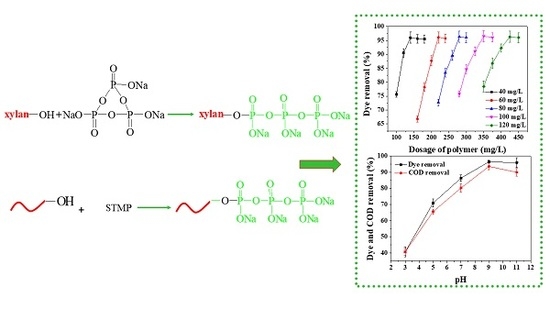
Preparation and Application of Phosphorylated Xylan as a Flocculant for Cationic Ethyl Violet Dye
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
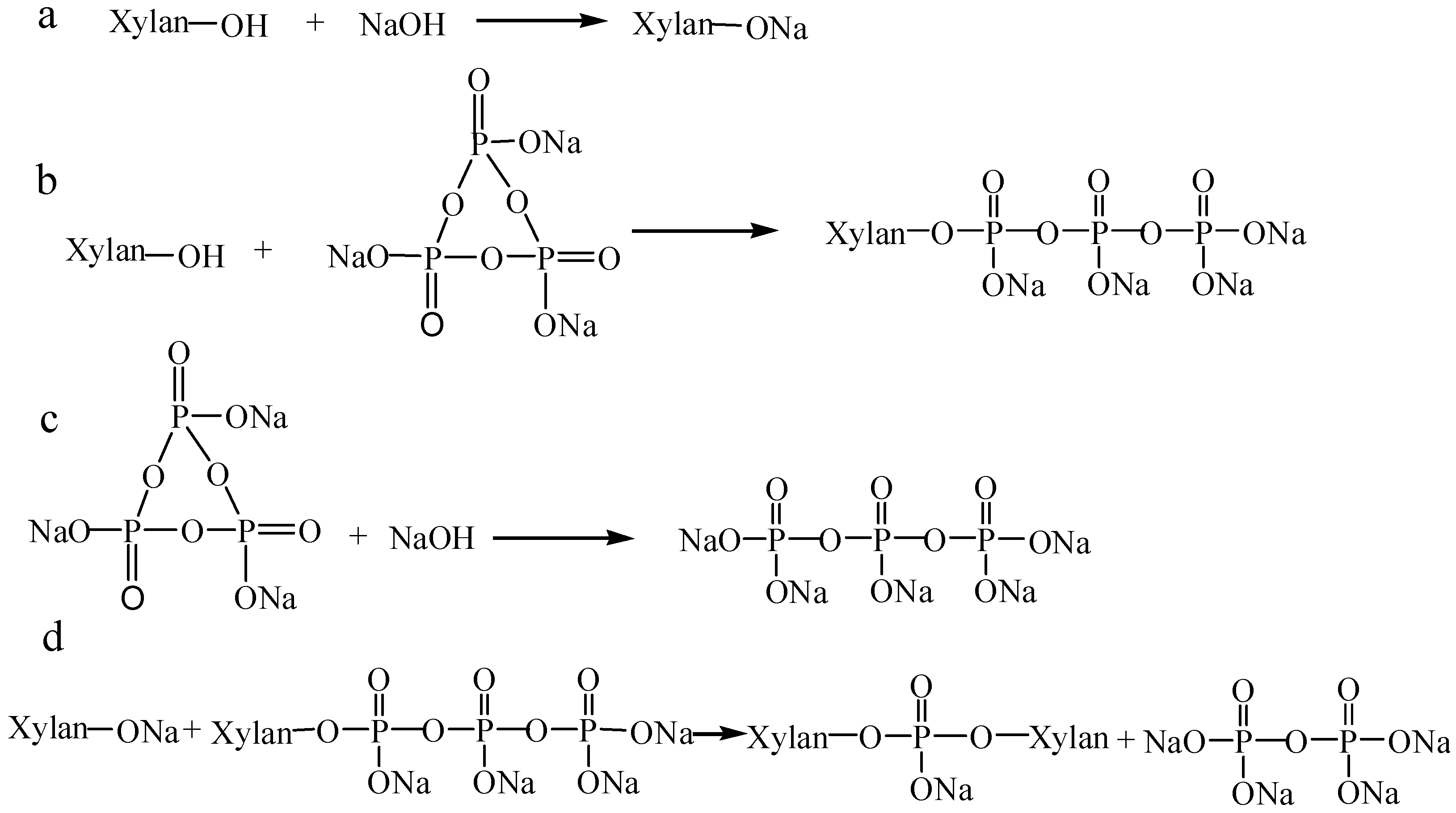
2.2. Phosphorylation of Xylan
2.3. Experimental Design
2.4. Analytical Methods
2.4.1. Fourier Transform Infrared (FTIR) Analysis
2.4.2. Charge Density Analysis
2.4.3. Determination of Phosphorus Content and Degree of Substitution (DS)
2.4.4. Molecular Weight Analysis
2.4.5. Elemental Analysis
2.4.6. Thermogravimetric Analysis
2.4.7. Viscosity Measurement
2.4.8. Flocculation Analysis
2.4.9. COD Analysis
3. Results
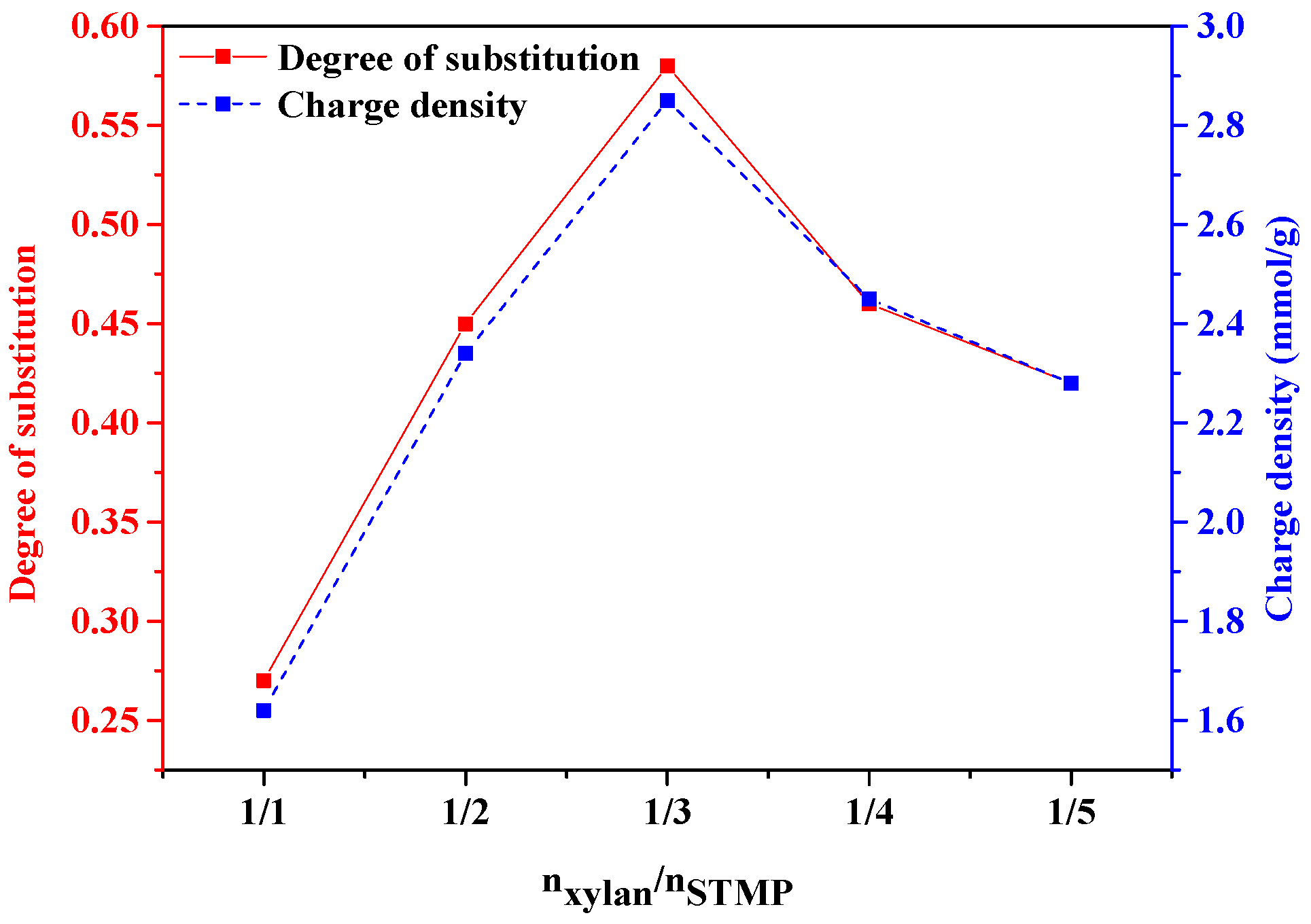
3.1. Influences of Reaction Conditions on DS and Charge Density of Phosphorylated Xylan
3.1.1. Molar Ratio of Xylan/STMP
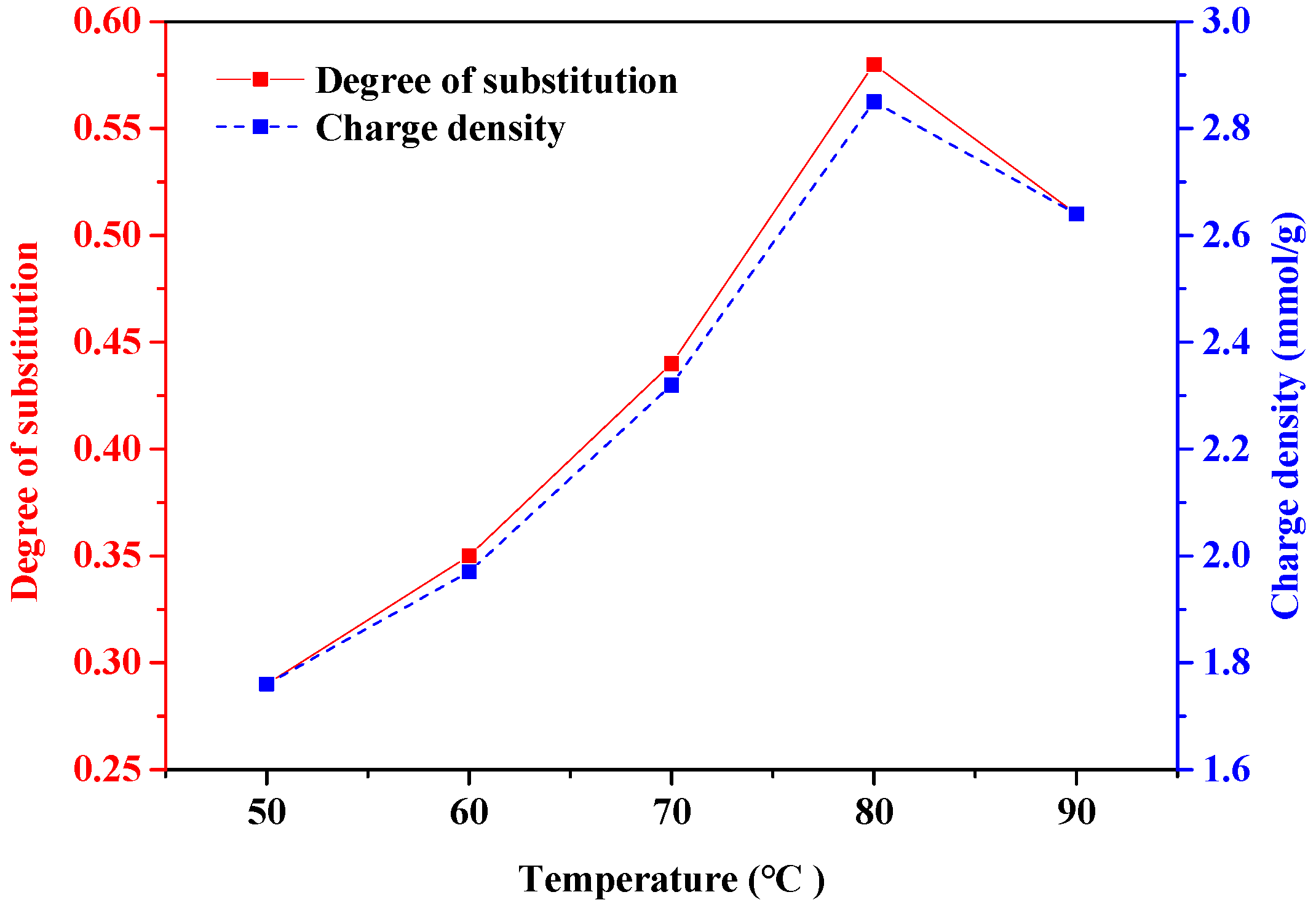
3.1.2. Temperature
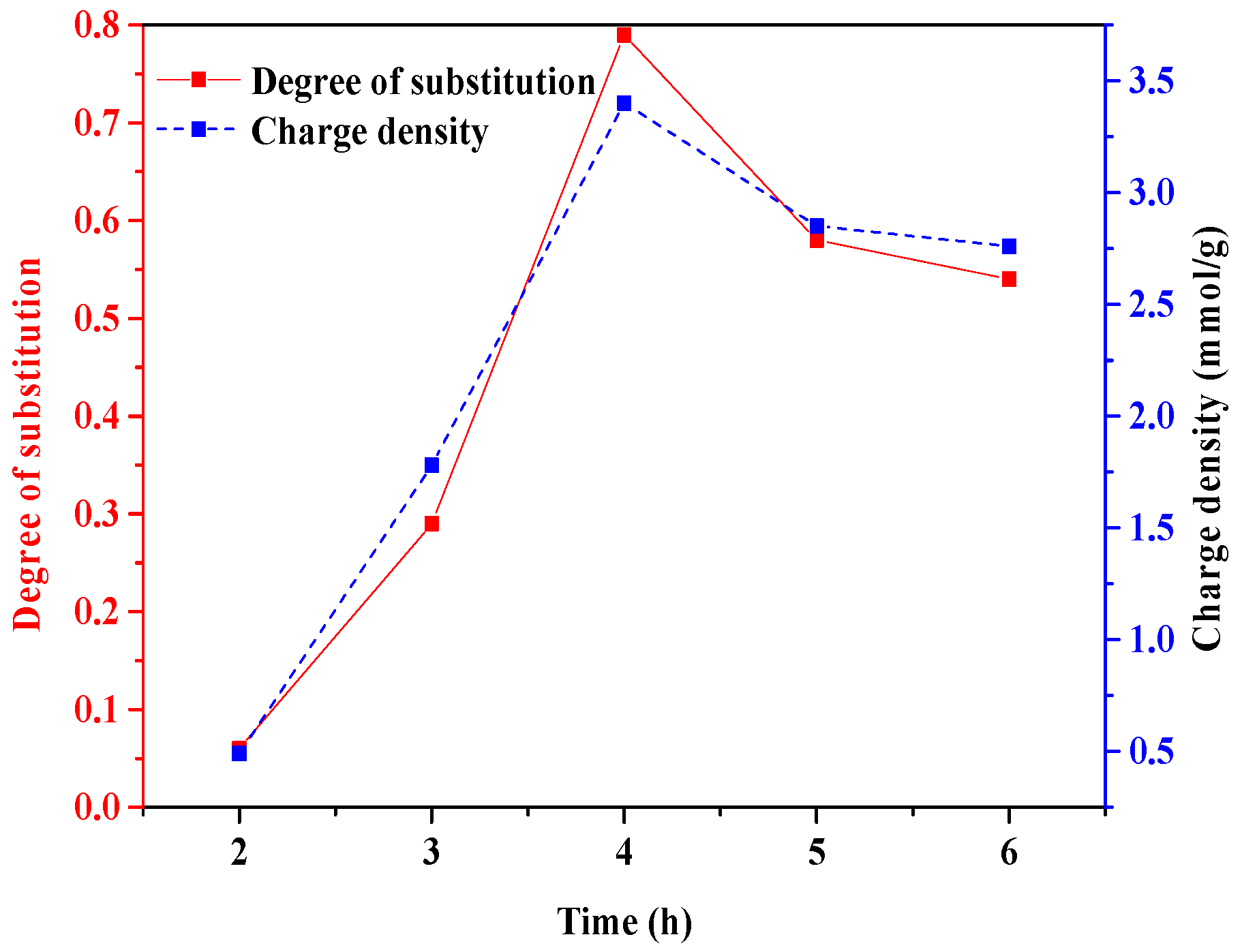
3.1.3. Time
3.2. Analysis of Variance (ANOVA)
3.3. Properties of Phosphorylated Xylan with the Maximum DS and Charge Density
3.4. Elemental Analysis
3.5. FTIR Analysis
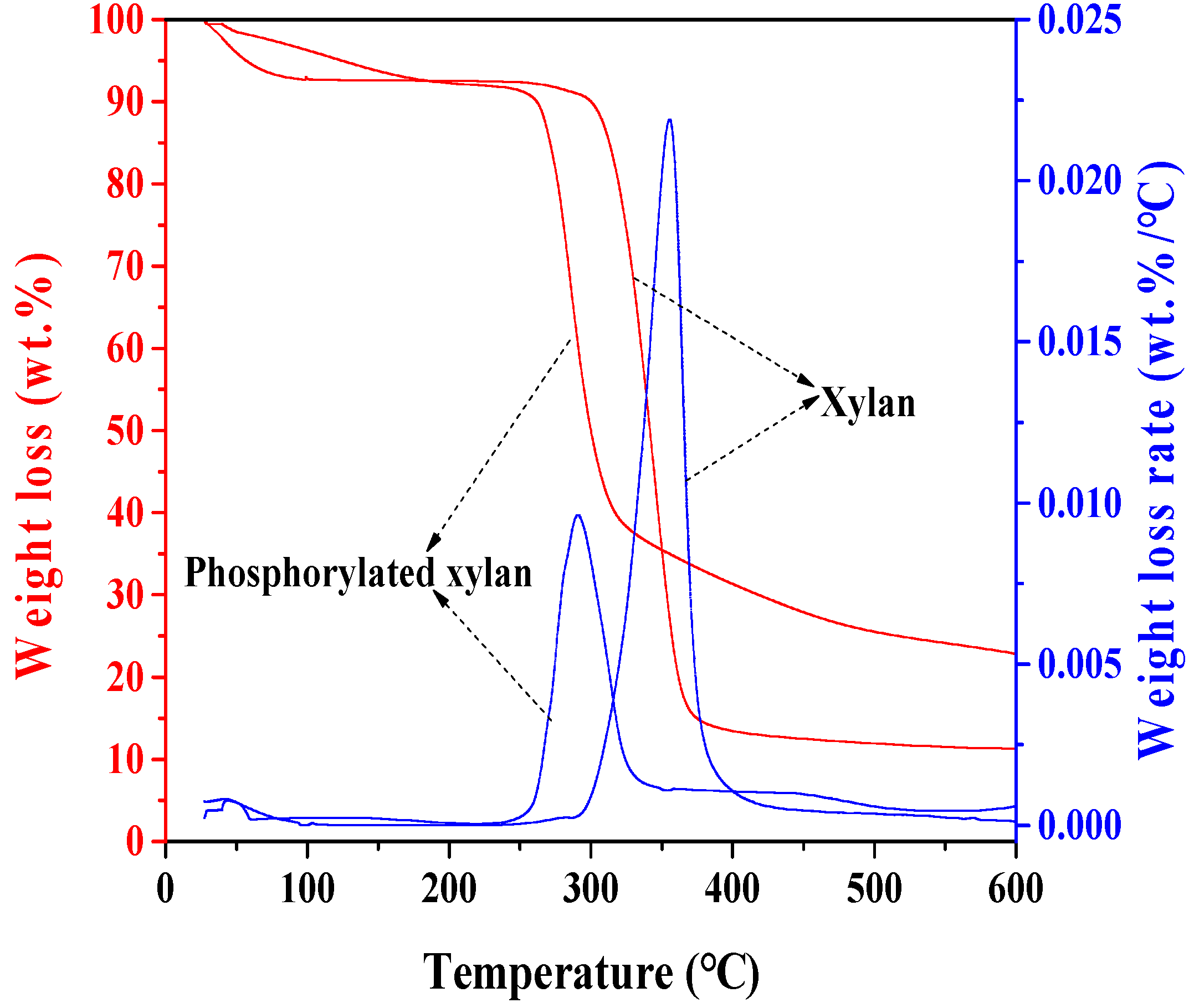
3.6. Thermogravimetric Analysis
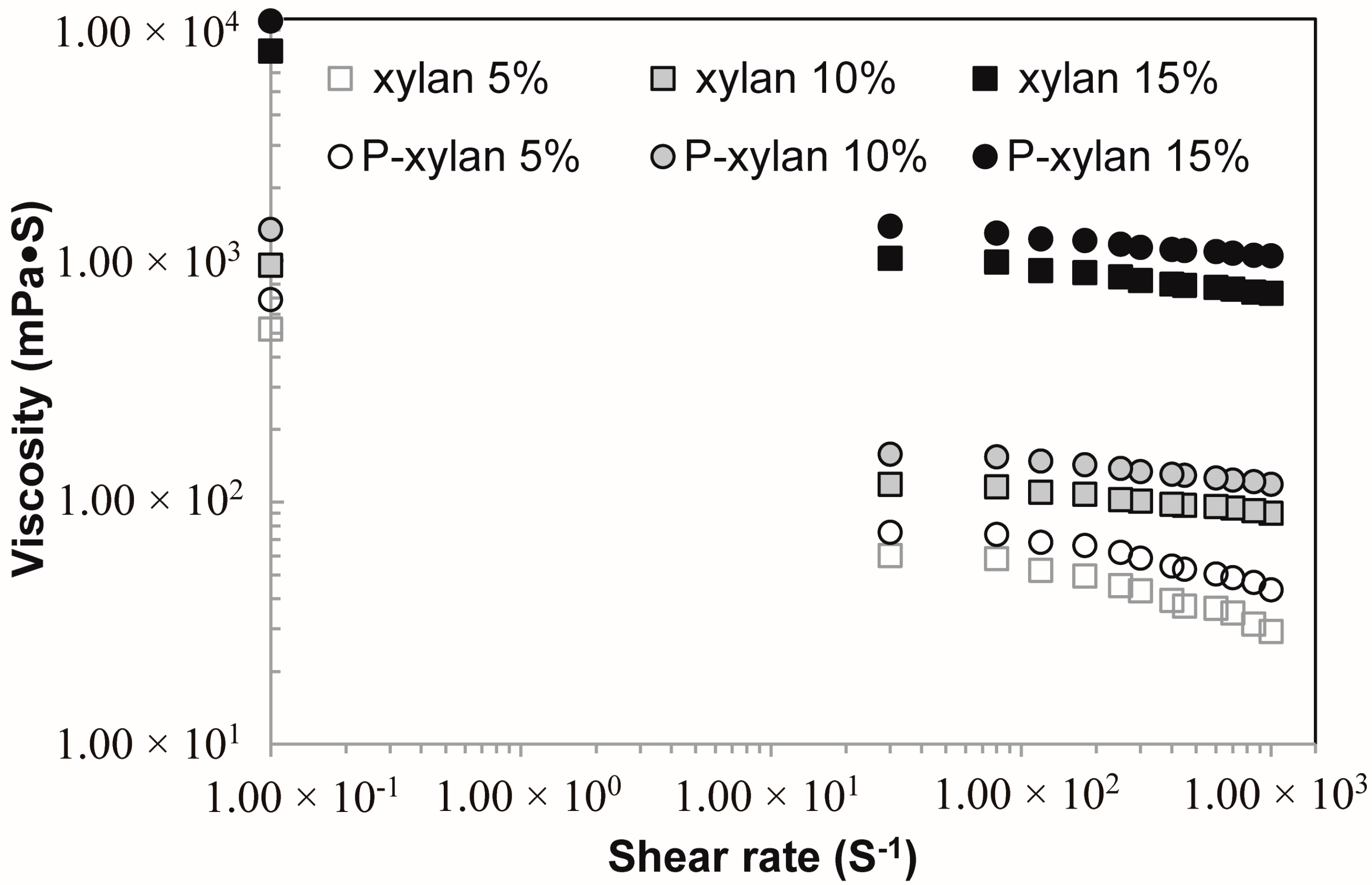
3.7. Viscosity Analysis of Xylan and Phophorylated Xylan Solutions
3.8. Flocculation Analysis
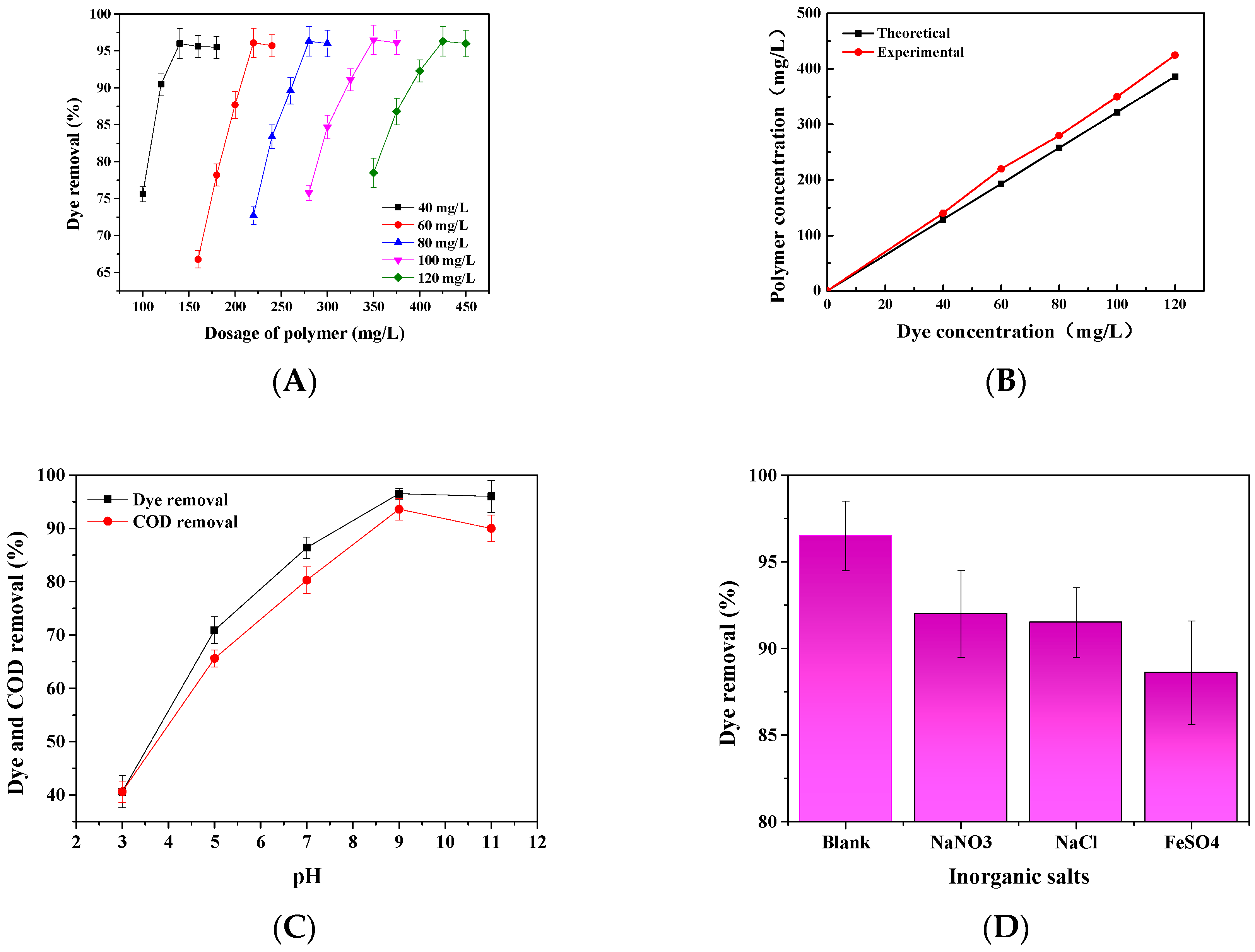
3.8.1. The Effect of Dye Concentrations and Phosphorylated Xylan Concentrations on Dye Removal
3.8.2. The Effect of pH on Dye and COD Removal
3.8.3. Inorganic Salts
3.9. Industrial Applicability
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hettrich, K.; Drechsler, U.; Loth, F.; Volkert, B. Preparation and characterization of water-soluble xylan ethers. Polymers 2017, 9, 129. [Google Scholar] [CrossRef]
- Mao, J.; Abushammala, H.; Hettegger, H.; Rosenau, T.; Laborie, M.P.; Mao, J.; Abushammala, H.; Hettegger, H.; Rosenau, T.; Laborie, M.P. Imidazole, a new tunable reagent for producing nanocellulose, part I: Xylan-coated CNCs and CNFs. Polymers 2017, 9, 473. [Google Scholar] [CrossRef]
