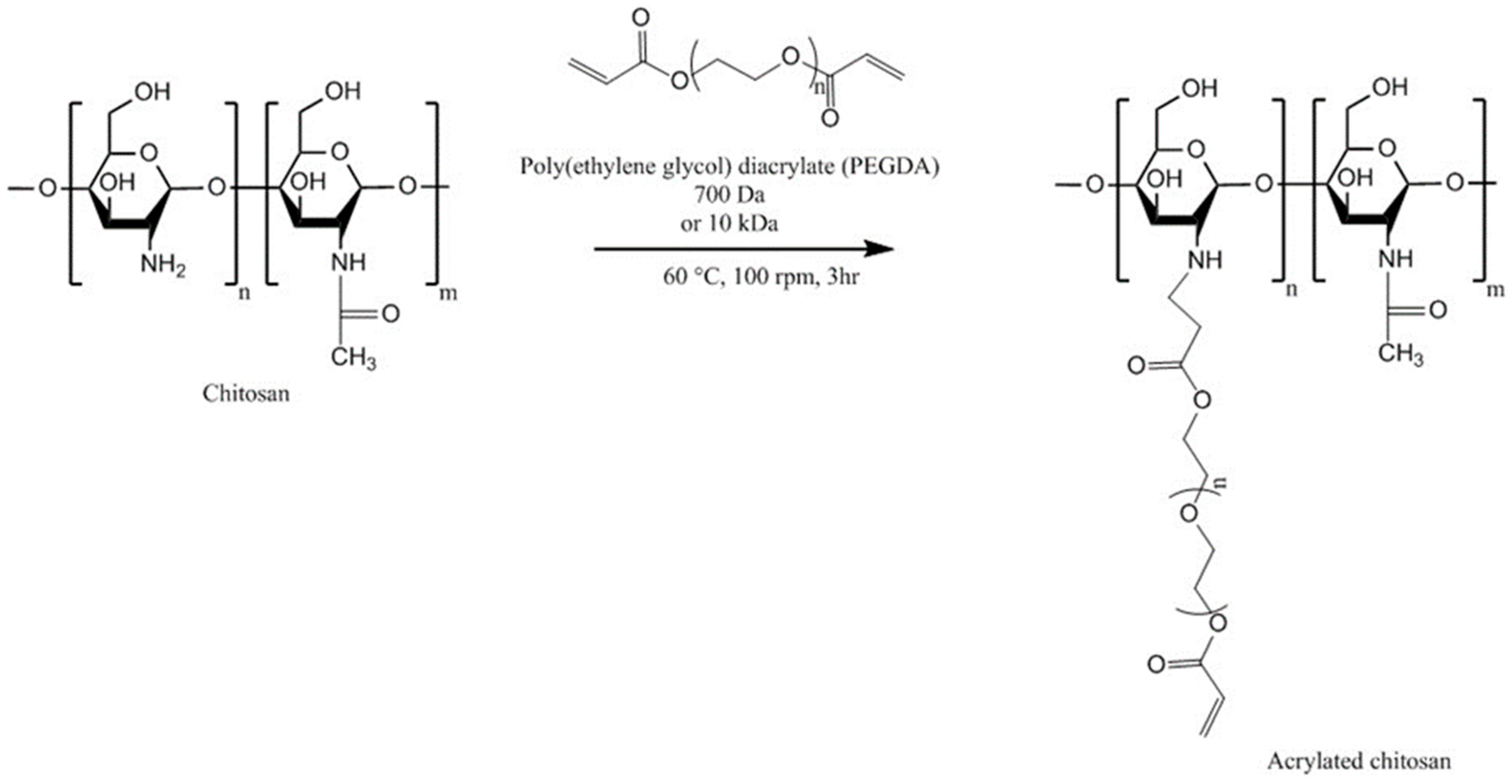

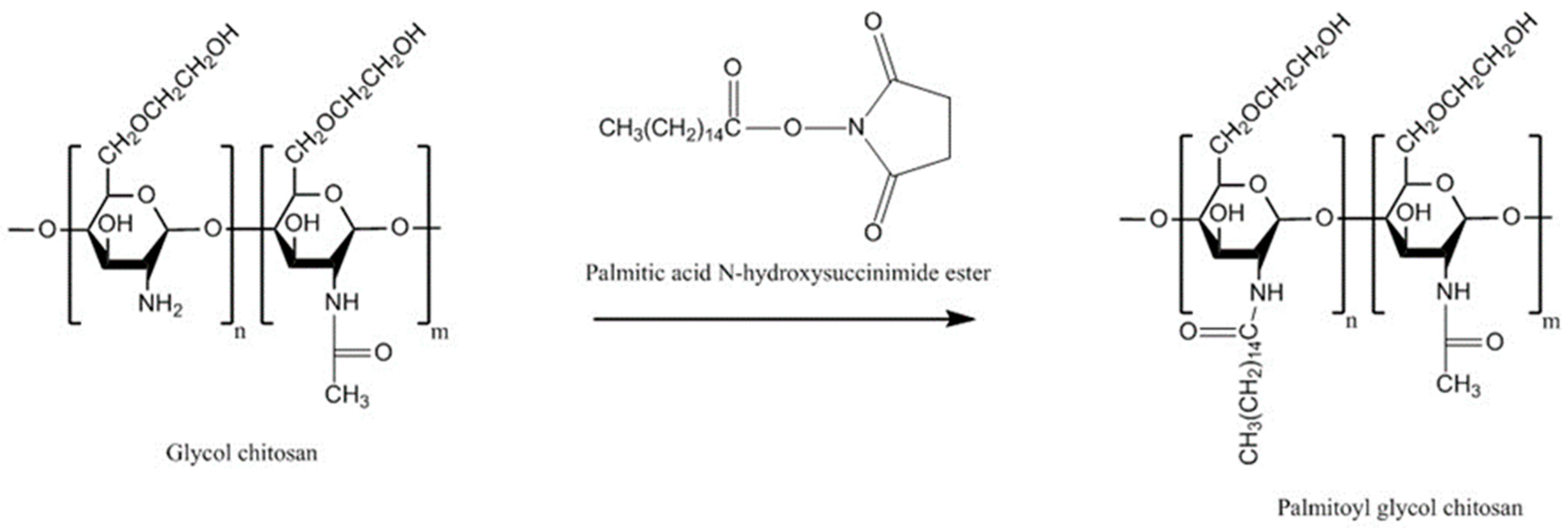

| Chitosan Derivatives | Advantages | Disadvantages | Drug | Route of Administration/Substrate | References |
|---|---|---|---|---|---|
| Trimethyl chitosan | Soluble at broad range of pHs (2–12), strong mucoadhesion; decreased TEER; increased paracellular permeability of basic or neutral macromolecules | Strong aggregation with anionic macromolecules such as heparin | Buserelin, ropinirole·HCl | Oral, small intestine, cattle nasal mucosa | [52,160,161] |
| N-carboxymethyl chitosan | Decreased TEER; increased paracellular permeability of anionic macromolecules | Insoluble at pH 3–7 (depending on the degree of substitution) due to its polyampholytic character | Low molecular weight heparin; Ofloxacin | Oral, rat small intestine; Ocular, rabbit eyes, in vivo | [73,76,82] |
| Chitosan-cysteine | Same mucoadhesion as unmodified chitosan, improved cohesion compared to unmodified chitosan, permeation enhancing effect | Susceptible to premature oxidation, undesirable side reactions led to the formation of (chitosan-cysteine-cysteine)n side chains | - | Oral, porcine intestinal mucosa | [25,84] |
