Preparation of the Yellow-Colored Aluminum Pigments with Double-Layer Structure Using a Crosslinked Copolymeric Dye
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of FGMAC
2.3. Preparation of PFMV
2.4. Preparation of Al@SiO2@PFMV
2.5. Characterization
3. Results and Discussion
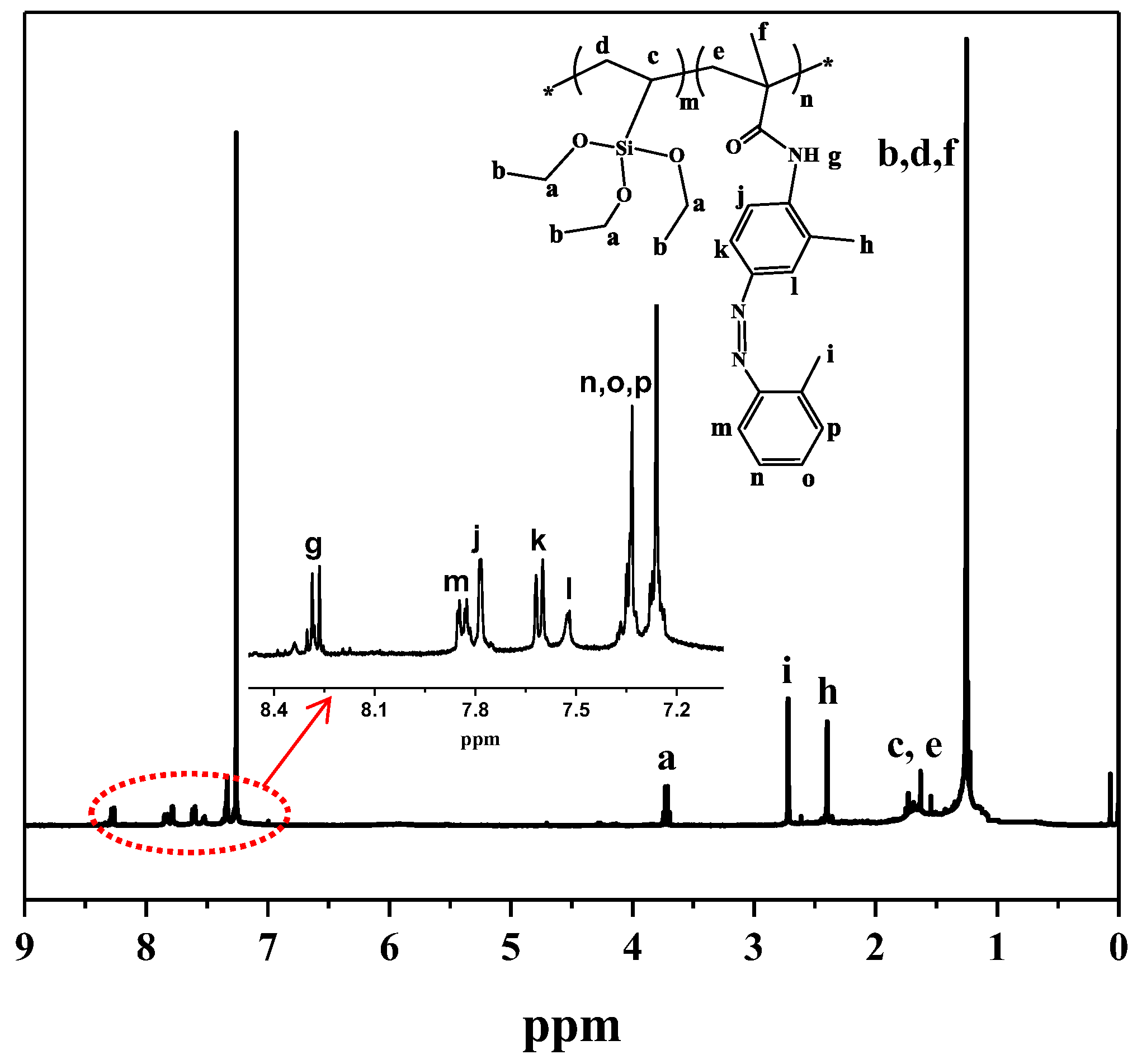
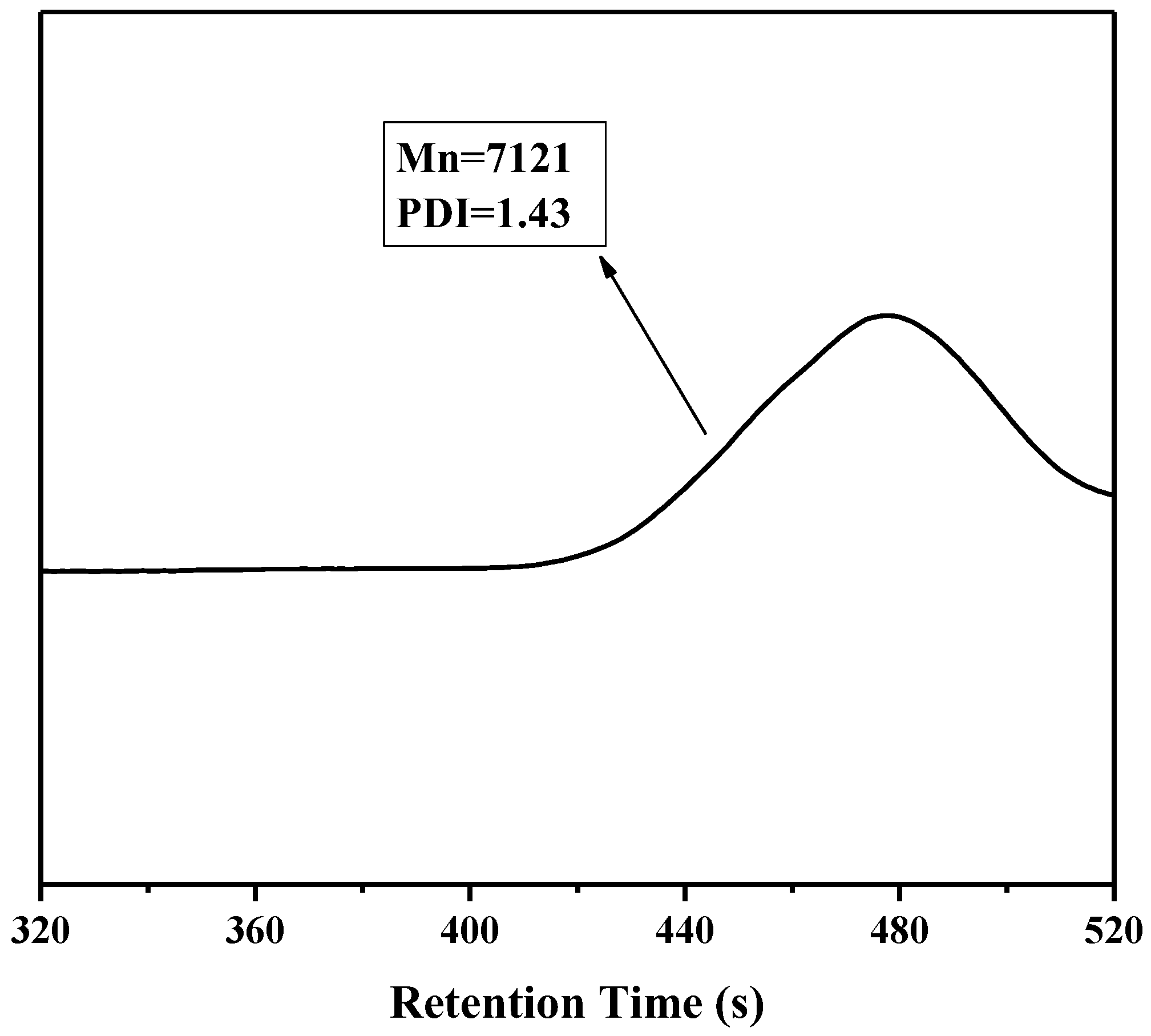
3.1. Preparation and Characterization of PFMV
3.2. Preparation and Characterization of the Al@SiO2@PFMV
3.2.1. FTIR Analysis
3.2.2. XPS Analysis
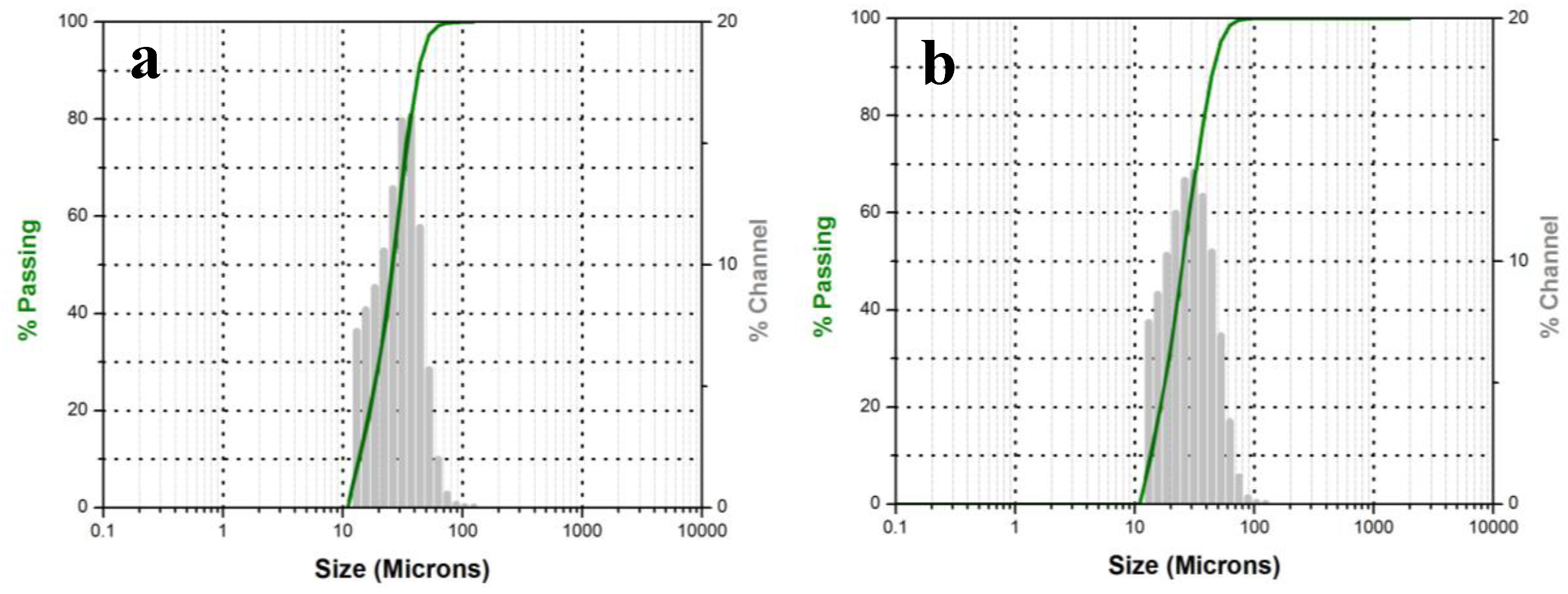
3.2.3. Particle Size Analysis
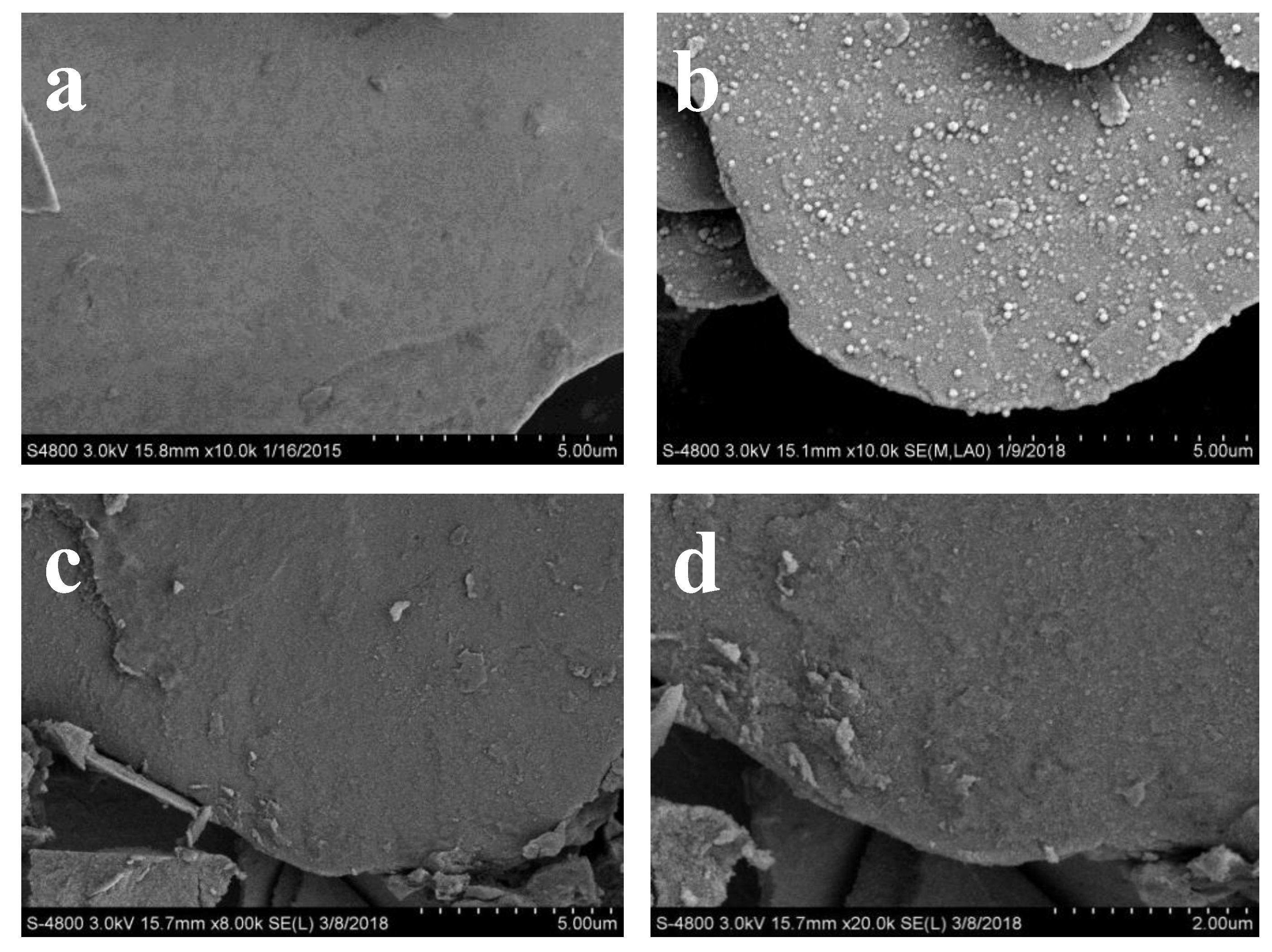
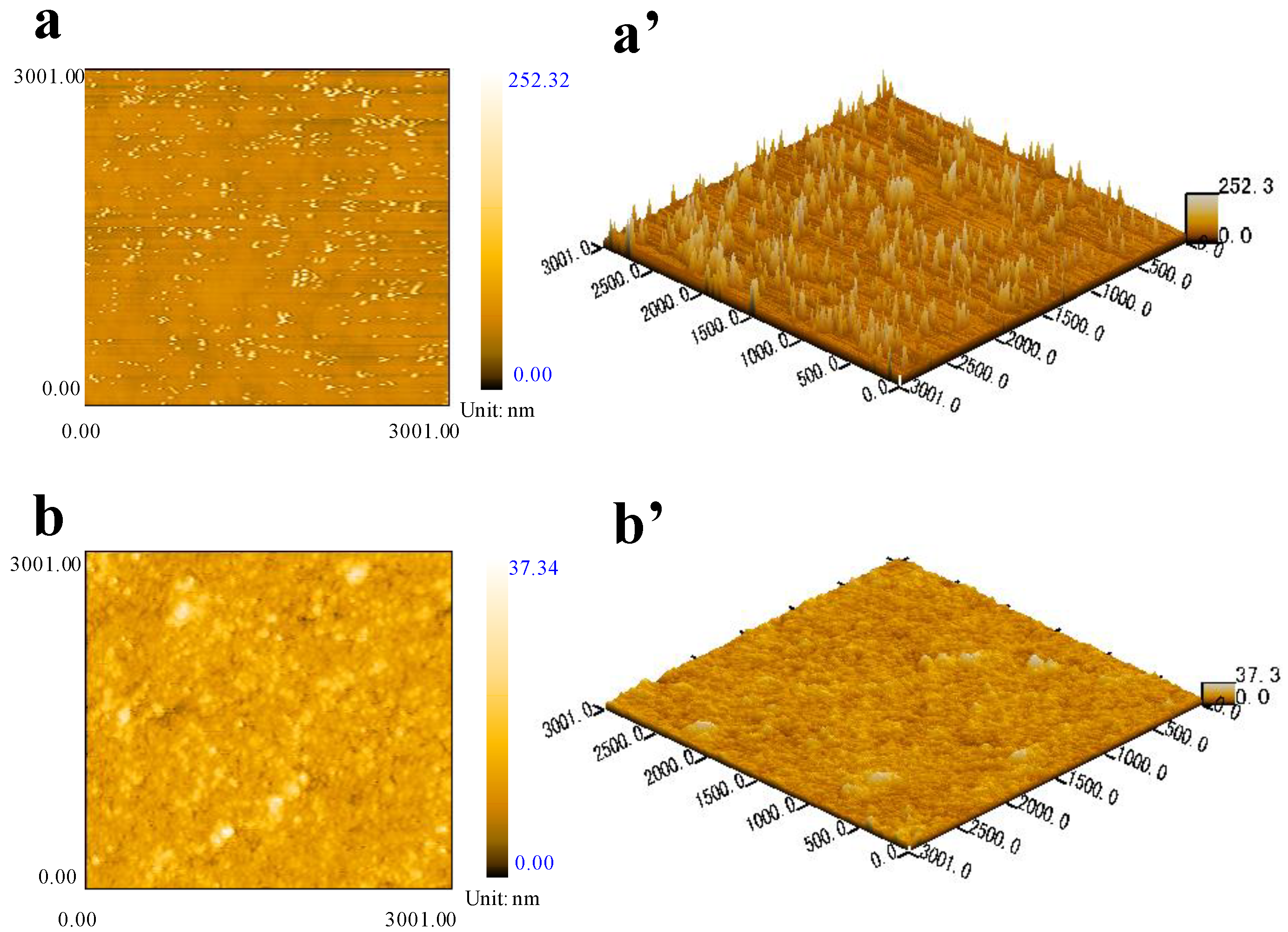
3.2.4. Morphologies of the Aluminum Pigments
3.2.5. TG Analysis
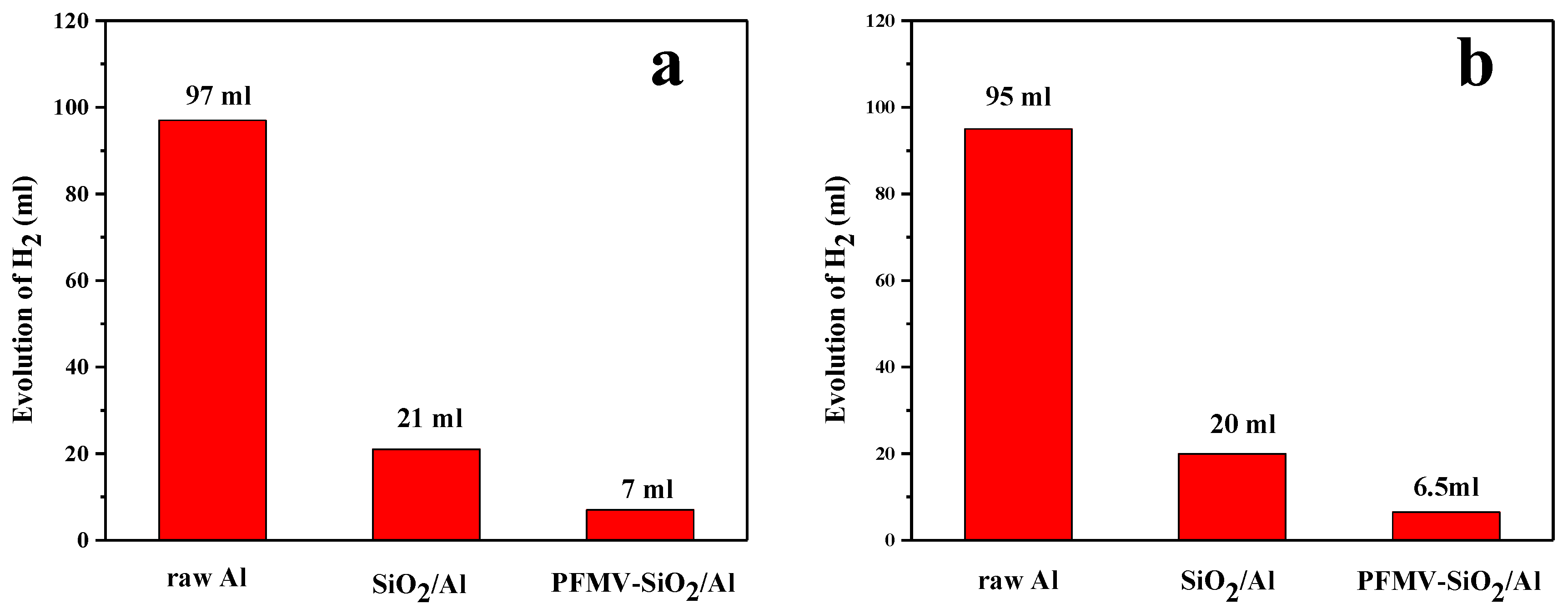
3.3. Corrosion Resistance Tests
3.4. Chromatism Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ma, Z.L.; Li, C.C.; Wei, H.M.; Ding, D.Q. Silica sol-gel anchoring on aluminum pigments surface for corrosion resistance based on aluminum oxidized by hydrogen peroxide. Dyes Pigment. 2015, 113, 730–736. [Google Scholar] [CrossRef]