- Bai, Y.-Y.; Xiao, L.-P.; Sun, R.-C. Microwave-assisted conversion of biomass derived hemicelluloses into xylo-oligosaccharides by novel sulfonated bamboo-based catalysts. Biomass Bioenergy 2015, 75, 245–253. [Google Scholar] [CrossRef]
- Daus, S.; Heinze, T. Xylan-based nanoparticles: Prodrugs for ibuprofen release. Macromol. Biosci. 2010, 10, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Hu, J.; Wei, L.; Du, Y.; Shi, X.; Zhang, L. Antioxidant and antimicrobial activity of maillard reaction products from xylan with chitosan/chitooligomer/glucosamine hydrochloride/taurine model systems. Food Chem. 2014, 148, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Chaa, L.; Joly, N.; Lequart, V.; Faugeron, C.; Mollet, J.-C.; Martin, P.; Morvan, H. Isolation, characterization and valorization of hemicelluloses from aristida pungens leaves as biomaterial. Carbohydr. Polym. 2008, 74, 597–602. [Google Scholar] [CrossRef]
- Willför, S.; Sundberg, K.; Tenkanen, M.; Holmbom, B. Spruce-derived mannans—A potential raw material for hydrocolloids and novel advanced natural materials. Carbohydr. Polym. 2008, 72, 197–210. [Google Scholar] [CrossRef]
- Marcelino, H.R.; Silva, A.E.D.; Gomes, M.C.S.; Oliveira, E.E.; Nagashima-Junior, T.; Pinheiro, G.S.; Silva, A.E.; Timoteo, A.R.D.S.; Agnez-Lima, L.F.; Ayala, A.P.; et al. Leads from physical, chemical, and thermal characterization on cytotoxic effects of xylan-based microparticles. Polymers 2015, 7, 2304–2315. [Google Scholar] [CrossRef]
- Wang, S.Y.; Li, H.L.; Ren, J.L.; Liu, C.F.; Peng, F.; Sun, R.C. Preparation of xylan citrate—A potential adsorbent for industrial wastewater treatment. Carbohydr. Polym. 2013, 92, 1960–1965. [Google Scholar]
