Nanoforest: Polyaniline Nanotubes Modified with Carbon Nano-Onions as a Nanocomposite Material for Easy-to-Miniaturize High-Performance Solid-State Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polyaniline Nanotube Matrix Synthesis
2.3. Synthesis of Pristine and Oxidized CNOs
2.4. Methods
3. Results and Discussion
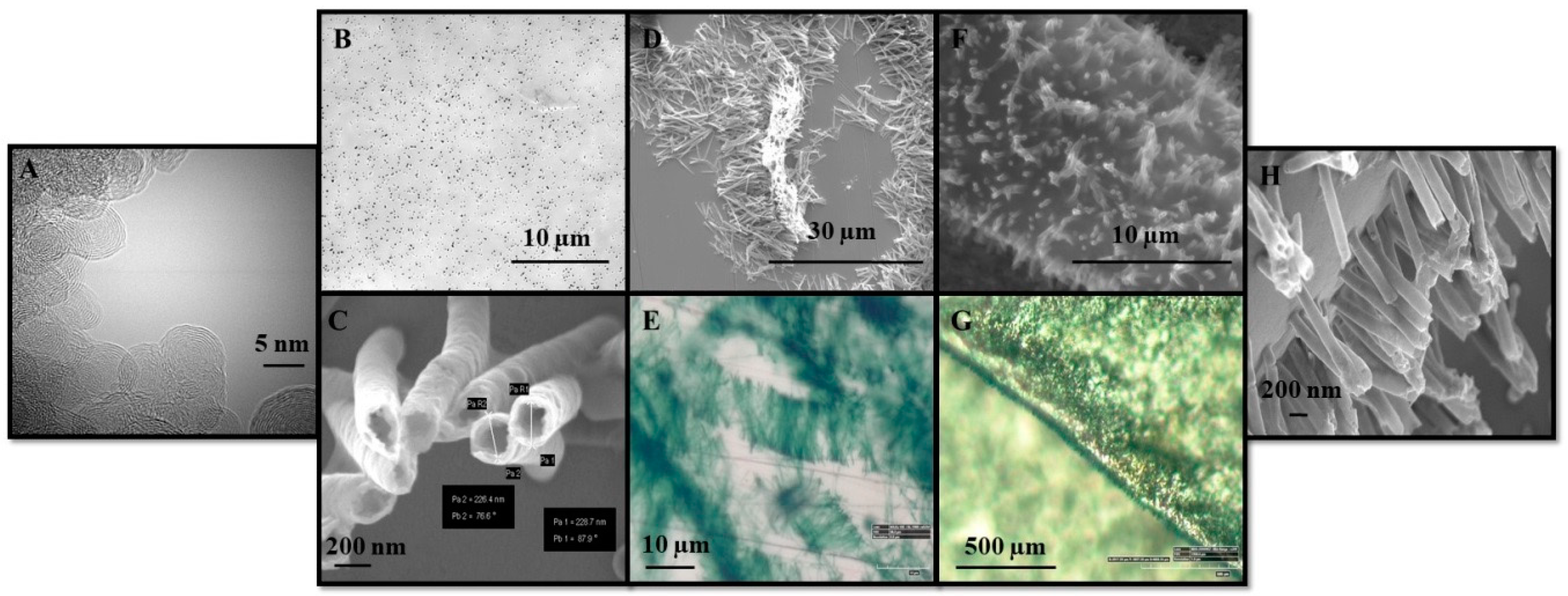
3.1. Nanocomposite PANINT/CNOsox Electrode Preparation Procedure
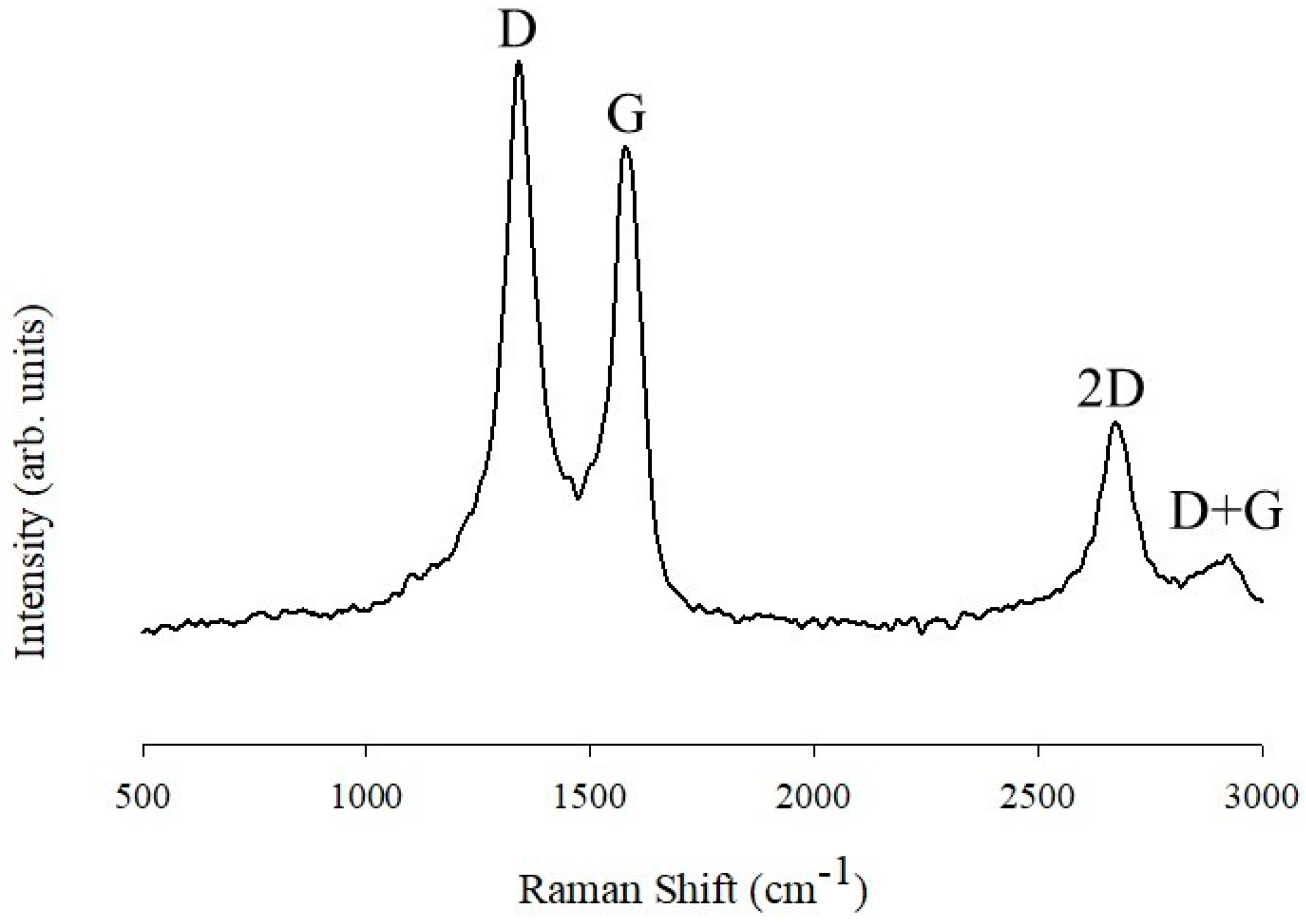
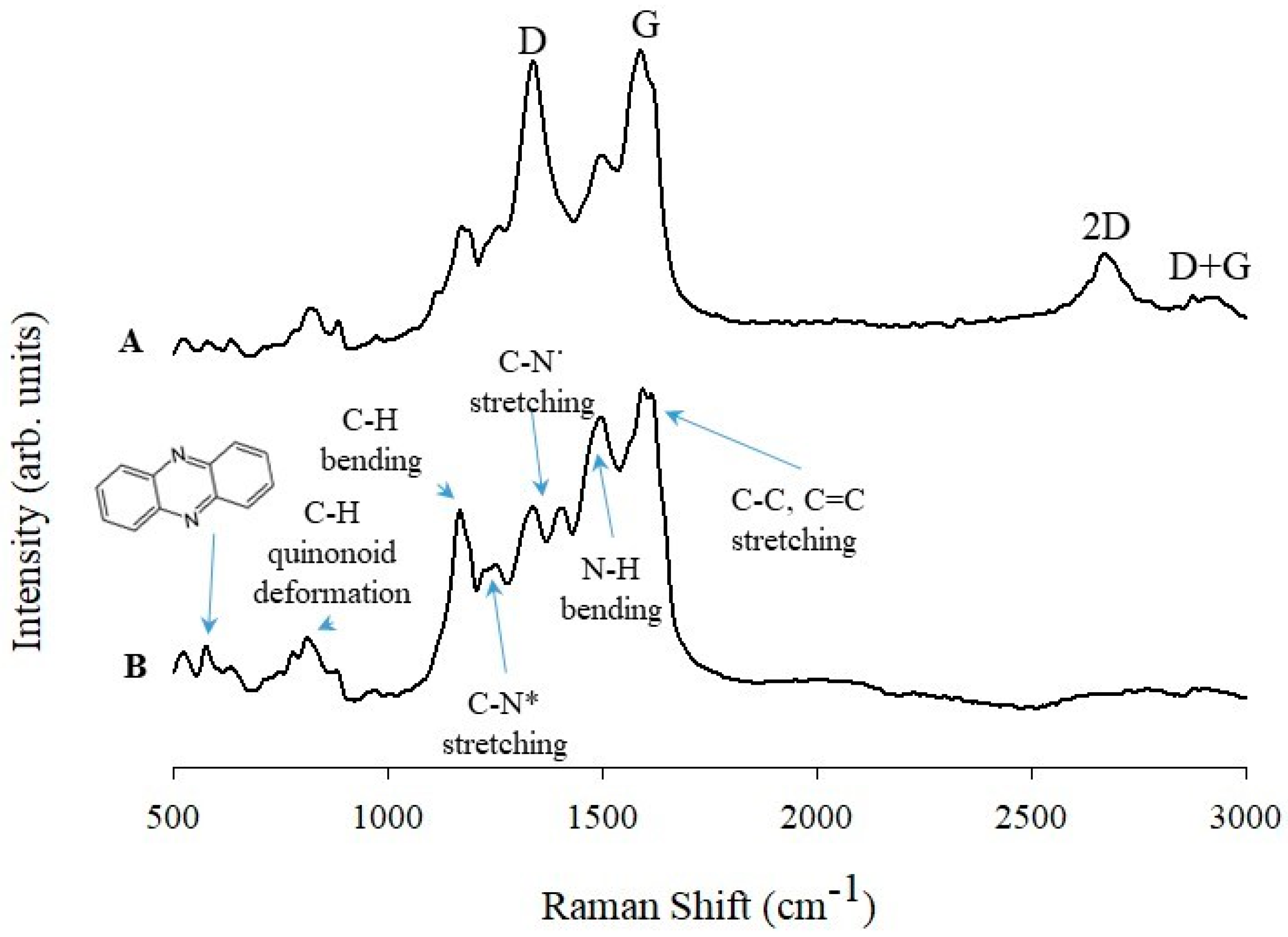
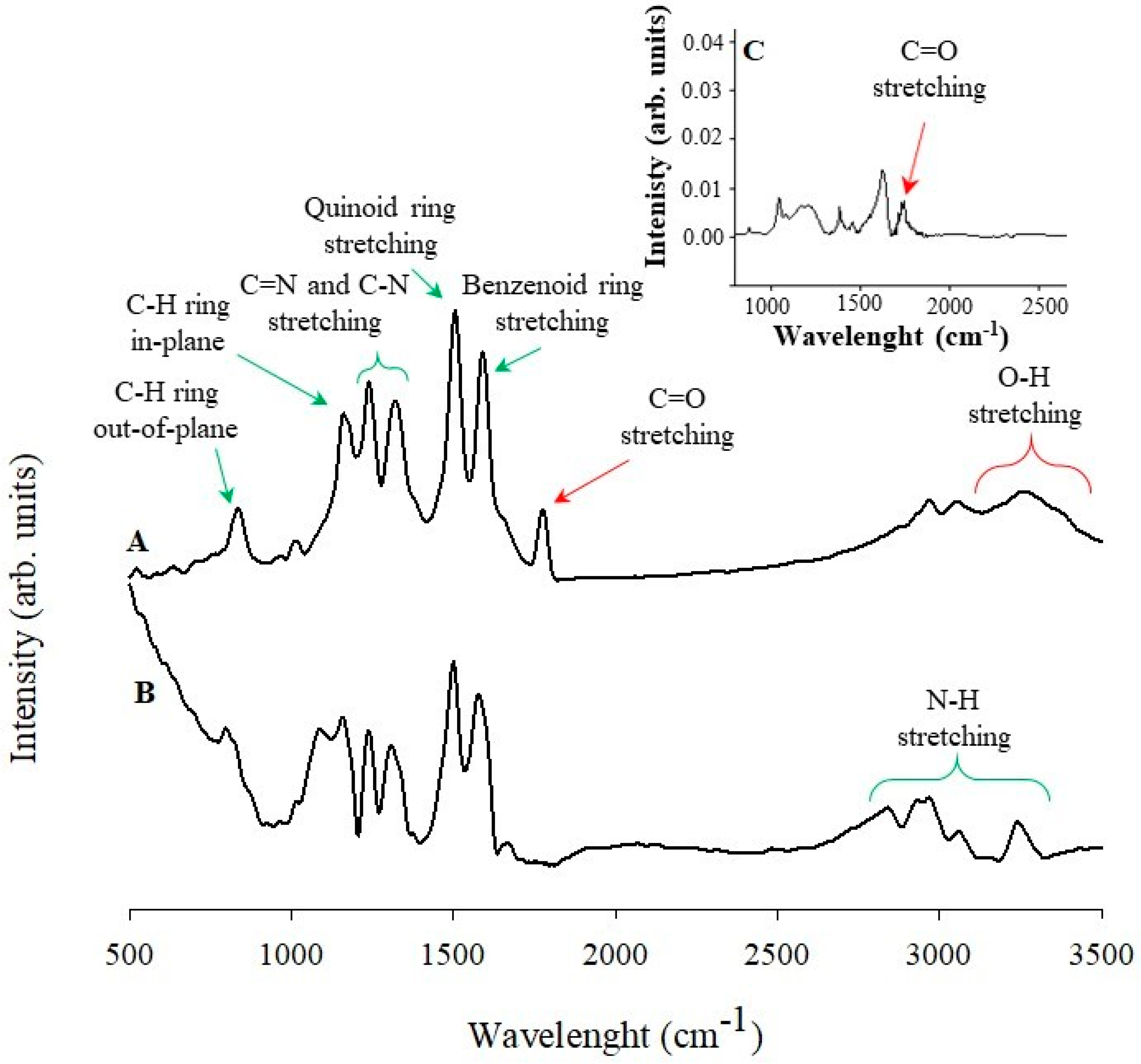
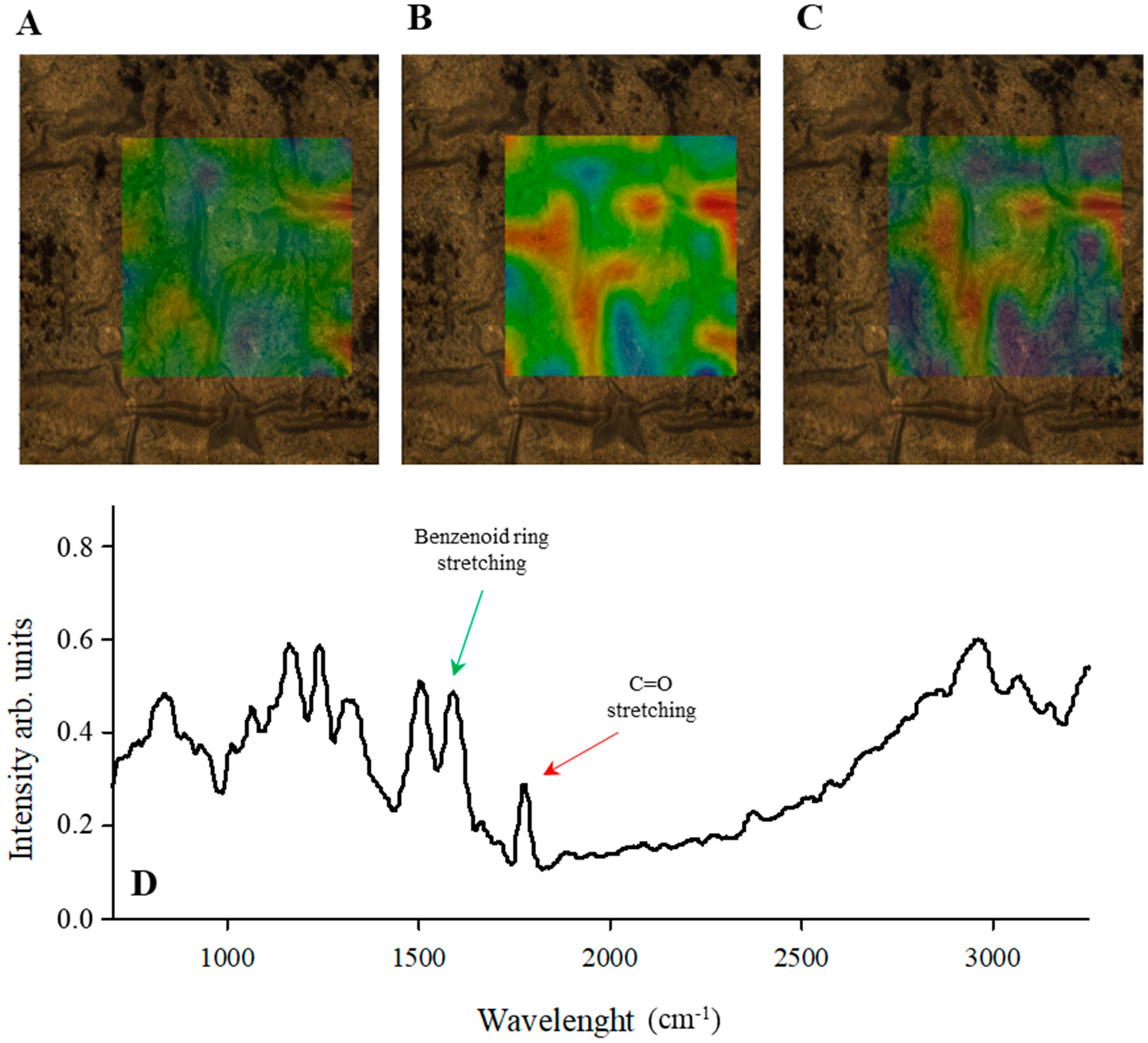
3.2. Raman and Infrared Spectroscopy Studies of PANINT/CNOsox
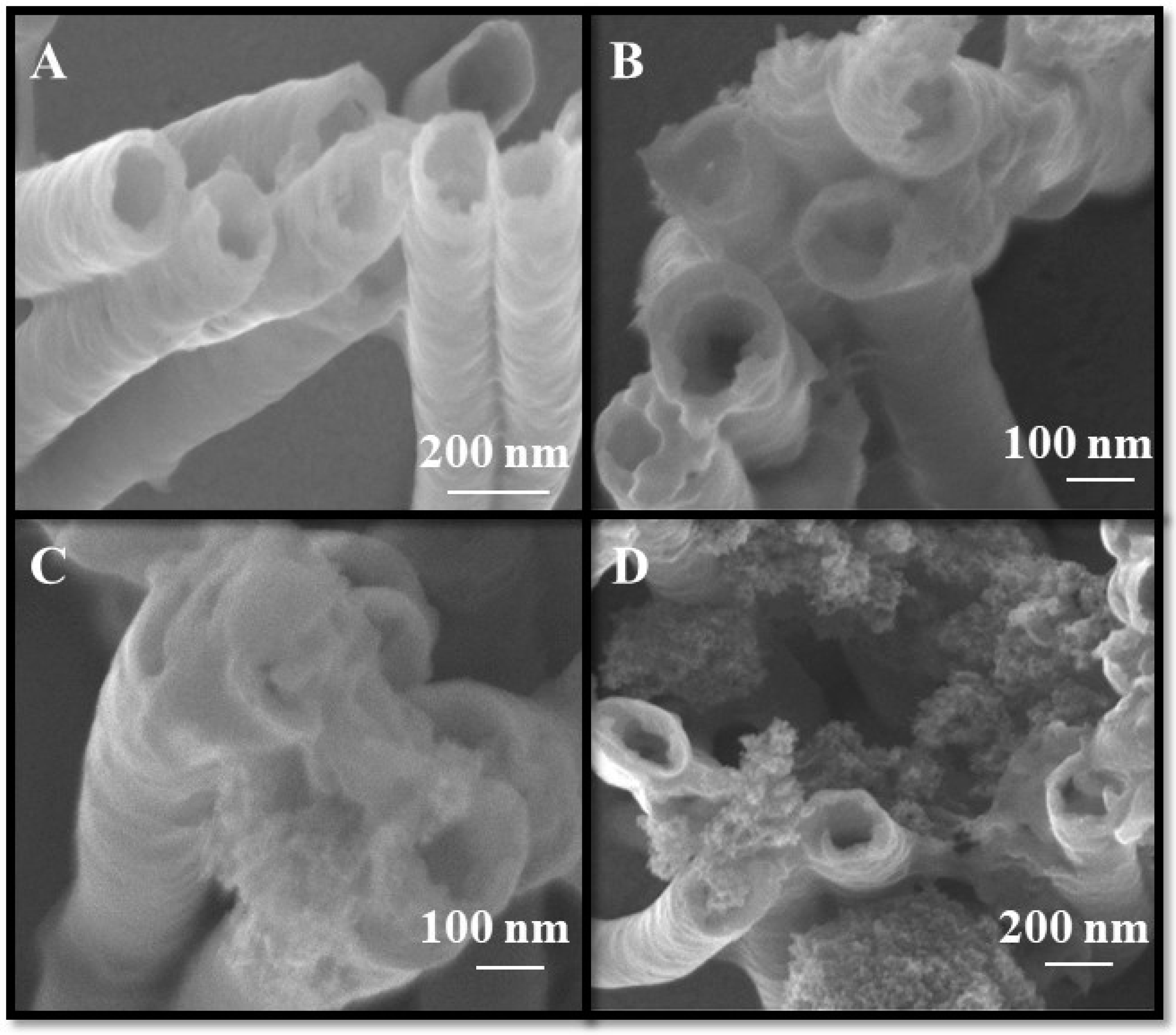
3.3. Nanocomposite Morphology Study
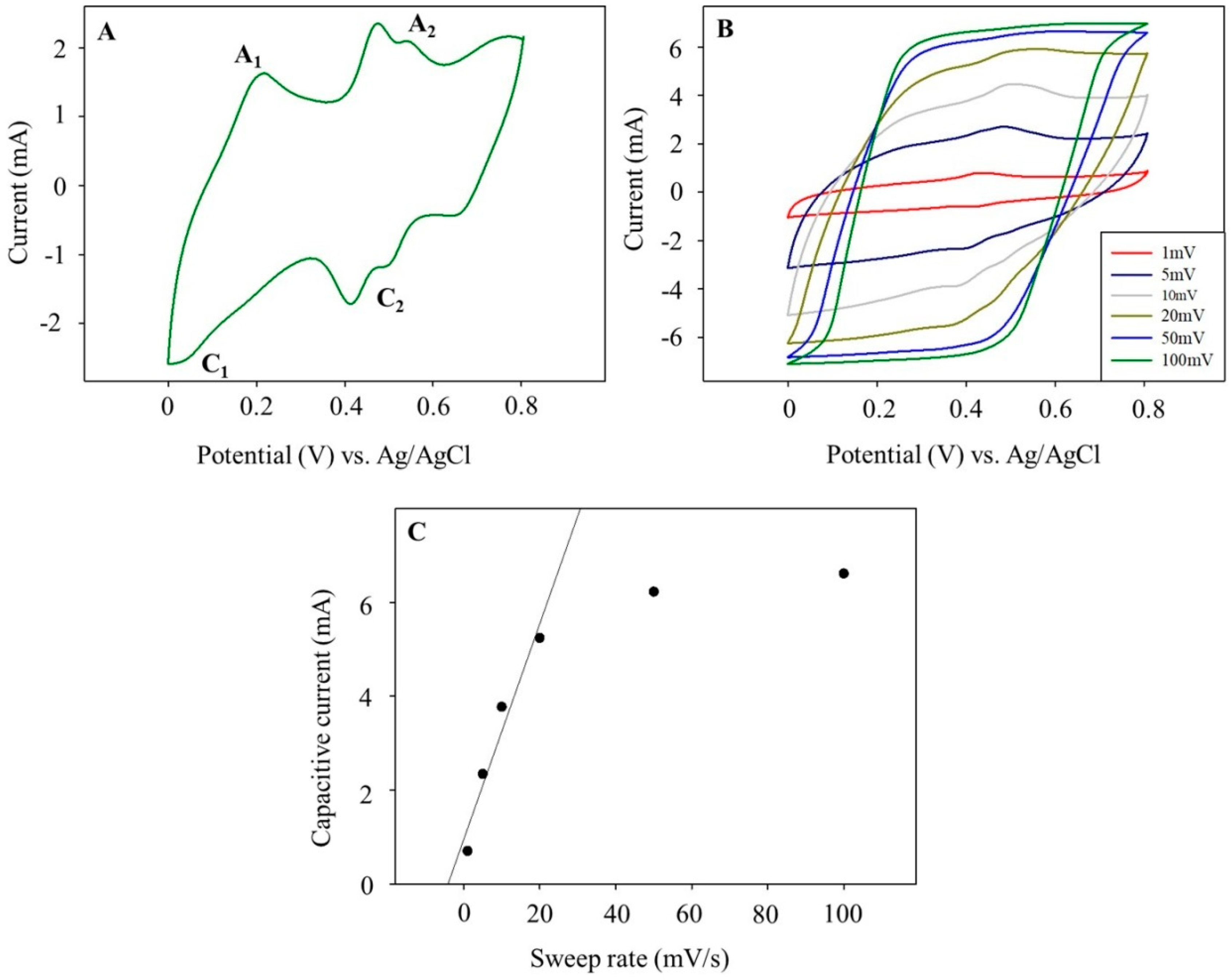
3.4. Voltammetric Studies of the PANINT/CNOsox Nanocomposite
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mosa, I.M.; Pattammattel, A.; Kadimisetty, K.; Pande, P.; El-Kady, M.F.; Bishop, G.W.; Novak, M.; Kaner, R.B.; Basu, A.K.; Kumar, C.V.; et al. Ultrathin Graphene–Protein Supercapacitors for Miniaturized Bioelectronics. Adv. Energy Mater. 2017, 7, 1700358. [Google Scholar] [CrossRef] [PubMed]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Ke, Q.; Wang, J. Graphene-based materials for supercapacitor electrodes—A review. J. Materiomics 2016, 2, 37–54. [Google Scholar] [CrossRef]
- Hong, S.-Y.; Park, S.-M. Electrochemistry of Conductive Polymers 36. pH Dependence of Polyaniline Conductivities Studied by Current-Sensing Atomic Force Microscopy. J. Phys. Chem. B 2005, 109, 9305–9310. [Google Scholar] [CrossRef]
- Prigodin, V.N.; Hsu, F.C.; Park, J.H.; Waldmann, O.; Epstein, A.J. Electron-ion interaction in doped conducting polymers. Phys. Rev. B 2008, 78, 035203. [Google Scholar] [CrossRef]
- Focke, W.W.; Wnek, G.E.; Wei, Y. Influence of oxidation state, pH, and counterion on the conductivity of polyaniline. J. Phys. Chem. 1987, 91, 5813–5818. [Google Scholar] [CrossRef]
- Parthasarathy, R.V.; Martin, C.R. Template-Synthesized Polyaniline Microtubules. Chem. Mater. 1994, 6, 1627–1632. [Google Scholar] [CrossRef]
- Chen, T.; Dai, L. Carbon nanomaterials for high-performance supercapacitors. Mater. Today 2013, 16, 272–280. [Google Scholar] [CrossRef]
- Grover, S.; Goel, S.; Marichi, R.B.; Sahu, V.; Singh, G.; Sharma, R.K. Polyaniline All Solid-State Pseudocapacitor: Role of Morphological Variations in Performance Evolution. Electrochim. Acta 2016, 196, 131–139. [Google Scholar] [CrossRef]