- Du, B.; Zhou, S.S.; Li, N.L. Research progress of coloring aluminum pigments by corrosion protection method. Procedia Environ. Sci. 2011, 10, 807–813. [Google Scholar] [CrossRef]
- Wang, K.; Wang, C.; Yin, Y.; Chen, K. Modification of Al pigment with graphene for infrared/visual stealth compatible fabric coating. J. Alloy. Compd. 2017, 690, 741–748. [Google Scholar] [CrossRef]
- Liu, H.; Ye, H.; Tang, X. Aluminum pigment encapsulated by in situ copolymerization of styrene and maleic acid. Appl. Surf. Sci. 2007, 254, 616–620. [Google Scholar] [CrossRef]
- Karlsson, P.; Palmqvist, A.E.C.; Holmberg, K. Surface modification for aluminium pigment inhibition. Adv. Colloid Interface Sci. 2006, 128, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, P.M.; Baeza, A.; Palmqvist, A.E.C.; Holmberg, K. Surfactant inhibition of aluminium pigments for waterborne printing inks. Corros. Sci. 2008, 50, 2282–2287. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, H.; Liu, H.; Han, K. Preparation and characterisation of aluminium pigments coated with silica for corrosion protection. Corros. Sci. 2011, 53, 1694–1699. [Google Scholar] [CrossRef]