- Kong, F.; Wang, S.; Price, J.T.; Konduri, M.K.; Fatehi, P. Water soluble kraft lignin–acrylic acid copolymer: Synthesis and characterization. Green Chem. 2015, 17, 4355–4366. [Google Scholar] [CrossRef]
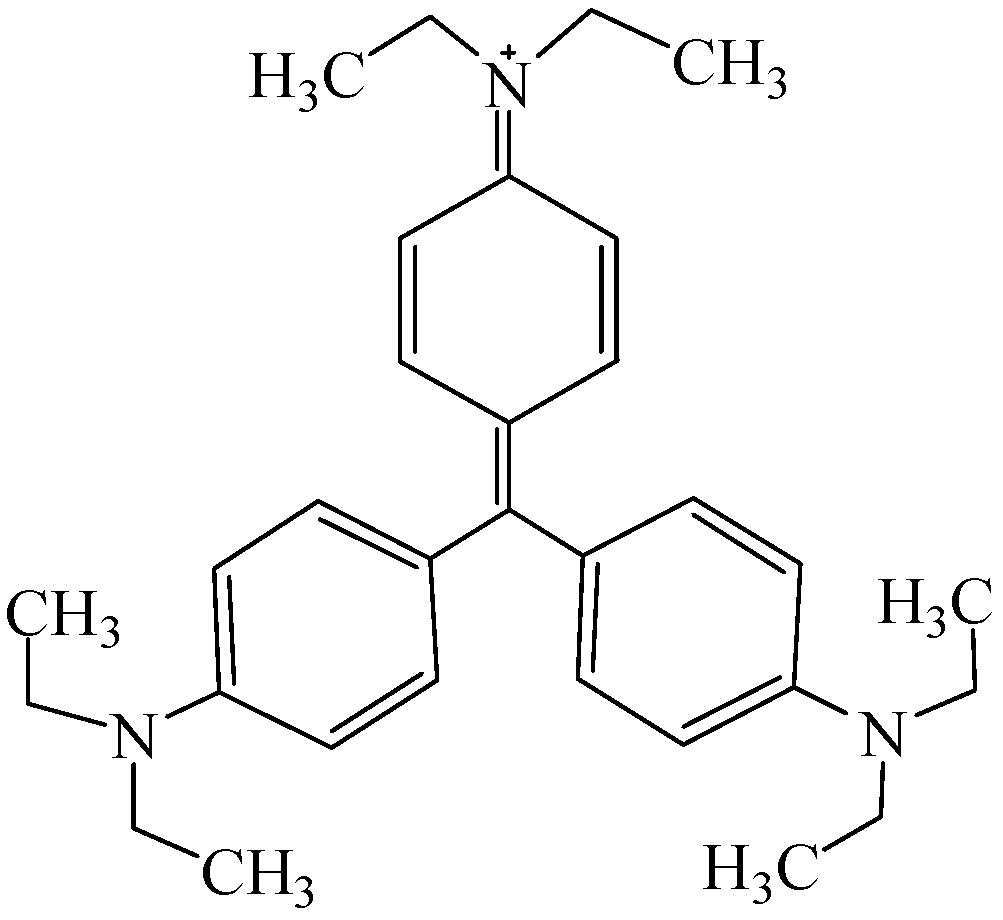
- Chen, C.Y.; Yen, S.H.; Chung, Y.C. Combination of photoreactor and packed bed bioreactor for the removal of ethyl violet from wastewater. Chemosphere 2014, 117, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.L.W.; Lu, C.S.; Lin, H.P.; Chen, J.Y.; Chen, C.C. Photocatalytic degradation of ethyl violet dye mediated by TiO2 under an anaerobic condition. J. Taiwan Inst. Chem. Eng. 2014, 45, 2469–2479. [Google Scholar] [CrossRef]
- Wang, N.; Feng, Z.; Ma, X.; Zheng, P. The modification of rectorite with carbon layers and trisodium trimetaphosphate for the removal of Pb2+. Appl. Clay Sci. 2017, 146, 115–121. [Google Scholar] [CrossRef]
- Igura, M.; Okazaki, M. Cadmium sorption characteristics of phosphorylated sago starch-extraction residue. J. Hazard. Mater. 2010, 178, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Dulong, V.; Lack, S.; Cerf, D.L.; Picton, L.; Vannier, J.P.; Muller, G. Hyaluronan-based hydrogels particles prepared by crosslinking with trisodium trimetaphosphate. Synthesis and characterization. Carbohydr. Polym. 2004, 57, 1–6. [Google Scholar] [CrossRef]
- Zhang, M.; Su, N.; Zhang, Q.; Wang, Y.; Li, J.; Ye, M. Phosphorylation and antiaging activity of polysaccharide from trichosanthes peel. J. Food Drug Anal. 2017, 25, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Ming, K.; Chen, Y.; Yao, F.; Shi, J.; Yang, J.; Du, H.; Wang, X.; Wang, Y.; Liu, J. Phosphorylated codonopsis pilosula polysaccharide could inhibit the virulence of duck hepatitis a virus compared with codonopsis pilosula polysaccharide. Int. J. Biol. Macromol. 2017, 94, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ni, Y.; Hu, X.; Li, Q. Effect of phosphorylation on antioxidant activities of pumpkin (Cucurbita pepo, Lady godiva) polysaccharide. Int. J. Biol. Macromol. 