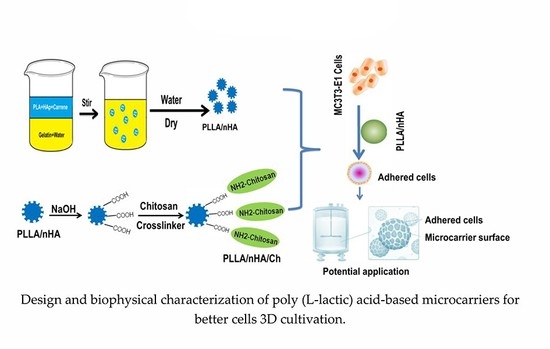
Design and Biophysical Characterization of Poly (l-Lactic) Acid Microcarriers with and without Modification of Chitosan and Nanohydroxyapatite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of PLLA, PLLA/nHA and PLLA/nHA/Ch Microcarriers
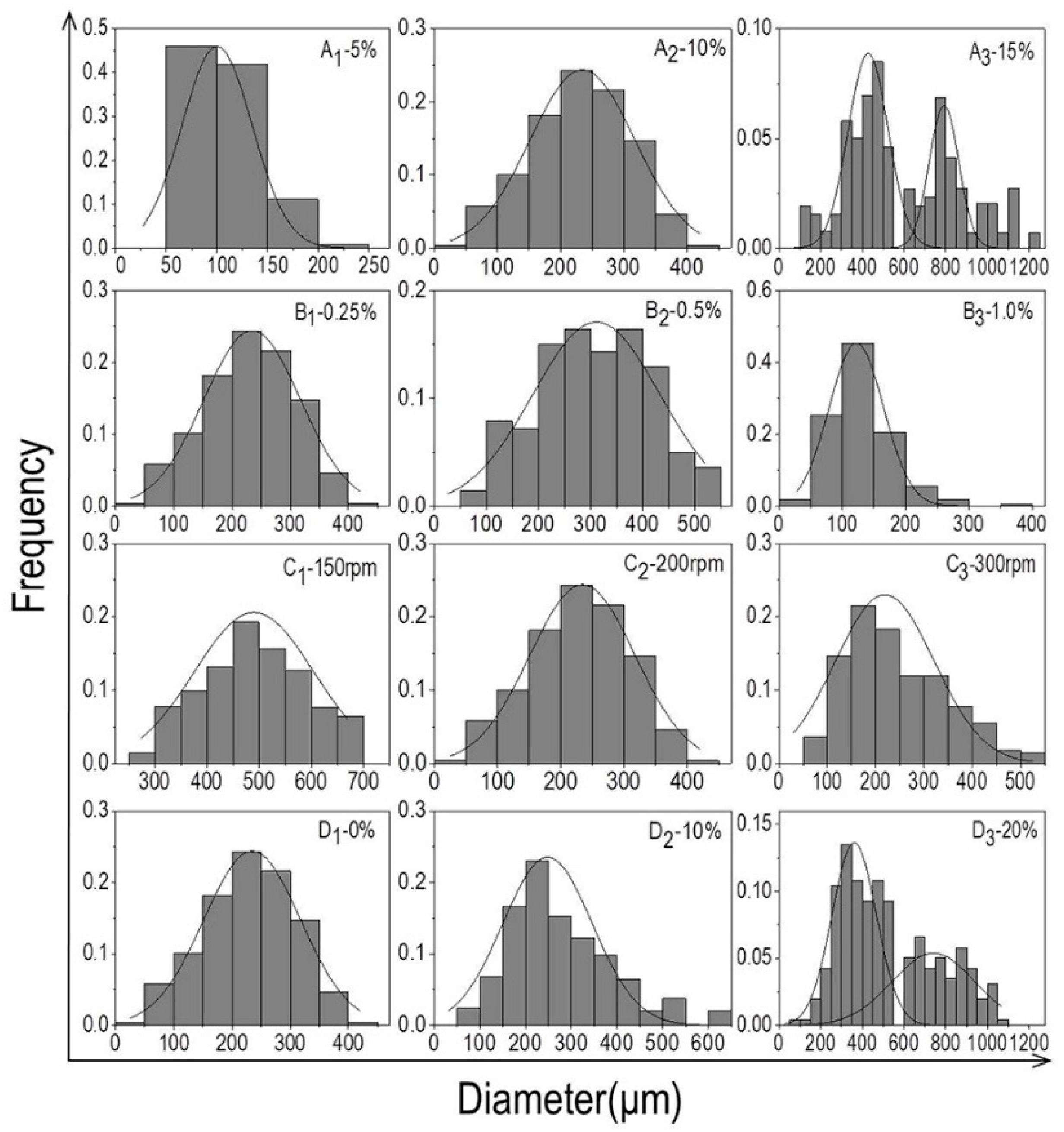
2.3. Analysis of Particle Size Distribution
2.4. Morphology Observation and Component Analysis of Microcarriers
2.5. Protein Adsorption Determination
2.6. MC3T3-E1 Cell Culture and Osteogenic Differentiation
2.7. Inoculation and Culture of MC3T3-E1 Cells on Microcarriers
2.8. Cell Adhesion, Proliferation, and Differentiation on Microcarriers
2.9. Statistical Analysis
3. Results and Discussion
3.1. The Particle Size Distribution of Microcarriers
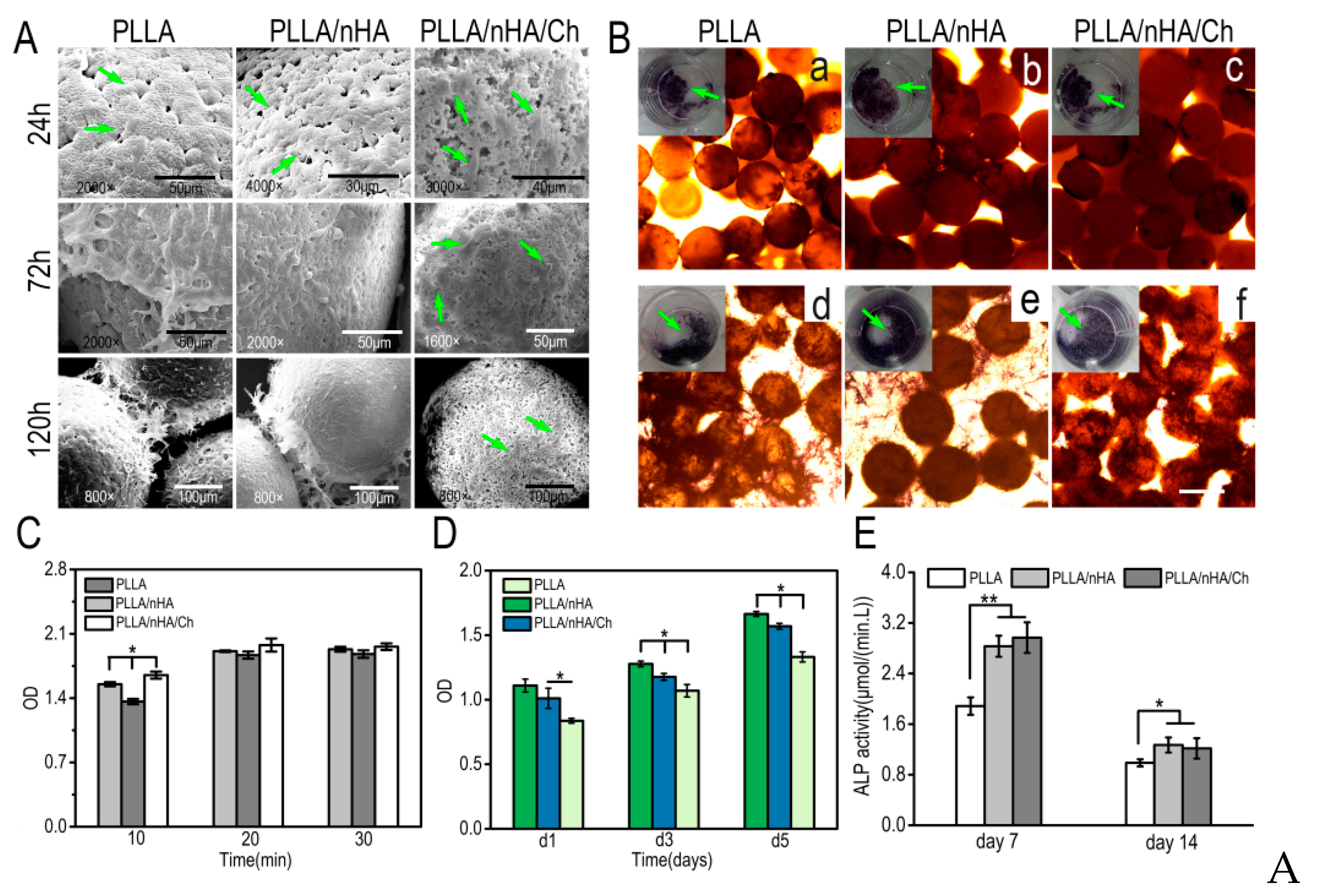
3.2. Morphology, Component Analysis, Protein Adsorption, and Degradation of Microcarriers
3.3. The Morphology and Osteogenic Differentiation of MC3T3-E1 Cells
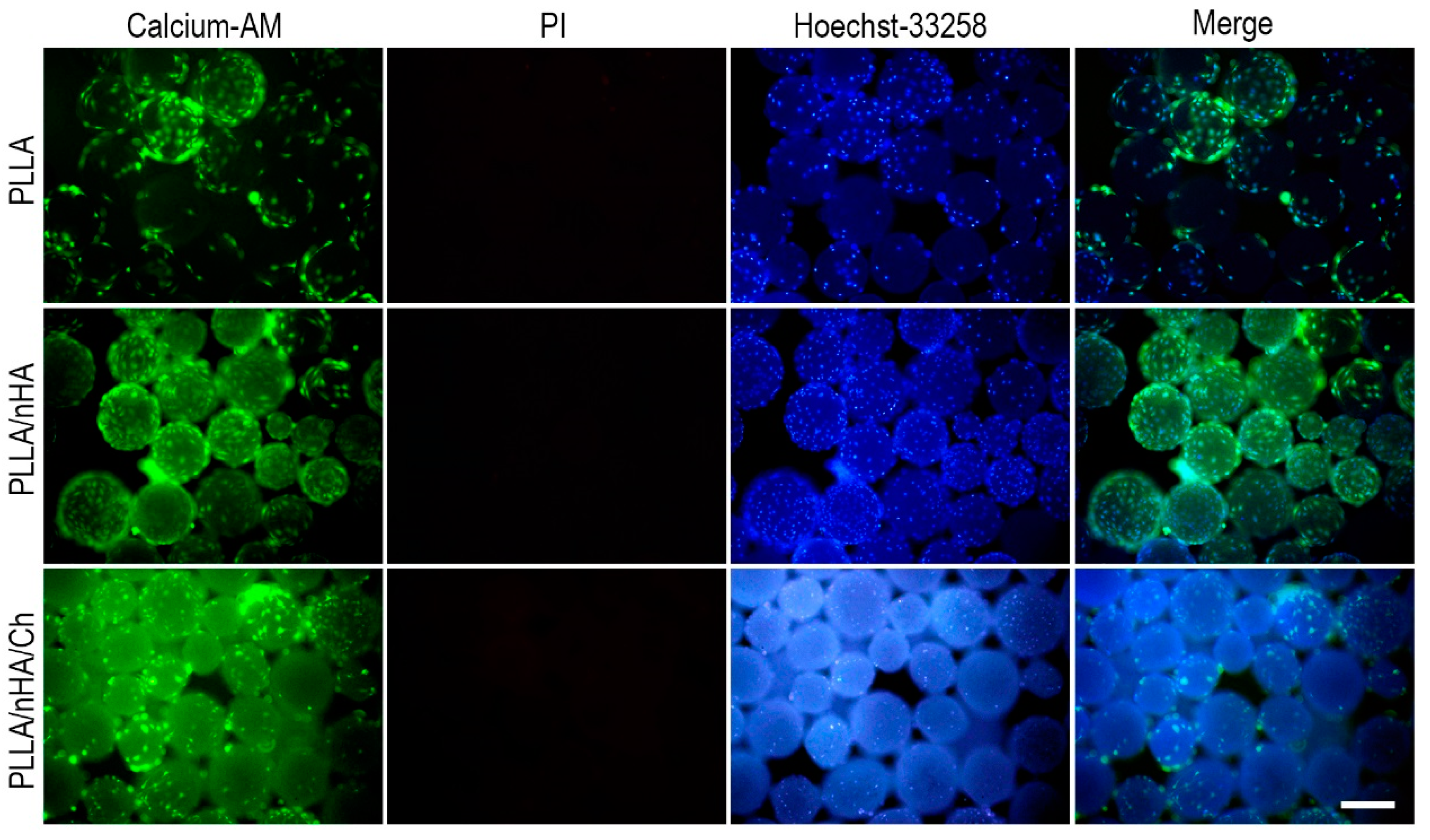
3.4. Live/Dead Staining of Cells on Microcarriers
3.5. Cell Adhesion, Detachment, and Differentiation on Microcarriers
