Contraction and Hydroscopic Expansion Stress of Dental Ion-Releasing Polymeric Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Water Absorption Dynamic Study
2.2. Water Sorption and Solubility
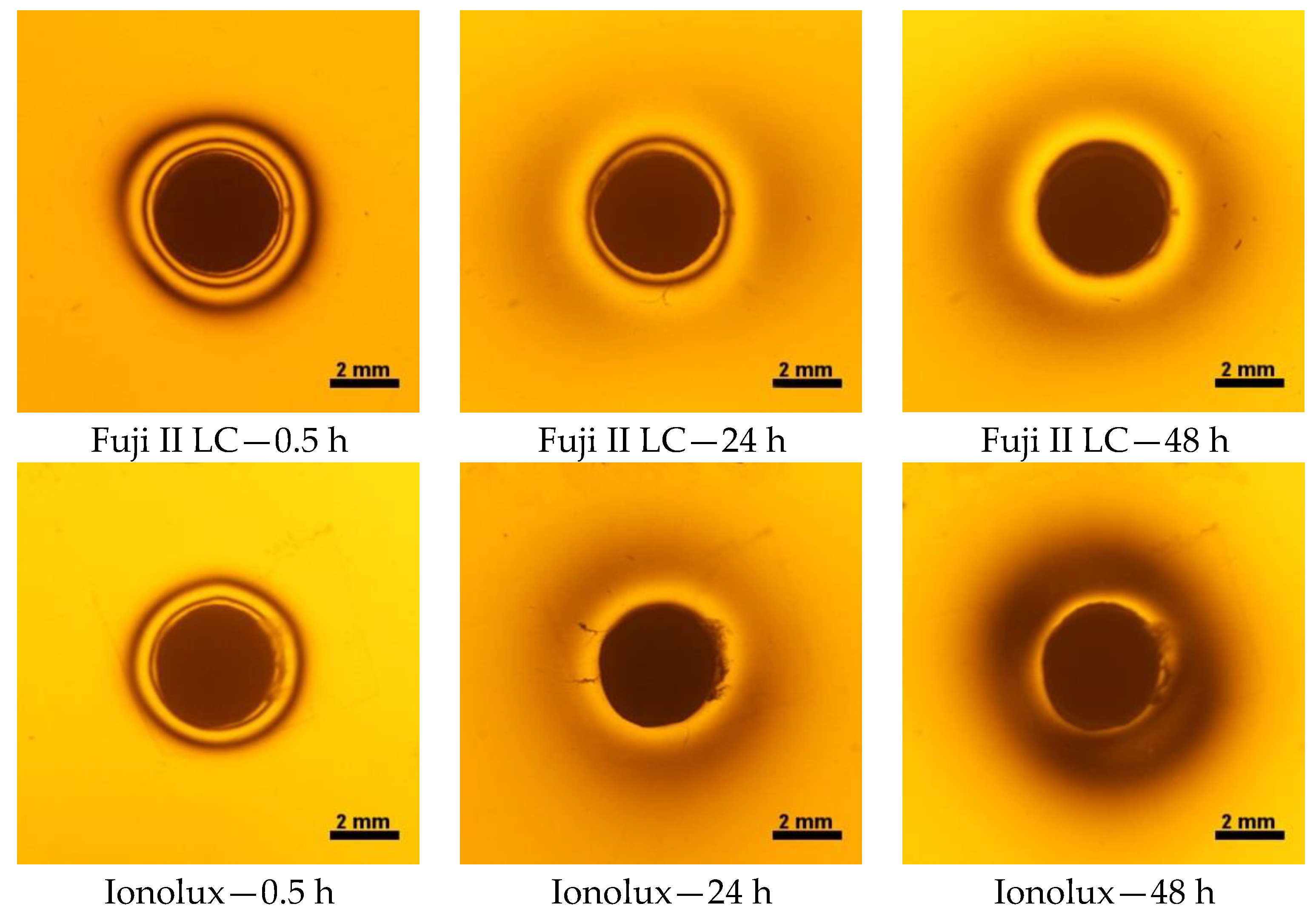