2015, 81, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Ghimici, L.; Suflet, D.M. Phosphorylated polysaccharide derivatives as efficient separation agents for zinc and ferric oxides particles from water. Sep. Purif. Technol. 2015, 144, 31–36. [Google Scholar] [CrossRef]
- Ke, M.; Yun, C.; Shi, J.; Yang, J.; Yao, F.; Du, H.; Wei, Z.; Bai, J.; Liu, J.; Wang, D. Effects of chrysanthemum indicum polysaccharide and its phosphate on anti-duck hepatitis a virus and alleviating hepatic injury. Int. J. Biol. Macromol. 2017, 102, 813–821. [Google Scholar]
- Rao, M.R.P.; Warrier, D.U.; Gaikwad, S.R.; Shevate, P.M. Phosphorylation of psyllium seed polysaccharide and its characterization. Int. J. Biol. Macromol. 2016, 85, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Woggum, T.; Sirivongpaisal, P.; Wittaya, T. Properties and characteristics of dual-modified rice starch based biodegradable films. Int. J. Biol. Macromol. 2014, 67, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Dulong, V.; Forbice, R.; Condamine, E.; Cerf, D.L.; Picton, L. Pullulan–stmp hydrogels: A way to correlate crosslinking mechanism, structure and physicochemical properties. Polym. Bull. 2011, 67, 455–466. [Google Scholar] [CrossRef]
- Konduri, M.K.; Fatehi, P. Synthesis and characterization of carboxymethylated xylan and its application as a dispersant. Carbohydr. Polym. 2016, 146, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Fu, H.; Xu, J.; Shang, J.; Cheng, Y. Physiochemical and biological properties of phosphorylated polysaccharides from dictyophora indusiata. Int. J. Biol. Macromol. 2015, 72, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Bhumkar, D.R.; Pokharkar, V.B. Studies on effect of ph on cross-linking of chitosan with sodium tripolyphosphate: A technical note. Aaps Pharmscitech 2006, 7, E138–E143. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhang, Y.; Fatehi, P. Sulfomethylated kraft lignin as a flocculant for cationic dye. Colloids Surf. A Physicochem. Eng. Asp. 2016, 503, 19–27. [Google Scholar] [CrossRef]