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wezel, A.L.V. Chapter 2–Microcarrier cultures of animal cells. Tissue Cult. 1973, 372–377. [Google Scholar] [CrossRef]
- Freiberg, S.; Zhu, X.X. Polymer microspheres for controlled drug release. Int. J. Pharm. 2004, 282, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, T.; Jiang, C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta Pharm. Sin. B 2018, 8, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Ventini, D.C.; Damiani, R.; Sousa, A.P.; De Oliveira, J.E.; Peroni, C.N.; Ribela, M.T.; Pereira, C.A. Improved bioprocess with CHO-hTSH cells on higher microcarriers concentration provides higher overall biomass and productivity for rhTSH. Appl. Biochem. Biotechnol. 2011, 164, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.W.; Huang, Y.Y.; Liu, Y.Z. Effects of the rate of solvent evaporation on the characteristics of drug loaded PLLA and PDLLA microspheres. Int. J. Pharm. 2001, 212, 161–169. [Google Scholar] [CrossRef]
- Liggins, R.T.; Burt, H.M. Paclitaxel loaded poly (l-lactic acid) (PLLA) microspheres: II. The effect of processing parameters on microsphere morphology and drug release kinetics. Int. J. Pharm. 2004, 281, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Aubert-Pouëssel, A.; Bibby, D.C.; Venier-Julienne, M.C.; Hindré, F.; Benoît, J.P. A novel in vitro delivery system for assessing the biological integrity of protein upon release from PLGA microspheres. Pharm. Res.-Dordr. 2002, 19, 1046–1051. [Google Scholar] [CrossRef]
- Sart, S.; Agathos, S.N.; Li, Y. Engineering stem cell fate with biochemical and biomechanical properties of microcarriers. Biotechnol. Progr. 2013, 29, 1354–1366. [Google Scholar] [CrossRef]
- Olmos, E.; Martin, C.; Loubiere, K.; Delaplace, G.; Marc, A. Critical agitation for microcarriers suspension in orbital shaken bioreactors: Experimental study and dimensional analysis. Chem. Eng. Sci. 2015, 122, 545–554. [Google Scholar] [CrossRef] [Green Version]
- Perez, R.A.; Riccardi, K.; Altankov, G.; Ginebra, M.P. Dynamic cell culture on calcium phosphate microcarriers for bone tissue engineering applications. J. Tissue Eng. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, C.; Olmos, E.; Balandras, F.; Tran, N.; Chevalot, I.; Guedon, E.; Marc, A. Investigation of growth conditions for the expansion of porcine mesenchymal stem cells on microcarriers in stirred cultures. Appl. Biochem. Biotechnol. 2014, 172, 1004–1017. [Google Scholar] [CrossRef]
- Rafiq, Q.A.; Brosnan, K.M.; Coopman, K.; Nienow, A.W.; Hewitt, C.J. Culture of human mesenchymal stem cells on microcarriers in a 5 l stirred-tank bioreactor. Biotechnol. Lett. 2013, 35, 1233–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chun, K.W.; Yoo, H.S.; Yoon, J.J.; Park, T.G. Biodegradable PLGA microcarriers for injectable delivery of chondrocytes: Effect of surface modification on cell attachment and function. Biotechnol. Prog. 2010, 20, 1797–1801. [Google Scholar] [CrossRef] [PubMed]
- Sivandzade, F.; Mashayekhan, S. Design and fabrication of injectable microcarriers composed of acellular cartilage matrix and chitosan. J. Biomater. Sci.-Polym. E 2018, 29, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Arora, A. Synthesis and Characterization of Novel Fluorescent Injectable Micro-Carriers for Tissue Regeneration (Doctoral Dissertation). 2014. Available online: http://oaktrust.library.tamu.edu/handle/1969.1/152694 (accessed on 28 April 2014).