2.3. Photoelastic Study
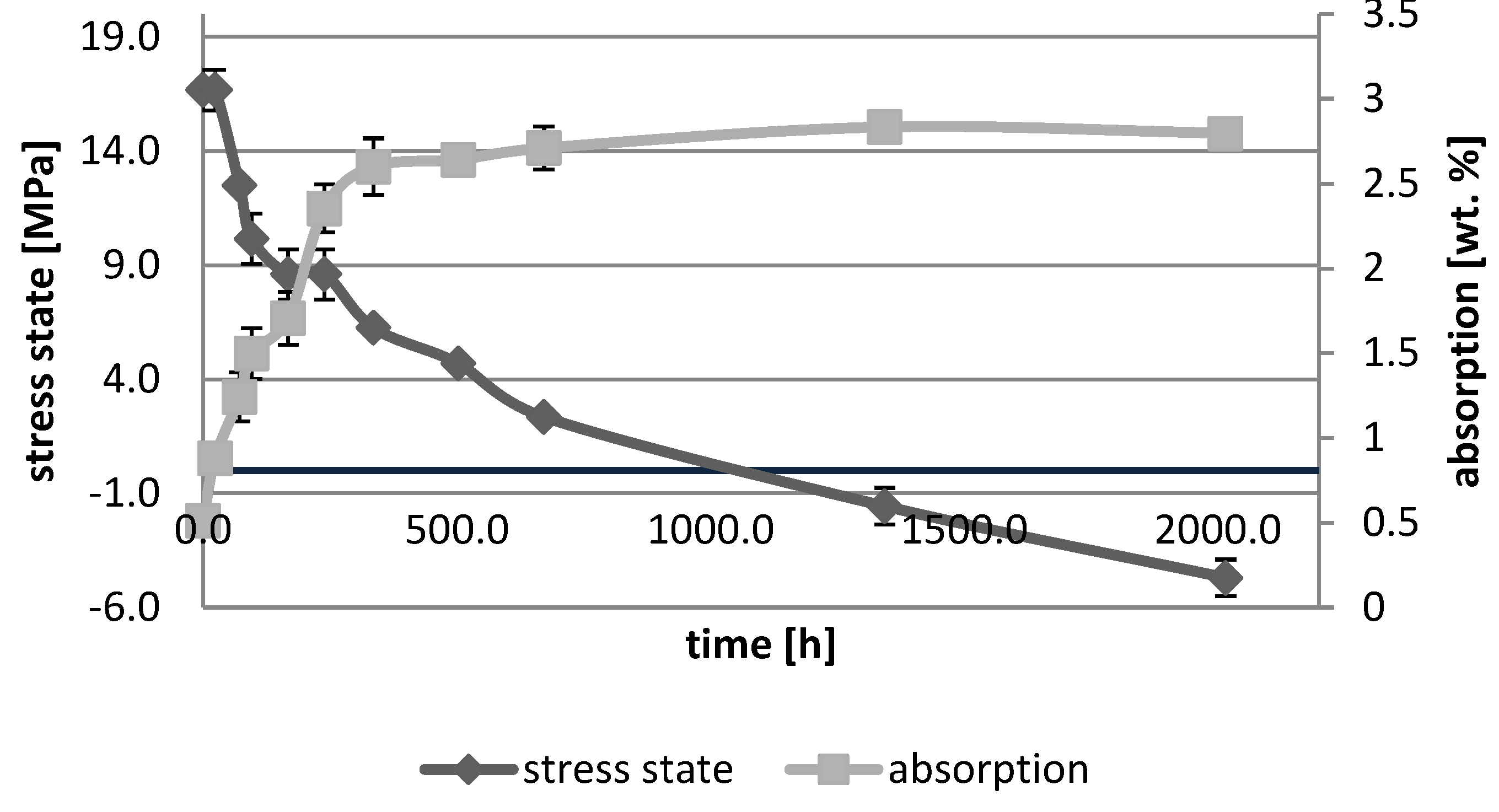
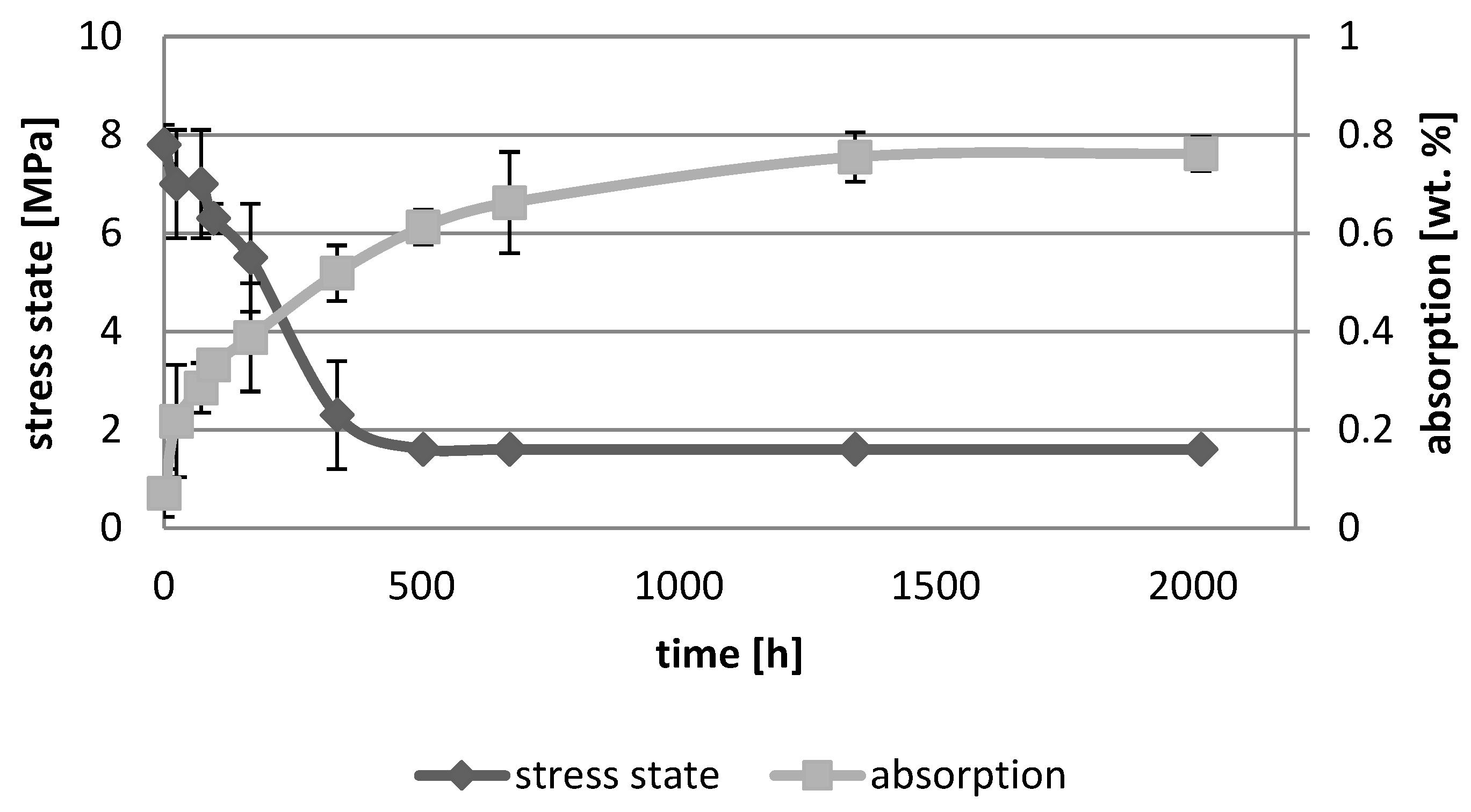