- Soyeon, J.; Jaewon, L. Optimization of pretreatment condition for ethanol production from oxalic acid pretreated biomass by response surface methodology. Ind. Crops Prod. 2016, 79, 1–6. [Google Scholar]
- Wei, D.; Cheng, W.; Wei, Y.; Zhang, L. Phosphorylated modification and in vitro antioxidant activity of radix hedysari polysaccharide. Glycoconj. J. 2012, 29, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Peng, F.; Sun, R.; Liu, C.; Cao, Z.; Luo, W.; Tang, J. Synthesis of cationic hemicellulosic derivatives with a low degree of substitution in dimethyl sulfoxide media. J. Appl. Polym. Sci. 2008, 109, 2711–2717. [Google Scholar] [CrossRef]
- Kurita, O.; Fujiwara, T.; Yamazaki, E. Characterization of the pectin extracted from citrus peel in the presence of citric acid. Carbohydr. Polym. 2008, 74, 725–730. [Google Scholar] [CrossRef]
- Hansen, N.M.; Plackett, D. Synthesis and characterization of birch wood xylan succinoylated in 1-n-butyl-3-methylimidazolium chloride. Polym. Chem. 2011, 2, 2010–2020. [Google Scholar] [CrossRef]
- Peng, P.; Zhai, M.; She, D.; Gao, Y. Synthesis and characterization of carboxymethyl xylan-g-poly(propylene oxide) and its application in films. Carbohydr. Polym. 2015, 133, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, C.; Zhang, A.; Sun, R.; Zhang, X.; Liu, C.; Zhang, A.; Sun, R.; Zhang, X.; Liu, C. Organic catalysis for ring-opening graft polymerization of p-dioxanone with xylan in ionic liquid. Polymers 2017, 9, 345. [Google Scholar] [CrossRef]