- Nikravesh, B.; Ramezanzadeh, B.; Sarabi, A.A.; Kasiriha, S.M. Evaluation of the corrosion resistance of an epoxy-polyamide coating containing different ratios of micaceous iron oxide/Al pigments. Corros. Sci. 2011, 53, 1592–1603. [Google Scholar] [CrossRef]
- Maayta, A.K.; Al-Rawashdeh, N.A.F. Inhibition of acidic corrosion of pure aluminum by some organic compounds. Corros. Sci. 2004, 46, 1129–1140. [Google Scholar] [CrossRef]
- Muller, B. Citric acid as corrosion inhibitor for aluminium pigment. Corros. Sci. 2004, 46, 159–167. [Google Scholar] [CrossRef]
- Mueller, B.; Fischer, S. Epoxy ester resins as corrosion inhibitors for aluminium and zinc pigments. Corros. Sci. 2006, 48, 2406–2416. [Google Scholar] [CrossRef]
- El-Etre, A.Y. Inhibition of acid corrosion of aluminum using vanillin. Corros. Sci. 2001, 43, 1031–1039. [Google Scholar] [CrossRef]
- Kiehl, A.; Greiwe, K. Encapsulated aluminium pigments. Prog. Org. Coat. 1999, 37, 179–183. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, S.L.; Kong, J.R.; Zhou, T.; Zuo, Y.J. Characterization of flaky aluminum pigments multi-coated by TiO2 and SiO2. Powder Technol. 2013, 237, 514–519. [Google Scholar] [CrossRef]