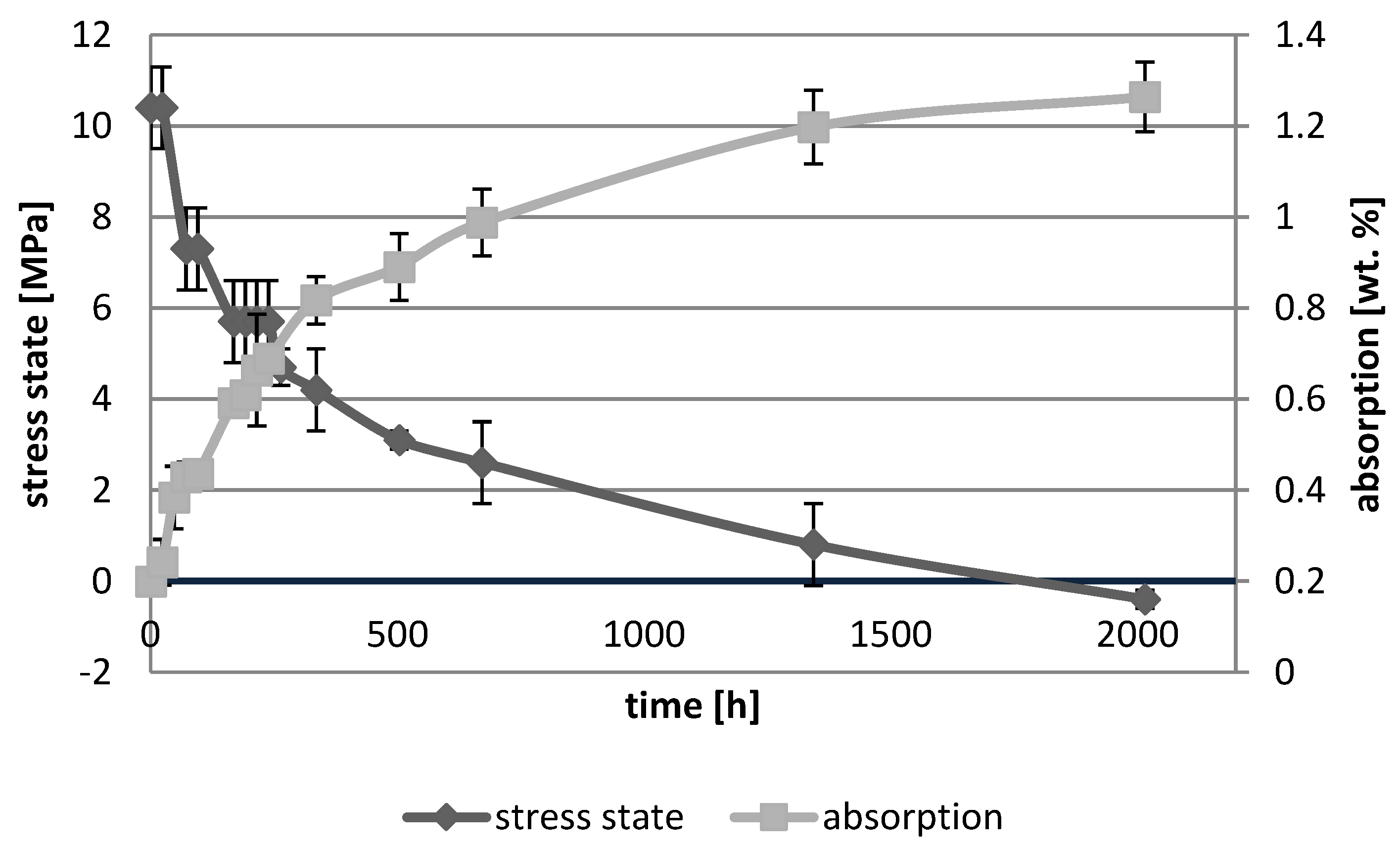
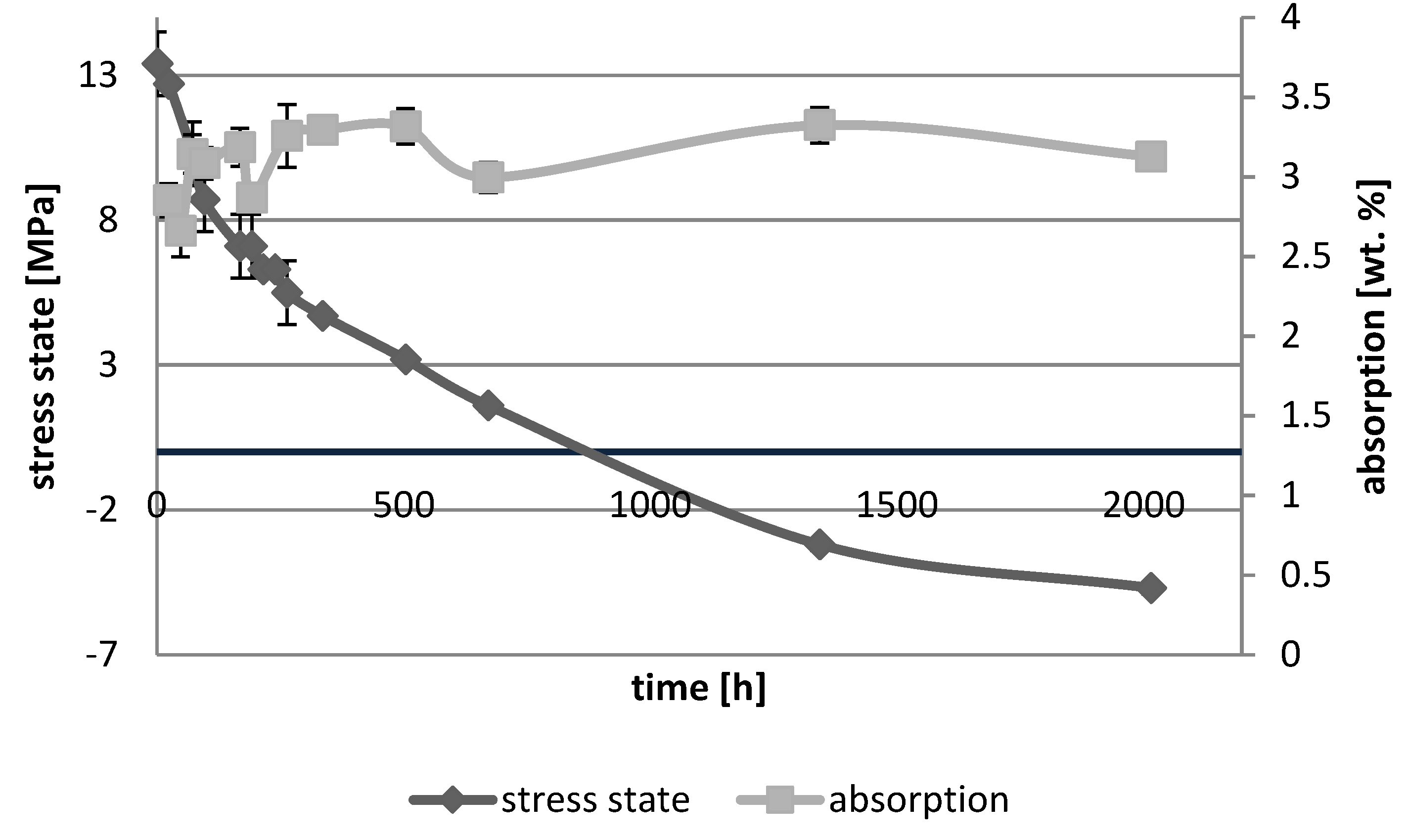
3. Results
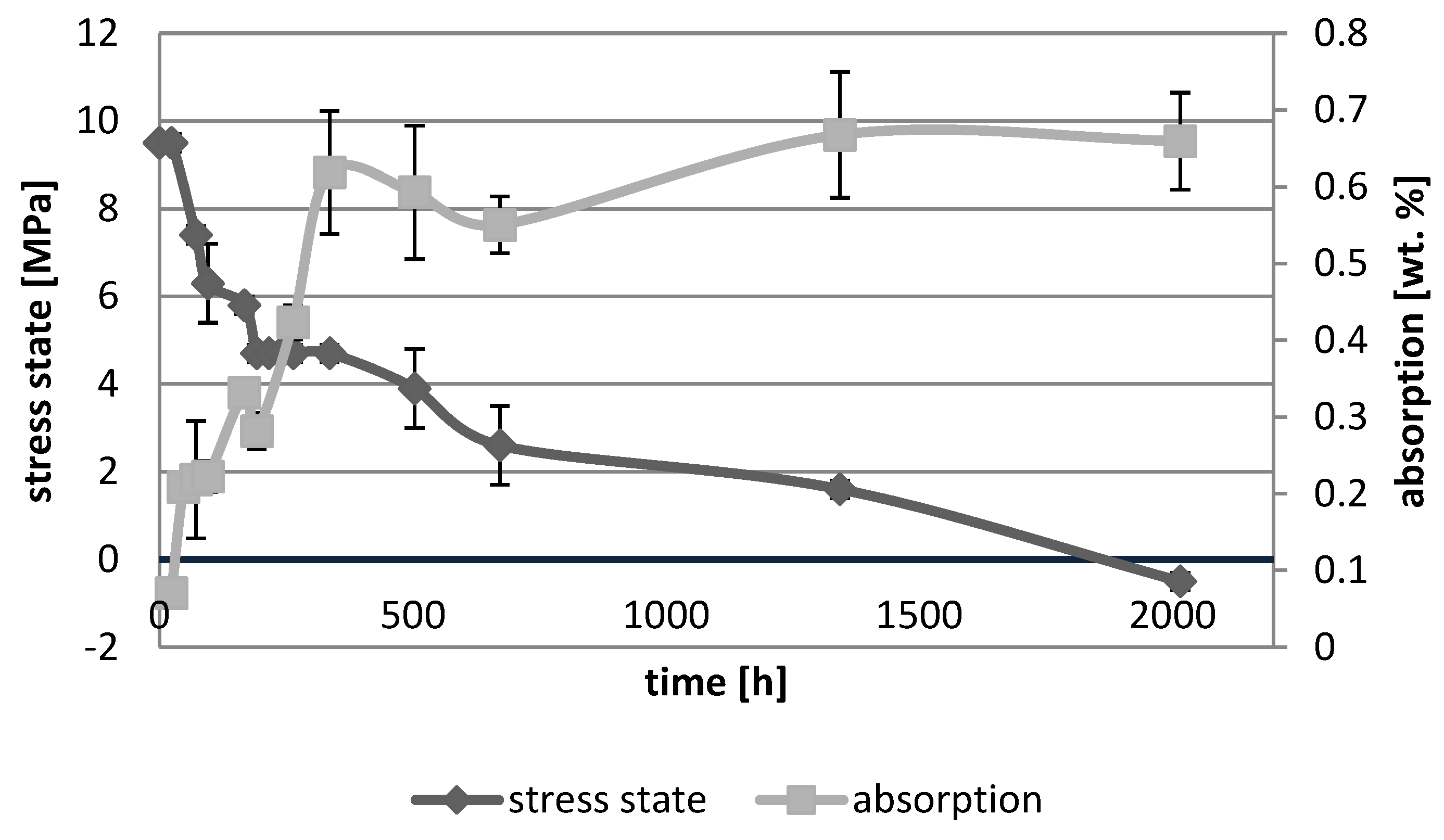