- Anderson, J.M.; Shive, M.S. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 2012, 64, 72–82. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, E.J.; Knowles, J.C.; Kim, H.W. Preparation of in situ hardening composite microcarriers: Calcium phosphate cement combined with alginate for bone regeneration. J. Biomater. Appl. 2014, 28, 1079. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Han, Y.; Yang, H.; Wang, G.; Lan, R.; Wang, J.Y. Preparation of microcarriers based on zein and their application in cell culture. Mater. Sci. Eng. C-Mater. 2016, 58, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.Y.; Ma, D.H. Ocular biocompatibility of gelatin microcarriers functionalized with oxidized hyaluronic acid. Mater. Sci. Eng. C-Mater. 2017, 72, 150–159. [Google Scholar] [CrossRef]
- Song, K.; Yang, Y.; Wu, S.; Zhang, Y.; Feng, S.; Wang, H.; Wang, Y.; Wang, L.; Liu, T. In vitro culture and harvest of BMMSCs on the surface of a novel thermo-sensitive glass microcarriers. Mater. Sci. Eng. C 2016, 58, 324–330. [Google Scholar] [CrossRef]
- Levato, R.; Visser, J.; Planell, J.A.; Engel, E.; Malda, J.; Mateostimoneda, M.A. Biofabrication of tissue constructs by 3d bioprinting of cell-laden microcarriers. Biofabrication 2014, 6, 035020. [Google Scholar] [CrossRef]
- Singh, B.; Singh, P.; Sutherland, A.J.; Pal, K. Control of shape and size of poly (lactic acid) microspheres based on surfactant and polymer concentration. Mater. Lett. 2017, 195, 48–51. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.P.; Su, C.H. Surface modification of electrospun PLLA nanofibers by plasma treatment and cationized gelatin immobilization for cartilage tissue engineering. Acta Biomater. 2011, 7, 234. [Google Scholar] [CrossRef] [PubMed]
- Szustakiewicz, K.; Stępak, B.; Antończak, A.J.; Maj, M.; Gazińska, M.; Kryszak, B.; Pigłowski, J. Femtosecond laser-induced modification of PLLA/hydroxypatite composite. Polym. Degrad. Stab. 2018, 149, 152–161. [Google Scholar] [CrossRef]
- Neděla, O.; Slepička, P.; Švorčík, V. Surface modification of polymer substrates for biomedical applications. Materials 2017, 10, 1115. [Google Scholar] [CrossRef] [PubMed]
- Rimpelová, S.; Peterková, L.; Kasálková, N.S.; Slepička, P.; Švorčík, V.; Ruml, T. Surface modification of biodegradable poly (l-lactic acid) by argon plasma: Fibroblasts and keratinocytes in the spotlight. Plasma Process. Polym. 2015, 11, 1057–1067. [Google Scholar] [CrossRef]
- Turon, P.; Valle, L.J.D.; Alemán, C.; Puiggalí, J. Chapter 2—Grafting of hydroxyapatite for biomedical applications. Biopolym. Graft. 2018, 45–80. [Google Scholar] [CrossRef]
- Pramanik, N.; Mishra, D.; Banerjee, I.; Maiti, T.K.; Bhargava, P.; Pramanik, P. Chemical synthesis, characterization, and biocompatibility study of hydroxyapatite/chitosan phosphate nanocomposite for bone tissue engineering applications. Int. J. Polym Mater. 2014. [Google Scholar] [CrossRef]