- Liu, H.; Ye, H.; Zhang, Y. Preparation and characterization of PMMA/flaky aluminum composite particle in the presence of MPS. Colloid. Surf. A 2008, 315, 1–6. [Google Scholar] [CrossRef]
- Liu, H.; Ye, H.; Zhang, Y.; Tang, X. Preparation and characterization of poly(trimethylolpropanetriacrylate)/flaky aluminum composite particle by in situ polymerization. Dyes Pigment. 2008, 79, 236–241. [Google Scholar] [CrossRef]
- Karlsson, P.M.; Esbjörnsson, N.B.; Holmberg, K. Admicellar polymerization of methyl methacrylate on aluminum pigments. J. Colloid Interface Sci. 2009, 337, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Batzilla, T.; Tulke, A. Preparation of encapsulated aluminum pigments by emulsion polymerization and their characterization. J. Coat. Technol. 1998, 70, 77–83. [Google Scholar] [CrossRef]
- Li, L.; Pi, P.; Wen, X.; Cheng, J.; Yang, Z. Optimization of sol-gel coatings on the surface of aluminum pigments for corrosion protection. Corros. Sci. 2008, 50, 795–803. [Google Scholar] [CrossRef]
- Zhu, H.; Qu, X.; Hu, Y.; Xie, H.; Chen, Z. Corrosion inhibition of flaky aluminium powders prepared through sol-gel process. Corros. Sci. 2011, 53, 481–486. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, Z.; Sheng, Y.; Thi, T.T.L. Flaky polyacrylic acid/aluminium composite particles prepared using in-situ polymerization. Dyes Pigment. 2010, 86, 155–160. [Google Scholar] [CrossRef]
- Joubert, M.; Save, M.; Mornet, S.; Lavaud, F.; Pellerin, V.; Morvan, F.; Tranchant, J.F.; Duguet, E.; Billon, L. Surface patterning of micron-sized aluminum flakes by seeded dispersion polymerization: Towards waterborne colored pigments by gold nanoparticles adsorption. Polymer 2014, 55, 762–771. [Google Scholar] [CrossRef]
- He, Y.; Li, H.; Ou, L.; Ding, F.; Zhan, Z.; Zhong, Y. Preparation and characterisation of water-based aluminium pigments modified with SiO2 and polymer brushes. Corros. Sci. 2016, 111, 802–810. [Google Scholar] [CrossRef]