- Yoon, H.; Jang, J. Conducting-Polymer Nanomaterials for High-Performance Sensor Applications: Issues and Challenges. Adv. Funct. Mater. 2009, 19, 1567–1576. [Google Scholar] [CrossRef]
- Sutar, D.S.; Major, S.S.; Srinivasa, R.S.; Yakhmi, J.V. Conformational morphology of polyaniline grown on self-assembled monolayer modified silicon. Thin Solid Films 2011, 520, 351–355. [Google Scholar] [CrossRef]
- Chaudhari, S.; Patil, P.P. Inhibition of nickel coated mild steel corrosion by electrosynthesized polyaniline coatings. Electrochim. Acta 2011, 56, 3049–3059. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Wang, R.; Tian, X. Novel route to polyaniline nanofibers from miniemulsion polymerization. J. Mater. Sci. 2011, 46, 1049–1052. [Google Scholar] [CrossRef]
- Cui, Z.; Coletta, C.; Rebois, R.; Baiz, S.; Gervais, M.; Goubard, F.; Aubert, P.H.; Dazzi, A.; Remita, S. Radiation-induced reduction–polymerization route for the synthesis of PEDOT conducting polymers. Radiat. Phys. Chem. 2016, 119, 157–166. [Google Scholar] [CrossRef]
- Dhawale, D.S.; Vinu, A.; Lokhande, C.D. Stable nanostructured polyaniline electrode for supercapacitor application. Electrochim. Acta 2011, 56, 9482–9487. [Google Scholar] [CrossRef]
- Martin, C.R. Template Synthesis of Electronically Conductive Polymer Nanostructures. Acc. Chem. Res. 1995, 28, 61–68. [Google Scholar] [CrossRef]
- Mazur, M.; Tagowska, M.; Pałys, B.; Jackowska, K. Template synthesis of polyaniline and poly(2-methoxyaniline) nanotubes: Comparison of the formation mechanisms. Electrochem. Commun. 2003, 5, 403–407. [Google Scholar] [CrossRef]
- Olejnik, P.; Gniadek, M.; Palys, B. Layers of polyaniline nanotubes deposited by langmuir–blodgett method. J. Phys. Chem. C 2012, 116, 10424–10429. [Google Scholar] [CrossRef]
- Long, Y.Z.; Li, M.M.; Gu, C.; Wan, M.; Duvail, J.L.; Liu, Z.; Fan, Z. Recent advances in synthesis, physical properties and applications of conducting polymer nanotubes and nanofibers. Prog. Polym. Sci. 2011, 36, 1415–1442. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, J.; Wan, M. Highly crystalline and thin polyaniline nanofibers oxidized by ferric chloride. Mater. Chem. Phys. 2009, 115, 275–279. [Google Scholar] [CrossRef]