- Ravichandran, R.; Venugopal, J.R.; Sundarrajan, S.; Mukherjee, S.; Ramakrishna, S. Precipitation of nanohydroxyapatite on PLLA/PBLG/collagen nanofibrous structures for the differentiation of adipose-derived stem cells to osteogenic lineage. Biomaterials 2012, 33, 846–855. [Google Scholar] [CrossRef]
- Wang, X.; Song, G.; Lou, T. Fabrication and characterization of nano-composite scaffold of PLLA/silane modified hydroxyapatite. Med. Eng. Phys. 2010, 32, 391–397. [Google Scholar] [CrossRef]
- Wang, J.L.; Chen, Q.; Du, B.B.; Cao, L.; Lin, H.; Fan, Z.Y.; Dong, J. Enhanced bone regeneration composite scaffolds of PLLA/β-TCP matrix grafted with gelatin and hap. Mat. Sci. Eng. C 2018, 87, 60–69. [Google Scholar] [CrossRef]
- Jiao, Y.; Liu, Z.C. Fabrication and characterization of PLLA-chitosan hybrid scaffolds with improved cell compatibility. J. Biomed. Mater. Res. A 2007, 80A, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Bo, D.; He, Y.; Luo, X.; Li, H. Degradation properties of chitosan microspheres/poly(l-lactic acid) composite in vitro and in vivo. Carbohydr. Polym. 2018, 193, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lao, L.; Tan, H.; Wang, Y.; Gao, C. Chitosan modified poly (l-lactide) microspheres as cell microcarriers for cartilage tissue engineering. Colloid Surf. B 2008, 66, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; McClements, D.J. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: Factors affecting particle size. Food Hydrocoll. 2011, 25, 1000–1008. [Google Scholar] [CrossRef]
- McClements, D.J. Food emulsions: Principles, practice, and techniques. In CRC Series in Contemporary Food Science Boca Raton, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Rosca, I.D.; Watari, F.; Uo, M. Microparticle formation and its mechanism in single and double emulsion solvent evaporation. J. Control. Release 2004, 99, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Gao, C.; Shi, Y.; Shen, J. Preparation of porous polylactide microspheres by emulsion-solvent evaporation based on solution induced phase separation. Polym. Adv. Technol. 2005, 16, 622–627. [Google Scholar] [CrossRef]
- Kandori, K.; Oda, S.; Fukusumi, M.; Morisada, Y. Synthesis of positively charged calcium hydroxyapatite nanocrystals and their adsorption behavior of proteins. Colloid Surf. B 2009, 73, 140–145. [Google Scholar] [CrossRef]
- Kandori, K.; Kuroda, T.; Togashi, S.; Katayama, E. Preparation of calcium hydroxyapatite nanoparticles using microreactor and their characteristics of protein adsorption. J. Phys. Chem. B 2011, 115, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Kanjwal, M.A.; Sheikh, F.A.; Nirmala, R.; Macossay, J.; Kim, H.Y. Fabrication of poly (caprolactone) nanofibers containing hydroxyapatite nanoparticles and their mineralization in a simulated body fluid. Fiber Polym. 2011, 12, 50–56. [Google Scholar] [CrossRef]
- He, Y.; Qian, Z.; Zhang, H.; Liu, X. Alkaline degradation behavior of polyesteramide fibers: Surface erosion. Colloid Polym. Sci. 2004, 282, 972–978. [Google Scholar] [CrossRef]
- Lyu, S.P.; Schley, J.; Loy, B.; Lind, D.; Hobot, C.; Sparer, R.; Untereker, D. Kinetics and time−temperature equivalence of polymer degradation. Biomacromolecules 2007, 8, 2301–2310. [Google Scholar] [CrossRef] [PubMed]