| Chitosan-N-acetylcysteine | 50-fold longer retention time than unmodified chitosan, biodegradability as indicated by the reduction of its solution viscosity after addition of hen white egg | Susceptible to premature oxidation | - | Oral, flat faced-discs, porcine intestinal mucosa | [87] |
| Chitosan-TGA | Controlled drug release, longer disintegration time (up to 100-fold) and 26-fold longer mucoadhesion time against unmodified chitosan | Need of mediator such as EDAC | Clotrimazole | Vaginal, tablets, bovine vaginal mucosa | [162] |
| Chitosan-TBA | Strong mucoadhesion, permeation enhancing effect, controlled release, no need for mediator | Prone to oxidation. In addition, unintended cyclisation side reactions | Insulin, cefadroxil | Oral, tablets, porcine and rat intestinal mucosa | [108,163] |
| Chitosan-thioethylamidine | Much quicker synthetic reaction rate than chitosan-TBA (1.5 h vs. 24 h), 8.9-fold longer mucosal detachment time than unmodified chitosan, controlled release, no cyclisation side reactions as in chitosan-TBA | Stability issues | FITC-dextran | Oral, tablets, porcine intestinal mucosa | [88] |
| Chitosan-glutathione | Improved stability compared to unmodified chitosan, enhanced mucoadhesion (9.9-fold increased adhesion force and 55-fold longer adhesion time), 4.9-fold higher permeation-enhancing effect against unmodified chitosan, used as oxidative stress suppressant | Stability issues | Thymopentin | Oral, tablets, in vitro porcine rat intestinal mucosa; Oral nanoparticles, in vivo rats; Injectable hydrogels | [89,91,104] |
| Pre-activated (S-protected) thiolated chitosan | Improved stability and mucoadhesion compared to unmodified chitosan and unprotected thiolated chitosan | 2-fold less swelling than unmodified chitosan | Leuprolide; Antide | Oral, tablets, porcine intestinal mucosa Oral, rat intestinal mucosa | [111,112] |
| Acrylated chitosan | Strong mucoadhesion, water-soluble | Use of low molecular weight PEGDA results in a weaker mucoadhesion | - | Oral, porcine intestinal mucosa | [119] |