- Peng, X.-W.; Ren, J.-L.; Zhong, L.-X.; Peng, F.; Sun, R.-C. Xylan-rich hemicelluloses-graft-acrylic acid ionic hydrogels with rapid responses to PH, salt, and organic solvents. J. Agric. Food Chem. 2011, 59, 8208–8215. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Shao, L. Phosphorylation of corn starch in an ionic liquid. Starch Stärke 2009, 61, 702–708. [Google Scholar] [CrossRef]
- CH, Ü.; Kutlu, M.; Atıcı, O.G. Mannich reaction of polysaccharides: Xylan functionalization in aqueous basic medium. Carbohydr. Polym. 2015, 127, 19–27. [Google Scholar]
- Xin, X.; Xu, G.; Gong, H.; Bai, Y.; Tan, Y. Interaction between sodium oleate and partially hydrolyzed polyacrylamide: A rheological study. Colloids Surf. A Physicochem. Eng. Asp. 2008, 326, 1–9. [Google Scholar] [CrossRef]
- Patruyo, L.; Müller, A.; Saez, A. Shear and extensional rheology of solutions of modified hydroxyethyl celluloses and sodium dodecyl sulfate. Polymer 2002, 43, 6481–6493. [Google Scholar] [CrossRef]
- Fang, R.; Cheng, X.S.; Xu, X.R. Synthesis of lignin-base cationic flocculant and its application in removing anionic azo-dyes from simulated wastewater. Bioresour. Technol. 2010, 101, 7323–7329. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Bajpai, M. The flocculation performance of tamarindus mucilage in relation to removal of vat and direct dyes. Bioresour. Technol. 2006, 97, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Guibal, E.; Roussy, J. Coagulation and flocculation of dye-containing solutions using a biopolymer (chitosan). React. Funct. Polym. 2007, 67, 33–42. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, H.; Jiang, Z.; Cai, T.; Li, H.; Li, H.; Li, A.; Cheng, R. Flocculation of both anionic and cationic dyes in aqueous solutions by the amphoteric grafting flocculant carboxymethyl chitosan-graft-polyacrylamide. J. Hazard. Mater. 2013, 255, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Bajpai, M. Flocculation behaviour of model textile wastewater treated with a food grade polysaccharide. J. Hazard. Mater. 