- Rahy, A.; Yang, D.J. Synthesis of highly conductive polyaniline nanofibers. Mater. Lett. 2008, 62, 4311–4314. [Google Scholar] [CrossRef]
- Delvaux, M.; Duchet, J.; Stavaux, P.-Y.; Legras, R.; Demoustier-Champagne, S. Chemical and electrochemical synthesis of polyaniline micro- and nano-tubules. Synth. Met. 2000, 113, 275–280. [Google Scholar] [CrossRef]
- Boulanger, N.; Barbero, D.R. Ordered and Highly Conductive Carbon Nanotube Nano-Networks in a Semiconducting Polymer Film by Solution Processing. Adv. Electron. Mater. 2015, 1, 1400030. [Google Scholar] [CrossRef]
- Li, L.; Qiu, J.; Wang, S. Three-dimensional ordered nanostructures for supercapacitor electrode. Electrochim. Acta 2013, 99, 278–284. [Google Scholar] [CrossRef]
- Kiamahalleh, M.V.; Sata, S.A.; Buniran, S.; Sharif Zein, S.H. Remarkable Stability of Supercapacitor Material Synthesized by Manganese Oxide Filled in Multiwalled Carbon Nanotubes. Curr. Nanosci. 2010, 6, 553–559. [Google Scholar] [CrossRef]
- Mondal, C.; Ghosh, D.; Aditya, T.; Sasmal, A.K.; Pal, T. Mn3O4 nanoparticles anchored to multiwall carbon nanotubes: A distinctive synergism for high-performance supercapacitors. New J. Chem. 2015, 39, 8373–8380. [Google Scholar] [CrossRef]
- Wang, H.; Lin, J.; Shen, Z.X. Polyaniline (PANi) based electrode materials for energy storage and conversion. J. Sci. Adv. Mater. Devices 2016, 1, 225–255. [Google Scholar] [CrossRef]
- Papathanassiou, A.N.; Mykhailiv, O.; Echegoyen, L.; Sakellis, I.; Plonska-Brzezinska, M.E. Electric properties of carbon nano-onion/polyaniline composites: A combined electric modulus and ac conductivity study. J. Phys. Appl. Phys. 2016, 49, 285305. [Google Scholar] [CrossRef]
- Mykhailiv, O.; Imierska, M.; Petelczyc, M.; Echegoyen, L.; Plonska-Brzezinska, M.E. Chemical versus Electrochemical Synthesis of Carbon Nano-onion/Polypyrrole Composites for Supercapacitor Electrodes. Chem. Eur. J. 2015, 21, 5783–5793. [Google Scholar] [CrossRef] [PubMed]
- Papathanassiou, A.N.; Plonska-Brzezinska, M.E.; Mykhailiv, O.; Echegoyen, L.; Sakellis, I. Combined high permittivity and high electrical conductivity of carbon nano-onion/polyaniline composites. Synth. Met. 2015, 209, 583–587. [Google Scholar] [CrossRef]
- Grądzka, E.; Winkler, K.; Borowska, M.; Plonska-Brzezinska, M.E.; Echegoyen, L. Comparison of the electrochemical properties of thin films of MWCNTs/C60-Pd, SWCNTs/C60-Pd and ox-CNOs/C60-Pd. Electrochim. Acta 2013, 96, 274–284. [Google Scholar] [CrossRef]