- Tawiah, B.; Asinyo, B.K.; Badoe, W.; Zhang, L.; Fu, S. Phthalocyanine green aluminum pigment prepared by inorganic acid radical/radical polymerization for waterborne textile applications. Int. J. Ind. Chem. 2016, 8, 1–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, H.; Liu, H.; Han, K. Preparation and characterization of colored aluminum pigments Al/SiO2/Fe2O3 with double-layer structure. Powder Technol. 2012, 229, 206–213. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, H.; Liu, H. Preparation and characterization of blue color aluminum pigments Al/SiO2/PB with double-layer structure. Powder Technol. 2012, 217, 614–618. [Google Scholar] [CrossRef]
- Tawiah, B.; Narh, C.; Li, M.; Zhang, L.; Fu, S. Polymer-encapsulated colorful Al pigment with high NIR and UV reflectance and their application in textiles. Ind. Eng. Chem. Res. 2015, 54, 11858–11865. [Google Scholar] [CrossRef]
- Joubert, M.; Khoukh, A.; Tranchant, J.F.; Morvan, F.; Billon, L. Hybrid aluminum colored pigments based on gradient copolymers design. Macromol. Chem. Phys. 2009, 210, 1544–1555. [Google Scholar] [CrossRef]
- Altomare, A.; Ciardelli, F.; Faralli, G.; Solaro, R. Synthesis of reactive polymeric dyes as textile auxiliaries. Macromol. Mater. Eng. 2003, 288, 679–692. [Google Scholar] [CrossRef]