2005, 118, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fatehi, P.; Ni, Y. Adsorption of lignocelluloses dissolved in prehydrolysis liquor of kraft-based dissolving pulp process on oxidized activated carbons. Ind. Eng. Chem. Res. 2011, 50, 11706–11711. [Google Scholar] [CrossRef]
- Liu, X.; Fatehi, P.; Ni, Y. Removal of inhibitors from pre-hydrolysis liquor of kraft-based dissolving pulp production process using adsorption and flocculation processes. Bioresour. Technol. 2012, 116, 492–496. [Google Scholar] [CrossRef] [PubMed]

| Run | Xylan/STMP, mol/mol | Temperature, °C | Time, h | Phosphorus Content, % | DS | Charge Density, mmol/g |
|---|---|---|---|---|---|---|
| 1 | 1/3 | 70.00 | 4.00 | 10.41 | 0.76 | 3.30 |
| 2 | 1/3 | 70.00 | 4.00 | 10.41 | 0.76 | 3.30 |
| 3 | 1/3 | 70.00 | 4.00 | 10.41 | 0.76 | 3.30 |
| 4 | 1/5 | 70.00 | 2.00 | 2.53 | 0.12 | 0.92 |
| 5 | 1/3 | 70.00 | 4.00 | 10.41 | 0.76 | 3.30 |
| 6 | 1/1 | 50.00 | 4.00 | 5.21 | 0.28 | 1.62 |
| 7 | 1/3 | 50.00 | 6.00 | 5.64 | 0.31 | 1.81 |
| 8 | 1/5 | 90.00 | 4.00 | 8.82 | 0.58 | 2.85 |
| 9 | 1/3 | 90.00 | 2.00 | 1.75 | 0.08 | 0.65 |
| 10 | 1/3 | 70.00 | 4.00 | 10.41 | 0.76 | 3.30 |
| 11 | 1/1 | 90.00 | 4.00 | 5.06 | 0.27 | 1.62 |
| 12 | 1/5 | 70.00 | 6.00 | 8.41 | 0.54 | 2.76 |
| 13 | 1/3 | 90.00 | 6.00 | 8.10 | 0.51 | 2.64 |
| 14 | 1/1 | 70.00 | 2.00 | 1.33 | 0.06 | 0.49 |
| 15 | 1/1 | 70.00 | 6.00 | 4.44 | 0.23 | 1.45 |
| 16 | 1/5 | 50.00 | 4.00 | 6.19 | 0.35 | 1.97 |
| 17 | 1/3 | 50.00 | 2.00 | 0.91 | 0.04 | 0.32 |
| Source | Sum of Squares | Mean Square | F-Value | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| DS | Charge Density, mmol/g | DS | Charge Density, mmol/g | DS | Charge Density, mmol/g | DS | Charge Density, mmol/g | |
| Model | 1.20 | 18.66 | 0.13 | 2.07 | 592.23 | 204.97 | 0.0001 | 0.0001 |