- Gupta, V.; Miura, N. Polyaniline/single-wall carbon nanotube (PANI/SWCNT) composites for high performance supercapacitors. Electrochim. Acta 2006, 52, 1721–1726. [Google Scholar] [CrossRef]
- Zhou, Y.; Qin, Z.Y.; Li, L.; Zhang, Y.; Wei, Y.L.; Wang, L.F.; Zhu, M.F. Polyaniline/multi-walled carbon nanotube composites with core–shell structures as supercapacitor electrode materials. Electrochim. Acta 2010, 55, 3904–3908. [Google Scholar] [CrossRef]
- Ning, G.; Li, T.; Yan, J.; Xu, C.; Wei, T.; Fan, Z. Three-dimensional hybrid materials of fish scale-like polyaniline nanosheet arrays on graphene oxide and carbon nanotube for high-performance ultracapacitors. Carbon 2013, 54, 241–248. [Google Scholar] [CrossRef]
- Lee, T.; Yun, T.; Park, B.; Sharma, B.; Song, H.-K.; Kim, B.-S. Hybrid multilayer thin film supercapacitor of graphene nanosheets with polyaniline: Importance of establishing intimate electronic contact through nanoscale blending. J. Mater. Chem. 2012, 22, 21092–21099. [Google Scholar] [CrossRef]
- Al-Jishi, R.; Dresselhaus, G. Lattice-dynamical model for graphite. Phys. Rev. B 1982, 26, 4514–4522. [Google Scholar] [CrossRef]
- Bacon, R. Growth, Structure, and Properties of Graphite Whiskers. J. Appl. Phys. 1960, 31, 283–290. [Google Scholar] [CrossRef]
- Banhart, F. Structural transformations in carbon nanoparticles induced by electron irradiation. Phys. Solid State 2002, 44, 399–404. [Google Scholar] [CrossRef] [Green Version]
- Bystrzejewski, M.; Rummeli, M.H.; Gemming, T.; Lange, H.; Huczko, A. Catalyst-free synthesis of onion-like carbon nanoparticles. New Carbon Mater. 2010, 25, 1–8. [Google Scholar] [CrossRef]
- Cabioc’h, T.; Jaouen, M.; Rivière, J.P.; Delafond, J.; Hug, G. Characterization and growth of carbon phases synthesized by high temperature carbon ion implantation into copper. Diam. Relat. Mater. 1997, 6, 261–265. [Google Scholar] [CrossRef]
- Palkar, A.; Melin, F.; Cardona, C.M.; Elliott, B.; Naskar, A.K.; Edie, D.D.; Kumbhar, A.; Echegoyen, L. Reactivity Differences between Carbon Nano Onions (CNOs) Prepared by Different Methods. Chem. Asian J. 2007, 2, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Gruen, D.M.; Shenderova, O.A.; Vul’, A.Y. (Eds.) Synthesis, Properties and Applications of Ultrananocrystalline Diamond; Springer: Berlin/Heidelberg, Germany, 2005; Volume 192. [Google Scholar]