- Tawiah, B.; Zhang, L.; Tian, A.; Fu, S.S. Coloration of aluminum pigment using SiO2 and γ-glycidoxypropyltrimethoxysilane with dichlorotriazine reactive dye. Pigment Resin Technol. 2016, 45, 335–345. [Google Scholar] [CrossRef]







| Sample | Elemental Contents (PP, at%) | ||||
|---|---|---|---|---|---|
| Al | C | O | Si | N | |
| raw Al | 49.78 | 25.4 | 24.82 | / | / |
| Al@SiO2 | 3.93 | 12.25 | 42.6 | 41.22 | / |
| Al@SiO2@PFMV | 3.74 | 40.02 | 24.83 | 24.38 | 7.03 |
| Samples | Rq (nm) | Ra (nm) | Rmax (nm) |
|---|---|---|---|
| Al@SiO2 | 9.64 | 9.52 | 233.6 |
| Al@SiO2@PFMV | 1.65 | 1.62 | 33.3 |
| Samples | Aluminum Pigments | Al@SiO2 | Al@SiO2@PFMV | |
|---|---|---|---|---|
| 15° | L* | 159.3 | 136.86 | 111.6 |
| a* | −0.31 | −0.18 | −1.61 | |
| b* | −2.68 | −0.53 | 14.04 | |
| 25° | L* | 109.68 | 109.82 | 94.04 |
| a* | −1.03 | −0.73 | −2 | |
| b* | −1.79 | −0.27 | 15.03 | |
| 45° | L* | 52.35 | 67.04 | 68.64 |
| a* | −0.44 | −0.38 | −1.57 | |
| b* | −1.19 | −0.27 | 14.56 | |
| 75° | L* | 31.73 | 41.27 | 49.5 |