| xylan/STMP (A) | 0.070 | 1.38 | 0.070 | 1.38 | 312.50 | 136.22 | 0.0001 | 0.0001 |
| Temperature (B) | 0.026 | 0.52 | 0.026 | 0.52 | 117.56 | 51.43 | 0.0001 | 0.0002 |
| Time (C) | 0.21 | 4.93 | 0.21 | 4.93 | 924.50 | 487.41 | 0.0001 | 0.0001 |
| AB | 0.014 | 0.19 | 0.014 | 0.19 | 64.00 | 19.14 | 0.0001 | 0.0033 |
| AC | 0.016 | 0.19 | 0.016 | 0.19 | 69.44 | 19.14 | 0.0001 | 0.0033 |
| BC | 0.006 | 0.063 | 0.006 | 0.063 | 28.44 | 6.18 | 0.0011 | 0.0419 |
| A2 | 0.16 | 1.61 | 0.16 | 1.61 | 702.49 | 158.74 | 0.0001 | 0.0001 |
| B2 | 0.16 | 1.88 | 0.16 | 1.88 | 720.73 | 185.48 | 0.0001 | 0.0001 |
| C2 | 0.46 | 6.87 | 0.46 | 6.87 | 2022.49 | 679.40 | 0.0001 | 0.0001 |
| Samples | Charge Density (mmol/g) | Mw (g/mol) | Mn (g/mol) | Mw/Mn | Degree of Polymerization |
|---|---|---|---|---|---|
| Unmodified Xylan | −0.12 | 20,800 | 11,850 | 1.76 | 90 |
| Phosphorylated Xylan | −3.40 | 23,500 | 18,600 | 1.26 | 81 |
| Samples | P, wt % | C, wt % | H, wt % | O, wt % | Formula |
|---|---|---|---|---|---|
| Xylan | 0.03 | 42.50 | 5.80 | 47.10 | C5H8.22O4.13P0.001 |
| Phosphorylated xylan | 31.81 | 26.22 | 3.15 | 70.90 | C5H7.21O10.13P2.34 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Xu, D.; Xia, N.; Zhao, X.; Kong, F.; Wang, S.; Fatehi, P. Preparation and Application of Phosphorylated Xylan as a Flocculant for Cationic Ethyl Violet Dye. Polymers 2018, 10, 317. https://doi.org/10.3390/polym10030317
Liu Z, Xu D, Xia N, Zhao X, Kong F, Wang S, Fatehi P. Preparation and Application of Phosphorylated Xylan as a Flocculant for Cationic Ethyl Violet Dye. Polymers. 2018; 10(3):317. https://doi.org/10.3390/polym10030317
Chicago/Turabian StyleLiu, Zhongming, Dingding Xu, Nannan Xia, Xin Zhao, Fangong Kong, Shoujuan Wang, and Pedram Fatehi. 2018. "Preparation and Application of Phosphorylated Xylan as a Flocculant for Cationic Ethyl Violet Dye" Polymers 10, no. 3: 317. https://doi.org/10.3390/polym10030317