- Plonska-Brzezinska, M.E.; Echegoyen, L. Carbon nano-onions for supercapacitor electrodes: Recent developments and applications. J. Mater. Chem. A 2013, 1, 13703–13714. [Google Scholar] [CrossRef]
- Mykhailiv, O.; Zubyk, H.; Plonska-Brzezinska, M.E. Carbon nano-onions: Unique carbon nanostructures with fascinating properties and their potential applications. Inorg. Chim. Acta 2017, 468, 49–66. [Google Scholar] [CrossRef]
- Bartelmess, J.; Giordani, S. Carbon nano-onions (multi-layer fullerenes): Chemistry and applications. Beilstein J. Nanotechnol. 2014, 5, 1980–1998. [Google Scholar] [CrossRef] [PubMed]
- Bobrowska, D.M.; Brzezinski, K.; Echegoyen, L.; Plonska-Brzezinska, M.E. A new perspective on carbon nano-onion/nickel hydroxide/oxide composites: Physicochemical properties and application in hybrid electrochemical systems. Fuller. Nanotub. Carbon Nanostruct. 2017, 25, 193–203. [Google Scholar] [CrossRef]
- Bobrowska, D.M.; Czyrko, J.; Brzezinski, K.; Echegoyen, L.; Plonska-Brzezinska, M.E. Carbon nano-onion composites: Physicochemical characteristics and biological activity. Fuller. Nanotub. Carbon Nanostruct. 2017, 25, 185–192. [Google Scholar] [CrossRef]
- Kuznetsov, V.L.; Chuvilin, A.L.; Moroz, E.M.; Kolomiichuk, V.N.; Shaikhutdinov, S.K.; Butenko, Y.V.; Mal’kov, I.Y. Effect of explosion conditions on the structure of detonation soots: Ultradisperse diamond and onion carbon. Carbon 1994, 32, 873–882. [Google Scholar] [CrossRef]
- Wong, S.S.; Woolley, A.T.; Joselevich, E.; Cheung, C.L.; Lieber, C.M. Covalently-Functionalized Single-Walled Carbon Nanotube Probe Tips for Chemical Force Microscopy. J. Am. Chem. Soc. 1998, 120, 8557–8558. [Google Scholar] [CrossRef]
- Salzmann, C.G.; Llewellyn, S.A.; Tobias, G.; Ward, M.A.H.; Huh, Y.; Green, M.L.H. The Role of Carboxylated Carbonaceous Fragments in the Functionalization and Spectroscopy of a Single-Walled Carbon-Nanotube Material. Adv. Mater. 2007, 19, 883–887. [Google Scholar] [CrossRef]
- Pujals, D.C.; de Fuentes, O.A.; García, L.F.D.; Cazzanelli, E.; Caputi, L.S. Raman spectroscopy of polyhedral carbon nano-onions. Appl. Phys. A 2015, 120, 1339–1345. [Google Scholar] [CrossRef]