| a* | −0.52 | −0.7 | −1.34 | |
| b* | −0.94 | 0.55 | 16.59 | |
| 110° | L* | 26.22 | 34.11 | 41.51 |
| a* | −0.6 | −0.82 | −1.12 | |
| b* | −1.1 | 0.82 | 18.5 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Nie, S.; Xia, R.; Lv, Y.; Cao, M.; Chen, M.; Dong, Q.; Su, L.; Chen, P.; Yang, B.; et al. Preparation of the Yellow-Colored Aluminum Pigments with Double-Layer Structure Using a Crosslinked Copolymeric Dye. Polymers 2018, 10, 1097. https://doi.org/10.3390/polym10101097
Wang Z, Nie S, Xia R, Lv Y, Cao M, Chen M, Dong Q, Su L, Chen P, Yang B, et al. Preparation of the Yellow-Colored Aluminum Pigments with Double-Layer Structure Using a Crosslinked Copolymeric Dye. Polymers. 2018; 10(10):1097. https://doi.org/10.3390/polym10101097
Chicago/Turabian StyleWang, Zhicheng, Shuping Nie, Ru Xia, Yang Lv, Ming Cao, Ming Chen, Qiannian Dong, Lifen Su, Peng Chen, Bin Yang, and et al. 2018. "Preparation of the Yellow-Colored Aluminum Pigments with Double-Layer Structure Using a Crosslinked Copolymeric Dye" Polymers 10, no. 10: 1097. https://doi.org/10.3390/polym10101097