- Borsari, V.; Fini, G.W. Physical characterization of different-roughness titanium surfaces, with and without hydroxyapatite coating, and their effect on human osteoblast-like cells. J. Biomed. Mater. Res. B 2005, 75B, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Zan, Q.; Wang, C.; Dong, L.; Cheng, P.; Tian, J. Effect of surface roughness of chitosan-based microspheres on cell adhesion. Appl. Surf. Sci. 2008, 255, 401–403. [Google Scholar] [CrossRef]
- Hu, X.; Park, S.H.; Gil, E.S.; Xia, X.X.; Weiss, A.S.; Kaplan, D.L. The influence of elasticity and surface roughness on myogenic and osteogenic-differentiation of cells on silk-elastin biomaterials. Biomaterials 2011, 32, 8979–8989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Han, J.; Sun, Y.; Huang, Y.; Zhou, M. MC3T3-E1 cell response to stainless steel 316l with different surface treatments. Mat. Sci. Eng. C 2015, 56, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Sobczak-Kupiec, A.; Pluta, K.; Drabczyk, A.; Włoś, M.; Tyliszczak, B. Synthesis and characterization of ceramic-polymer composites containing bioactive synthetic hydroxyapatite for biomedical applications. Ceram. Int. 2018, 44, 13630–13638. [Google Scholar] [CrossRef]
- Swetha, M.; Sahithi, K.; Moorthi, A.; Srinivasan, N.; Ramasamy, K.; Selvamurugan, N. Biocomposites containing natural polymers and hydroxyapatite for bone tissue engineering. Int. J. Biol. Macromol. 2010, 47, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Shahin-Shamsabadi, A.; Hashemi, A.; Tahriri, M.; Bastami, F.; Salehi, M.; Abbas, F.M. Mechanical, material, and biological study of a PCL/bioactive glass bone scaffold: Importance of viscoelasticity. Mater. Sci. Eng. C 2018, 90, 280–288. [Google Scholar] [CrossRef]






| Microcarriers | PLLA | PLLA/nHA | PLLA/nHA/Ch |
|---|---|---|---|
| Mean diameter/μm | 291.9 ± 30.7 | 275.7 ± 30.6 | 269.4 ± 26.3 |
| Sphericity | ++ | +++ | + |
| Microcarriers | Elemental Fraction (wt %) | ||
|---|---|---|---|
| C | N | H | |
| PLLA | 41.93 ± 0.11 | 0 | 4.80 ± 0.03 |
| PLLA/nHA/Ch | 46.96 ± 0.13 | 0.03 ± 0.01 | 5.22 ± 0.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Song, K.; Chen, Y.; Wang, Y.; Shi, F.; Nie, Y.; Liu, T. Design and Biophysical Characterization of Poly (l-Lactic) Acid Microcarriers with and without Modification of Chitosan and Nanohydroxyapatite. Polymers 2018, 10, 1061. https://doi.org/10.3390/polym10101061
Li L, Song K, Chen Y, Wang Y, Shi F, Nie Y, Liu T. Design and Biophysical Characterization of Poly (l-Lactic) Acid Microcarriers with and without Modification of Chitosan and Nanohydroxyapatite. Polymers. 2018; 10(10):1061. https://doi.org/10.3390/polym10101061
Chicago/Turabian StyleLi, Liying, Kedong Song, Yongzhi Chen, Yiwei Wang, Fangxin Shi, Yi Nie, and Tianqing Liu. 2018. "Design and Biophysical Characterization of Poly (l-Lactic) Acid Microcarriers with and without Modification of Chitosan and Nanohydroxyapatite" Polymers 10, no. 10: 1061. https://doi.org/10.3390/polym10101061