- Bogdanov, K.; Fedorov, A.; Osipov, V.; Enoki, T.; Takai, K.; Hayashi, T.; Ermakov, V.; Moshkalev, S.; Baranov, A. Annealing-induced structural changes of carbon onions: High-resolution transmission electron microscopy and Raman studies. Carbon 2014, 73, 78–86. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, Y.; Torres, J.; Lee, S.H.; Yun, M. Facile and template-free method toward chemical synthesis of polyaniline film/nanotube structures. J. Polym. Sci. Part Polym. Chem. 2017, 55, 3973–3979. [Google Scholar] [CrossRef]
- Do Nascimento, G.M.; Temperini, M.R.L. Studies on the resonance Raman spectra of polyaniline obtained with near-IR excitation. J. Raman Spectrosc. 2008, 39, 772–778. [Google Scholar] [CrossRef]
- Tucceri, R.; Arnal, P.M.; Scian, A.N. Spectroscopic Characterization of Poly(ortho-Aminophenol) Film Electrodes: A Review Article. J. Spectrosc. 2013, 2013, 951604. [Google Scholar] [CrossRef]
- Ćirić-Marjanović, G.; Trchová, M.; Stejskal, J. The chemical oxidative polymerization of aniline in water: Raman spectroscopy. J. Raman Spectrosc. 2008, 39, 1375–1387. [Google Scholar] [CrossRef]
- Šeděnková, I.; Trchová, M.; Stejskal, J. Thermal degradation of polyaniline films prepared in solutions of strong and weak acids and in water—FTIR and Raman spectroscopic studies. Polym. Degrad. Stab. 2008, 93, 2147–2157. [Google Scholar] [CrossRef]
- Jabłońska, A.; Gniadek, M.; Pałys, B. Enhancement of Direct Electrocatalytic Activity of Horseradish Peroxidase on Polyaniline Nanotubes. J. Phys. Chem. C 2015, 119, 12514–12522. [Google Scholar] [CrossRef]
- Trchová, M.; Stejskal, J. Polyaniline: The infrared spectroscopy of conducting polymer nanotubes (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 1803–1817. [Google Scholar] [CrossRef] [Green Version]
- Trchová, M.; Morávková, Z.; Bláha, M.; Stejskal, J. Raman spectroscopy of polyaniline and oligoaniline thin films. Electrochim. Acta 2014, 122, 28–38. [Google Scholar] [CrossRef]
- Morsi, R.E.; Khamis, E.A.; Al-Sabagh, A.M. Polyaniline nanotubes: Facile synthesis, electrochemical, quantum chemical characteristics and corrosion inhibition efficiency. J. Taiwan Inst. Chem. Eng. 2016, 60, 573–581. [Google Scholar] [CrossRef]
- Plonska-Brzezinska, M.E.; Dubis, A.T.; Lapinski, A.; Villalta-Cerdas, A.; Echegoyen, L. Electrochemical Properties of Oxidized Carbon Nano-Onions: DRIFTS-FTIR and Raman Spectroscopic Analyses. ChemPhysChem 2011, 12, 2659–2668. [Google Scholar] [CrossRef]