| Half-acetylated chitosan | Better solubility at higher pHs (up to 7.4) compared to unmodified chitosan, sustained drug release | Less mucoadhesive compared to unmodified chitosan | Ibuprofen | Oral, porcine gastric mucosa | [35] |
| Palmitoyl glycol chitosan | Amphiphilic property, diminished erosion and slow hydration led to controlled release, control bioadhesive strength by changing the degree of palmitoylation | Potential problems with reproducibility with the degrees of substitution related to insolubility of the final product | FITC-dextran | Buccal/disc shaped gels, porcine buccal mucosa | [130] |
| Hexanoyl glycol chitosan | In situ gelling property, in vivo ocular retention, longer duration of action | - | Rhodamine, brimonidine | Ocular, rabbit, in vivo ocular tissues | [131] |
| Chitosan-enzyme inhibitors | Protects drugs from enzymatic degradation. Controlled antipain release over 6 h, mucoadhesive properties preserved | Potential stability issues | Insulin | Oral, flat-faced discs, porcine intestinal mucosa | [13] |
| Chitosan-EDTA | Better mucoadhesion than unmodified chitosan Inhibits Zn and Co-dependent proteases including carboxypeptidase A and aminopeptidase N | No Ca-dependent serine proteases inhibition | - | Oral, flat-faced discs, porcine intestinal mucosa | [140] |
| Chitosan-enzyme inhibitors-EDTA | Strong inhibitory action against serine proteases, Zn-dependent exopeptidases including carboxypeptidase A and B, aminopeptidase N | Less mucoadhesive than unmodified chitosan and chitosan-EDTA | - | Oral, flat-faced discs, porcine intestinal mucosa | [139] |
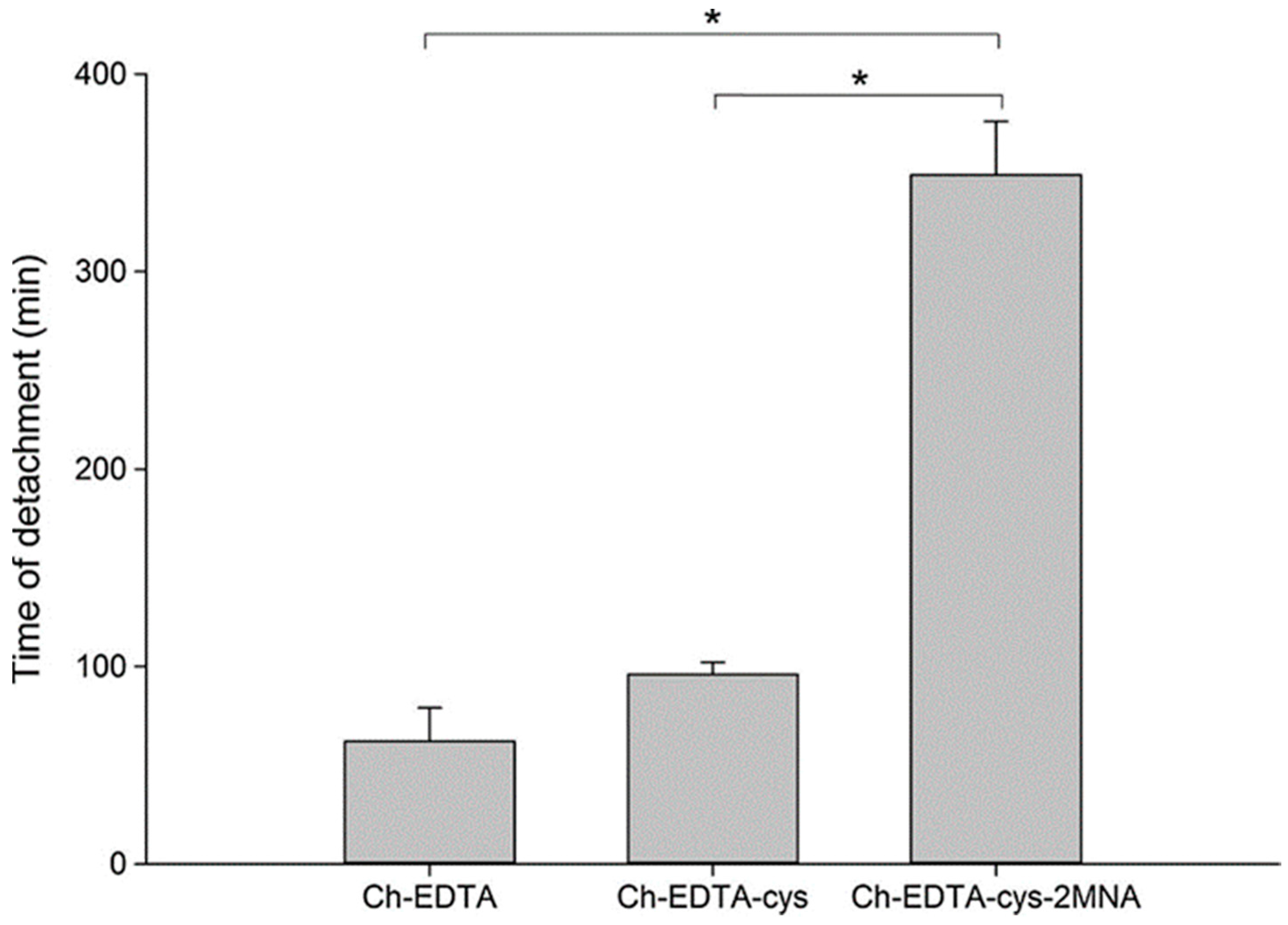
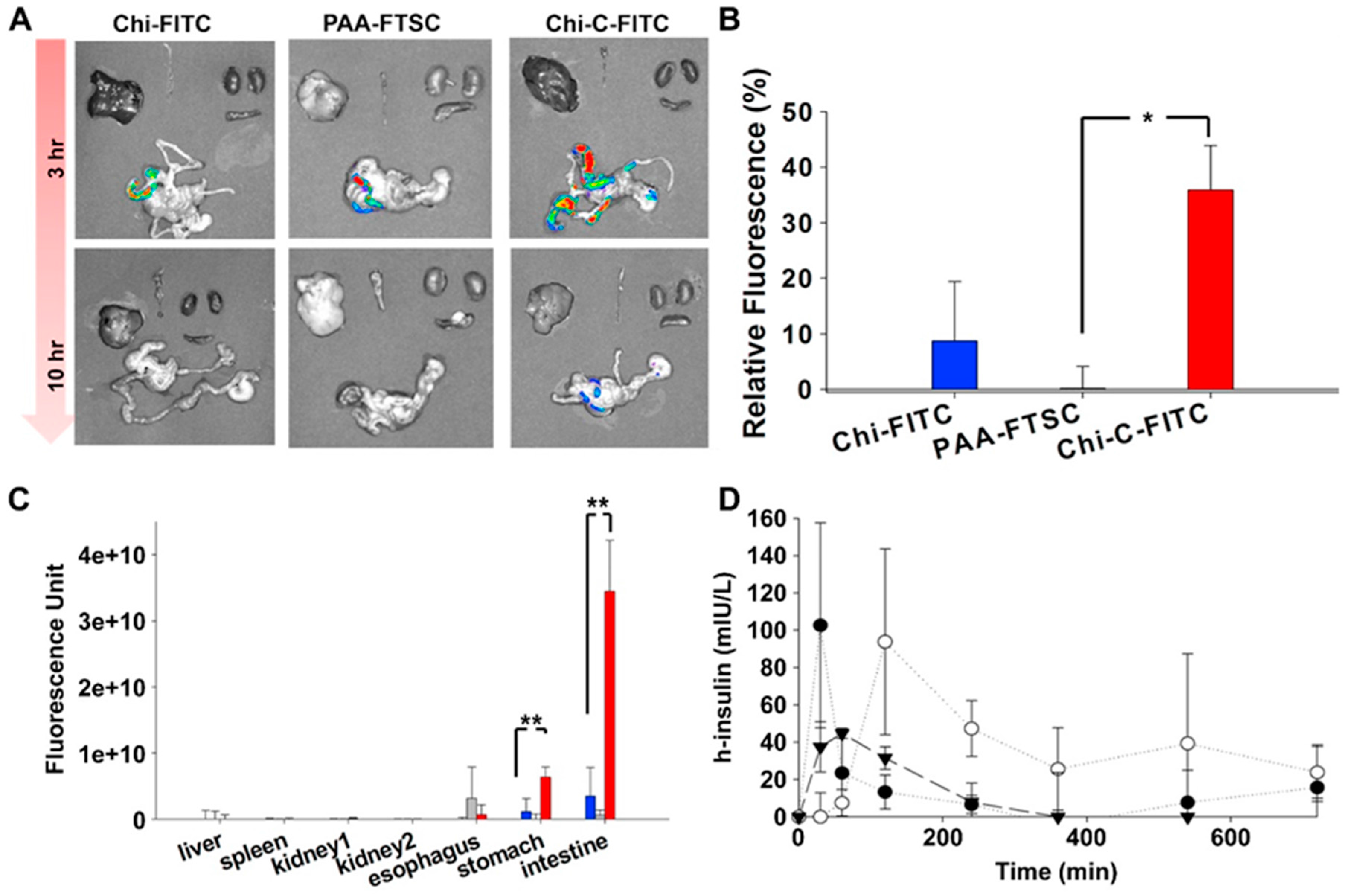
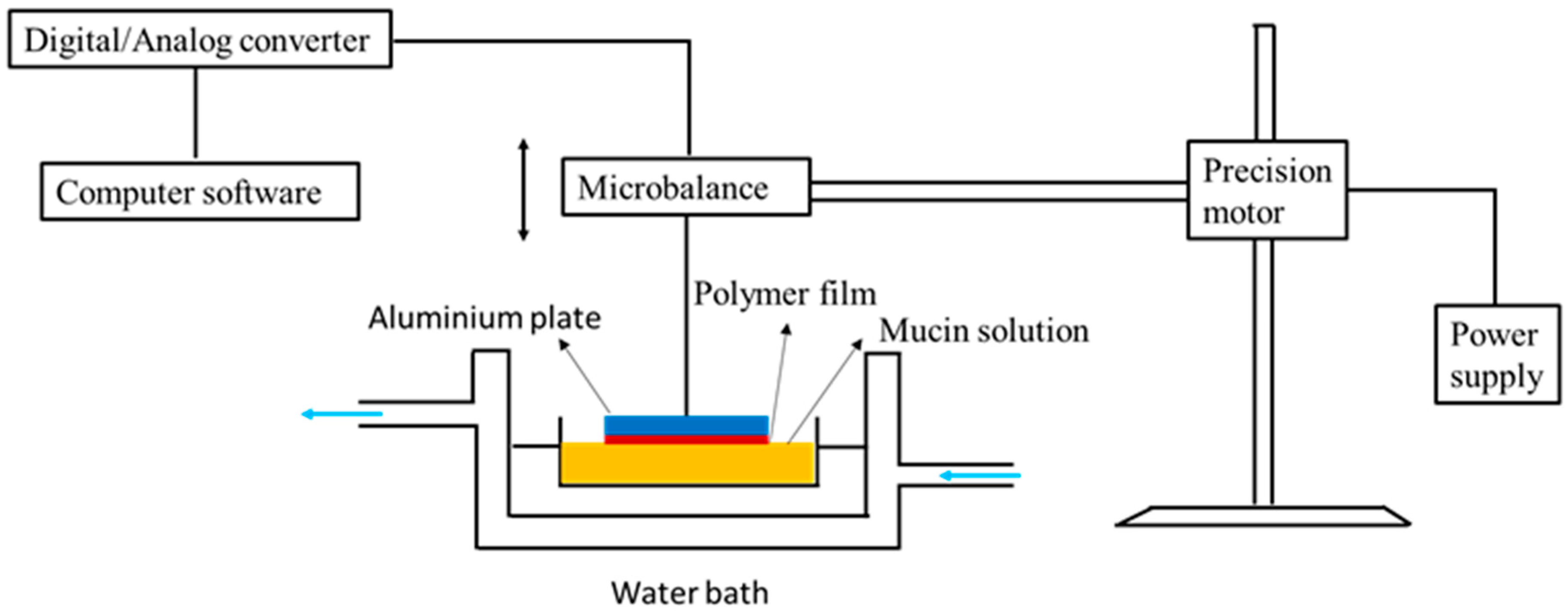
| Chitosan-catechol conjugate | Strong mucoadhesion, higher solubility at neutral pH, sustained drug release, improved therapeutic effect in vivo compared to unmodified chitosan | Poor mucoadhesion in acidic environment, optimum degree of substitution (7.2%) is required to achieve water-soluble product and formation of large gel-like aggregates has been observed for greater degree of substitution (12.7%) | Lidocaine; Sulfasalazine | Oral, mice gastrointestinal tract, porcine gastric mucin type II; Buccal, hydrogels, porcine and rabbit buccal mucosa; Rectal, hydrogels, mice rectal mucosa in vivo | [141,143,164,165] |
| Methyl pyrrolidinone chitosan | Greater mucoadhesion and penetration enhancing effect than unmodified chitosan | - | Acyclovir | Buccal and vaginal, porcine cheek or submaxillary bovine mucin, vaginal mucosa, or porcine gastric mucin | [153] |
| Chitosan-cyclodextrin | Inclusion ability, sustained release | Weaker mucoadhesion than the parent chitosan | - | Porcine gastric mucin | [156,157] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
M. Ways, T.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. https://doi.org/10.3390/polym10030267
M. Ways TM, Lau WM, Khutoryanskiy VV. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers. 2018; 10(3):267. https://doi.org/10.3390/polym10030267
Chicago/Turabian StyleM. Ways, Twana Mohammed, Wing Man Lau, and Vitaliy V. Khutoryanskiy. 2018. "Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems" Polymers 10, no. 3: 267. https://doi.org/10.3390/polym10030267