- Wang, S.; Ma, L.; Gan, M.; Fu, S.; Dai, W.; Zhou, T.; Sun, X.; Wang, H.; Wang, H. Free-standing 3D graphene/polyaniline composite film electrodes for high-performance supercapacitors. J. Power Sources 2015, 299, 347–355. [Google Scholar] [CrossRef]
- Kim, B.; Kwon, J.; Ko, J.; Park, J.; Too, C.; Wallace, G. Preparation and enhanced stability of flexible supercapacitor prepared from nafion/polyaniline nano-fiber. Synth. Met. 2010, 160, 94–98. [Google Scholar] [CrossRef]
- Dhawale, D.S.; Dubal, D.P.; Jamadade, V.S.; Salunkhe, R.R.; Lokhande, C.D. Fuzzy nanofibrous network of polyaniline electrode for supercapacitor application. Synth. Met. 2010, 160, 519–522. [Google Scholar] [CrossRef]
- Dhawale, D.S.; Salunkhe, R.R.; Jamadade, V.S.; Dubal, D.P.; Pawar, S.M.; Lokhande, C.D. Hydrophilic polyaniline nanofibrous architecture using electrosynthesis method for supercapacitor application. Curr. Appl. Phys. 2010, 10, 904–909. [Google Scholar] [CrossRef]
- Ismail, Y.A.; Chang, J.; Shin, S.R.; Mane, R.S.; Han, S.-H.; Kim, S.J. Hydrogel-Assisted Polyaniline Microfiber as Controllable Electrochemical Actuatable Supercapacitor. J. Electrochem Soc. 2009, 156, A313–A317. [Google Scholar] [CrossRef]
- Bélanger, D.; Ren, X.; Davey, J.; Uribe, F.; Gottesfeld, S. Characterization and Long-Term Performance of Polyaniline-Based Electrochemical Capacitors. J. Electrochem. Soc. 2000, 147, 2923–2929. [Google Scholar] [CrossRef]
- Otrokhov, G.; Pankratov, D.; Shumakovich, G.; Khlupova, M.; Zeifman, Y.; Vasil’eva, I.; Morozova, O.; Yaropolov, A. Enzymatic synthesis of polyaniline/multi-walled carbon nanotube composite with core shell structure and its electrochemical characterization for supercapacitor application. Electrochim. Acta 2014, 123, 151–157. [Google Scholar] [CrossRef]
- Zhou, S.; Mo, S.; Zou, W.; Jiang, F.; Zhou, T.; Yuan, D. Preparation of polyaniline/2-dimensional hexagonal mesoporous carbon composite for supercapacitor. Synth. Met. 2011, 161, 1623–1628. [Google Scholar] [CrossRef]
- Jang, J.; Bae, J.; Choi, M.; Yoon, S.-H. Fabrication and characterization of polyaniline coated carbon nanofiber for supercapacitor. Carbon 2005, 43, 2730–2736. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Feng, Y.; Feng, W. Electropolymerization of graphene oxide/polyaniline composite for high-performance supercapacitor. Electrochim. Acta 2013, 90, 95–100. [Google Scholar] [CrossRef]
- Xia, X.; Hao, Q.; Lei, W.; Wang, W.; Sun, D.; Wang, X. Nanostructured ternary composites of graphene/Fe2O3/polyaniline for high-performance supercapacitors. J. Mater. Chem. 2012, 22, 16844–16850. [Google Scholar] [CrossRef]


| Specific Capacitance (F g−1) | ||||
|---|---|---|---|---|
| Pristine PANINT | PANINT/CNOsox Composite | |||
| Sweep rate (mV s−1) | C1 | C2 | C1 | C2 |
| 1 | 237 | 269 | 795 | 946 |
| 5 | - | - | 741 | 681 |
| 10 | - | - | 616 | 614 |
| 20 | - | - | 431 | 441 |
| 50 | - | - | 213 | 200 |
| 100 | 53 | 70 | 115 | 169 |
| Material | Sweep Rate (mV s−1) | Potential Range (V) | Electrolyte | Specific Capacitance (F g−1) | References |
|---|---|---|---|---|---|
| PANI | 10 | −0.1–0.8 | 1 M H2SO4 | 503 | [15]. |
| PANI | 10 | −0.2–0.6 | Nafion | 269 | [64] |
| Nanofibrous PANI | 10 | −0.1–0.8 | 1 M H2SO4 | 839 | [65] |
| Nanofibrous PANI | 10 | −0.1–0.8 | 1 M H2SO4 | 861 | [66] |
| Hydrogel-assisted PANI microfiber | 10 | −0.2–0.8 | 1 M methane sulfonic acid | 703 | [67] |
| BF4-doped PANI | 50 | 0–0.75 | 4 M HBF | 74 | [68] |
| PANI/CNT | 5 | −0.1–0.7 | PVA/H3PO4 | 440 | [69] |
| PANI/MWCNT | 1 | 0–1.0 | 0.1 M H2SO4 | 560 | [33] |
| Mesoporous C/PANI | 2 | −0.1–1.0 | 1 M H2SO4 | 470 | [70] |
| PANI on CNF | 5 | 0–0.8 | 1 M H2SO4 | 264 | [71] |
| PANI/GO | 1 | −0.1–0.9 | 1 M H2SO4 | 1136 | [72] |
| G/Fe2O3/PANI | 1 | −1.0–0.1 | 1 M KOH | 638 | [73] |
| PANINT/CNOsox | 1 | 0–0.8 | 1 M H2SO4 | 946 | this work |
| PANINT/CNOsox | 10 | 0–0.8 | 1 M H2SO4 | 614 | this work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olejnik, P.; Gniadek, M.; Echegoyen, L.; Plonska-Brzezinska, M.E. Nanoforest: Polyaniline Nanotubes Modified with Carbon Nano-Onions as a Nanocomposite Material for Easy-to-Miniaturize High-Performance Solid-State Supercapacitors. Polymers 2018, 10, 1408. https://doi.org/10.3390/polym10121408
Olejnik P, Gniadek M, Echegoyen L, Plonska-Brzezinska ME. Nanoforest: Polyaniline Nanotubes Modified with Carbon Nano-Onions as a Nanocomposite Material for Easy-to-Miniaturize High-Performance Solid-State Supercapacitors. Polymers. 2018; 10(12):1408. https://doi.org/10.3390/polym10121408
Chicago/Turabian StyleOlejnik, Piotr, Marianna Gniadek, Luis Echegoyen, and Marta E. Plonska-Brzezinska. 2018. "Nanoforest: Polyaniline Nanotubes Modified with Carbon Nano-Onions as a Nanocomposite Material for Easy-to-Miniaturize High-Performance Solid-State Supercapacitors" Polymers 10, no. 12: 1408. https://doi.org/10.3390/polym10121408