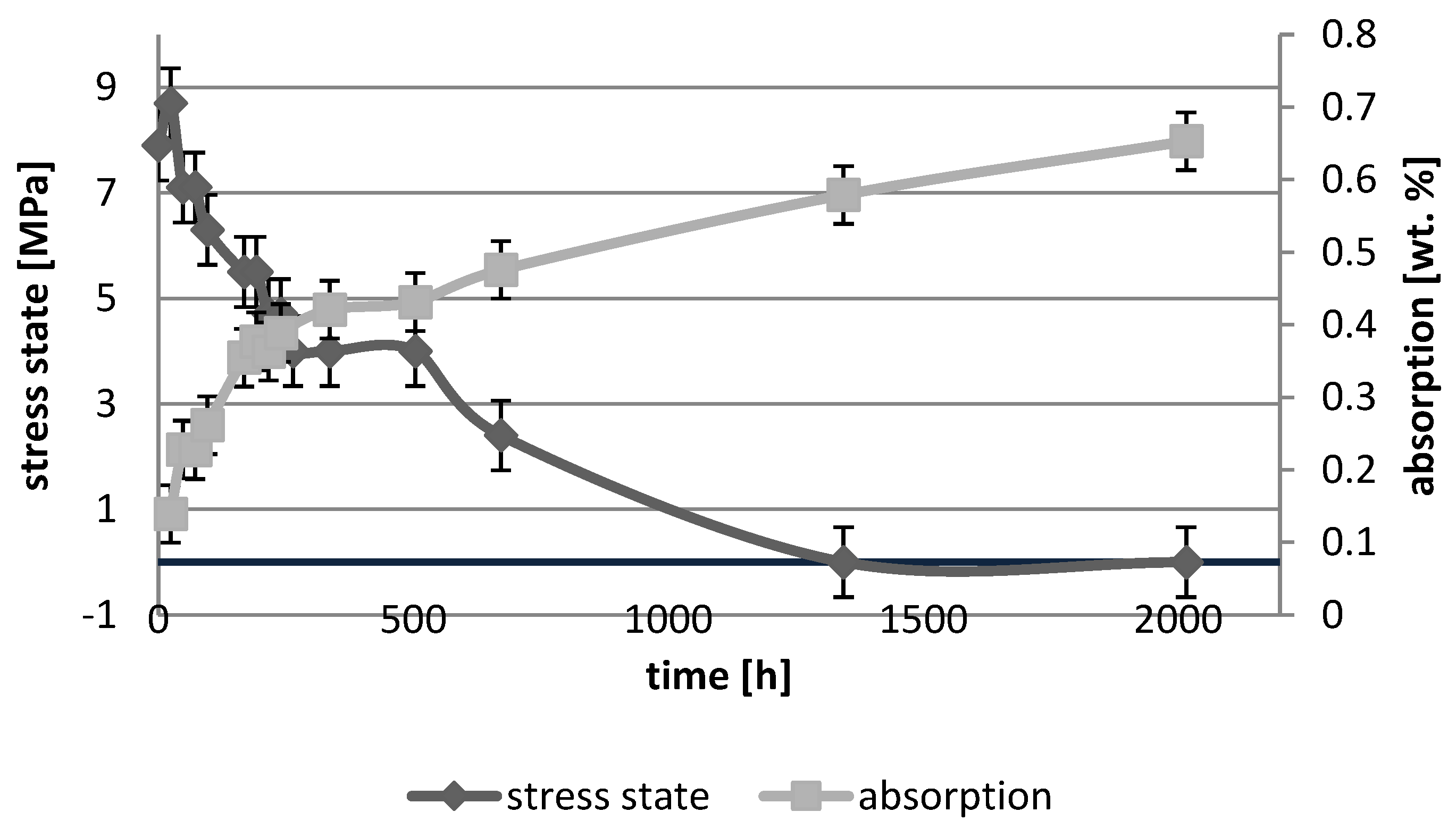
3.1. Absorption Dynamic Study
3.2. Water Sorption and Solubility
3.3. Photoelastic Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cramer, N.B.; Stansbury, J.W.; Bowman, C.N. Recent Advances and Developments in Composite Dental Restorative Materials. J. Dent. Res. 2011, 90, 402–416. [Google Scholar] [CrossRef] [PubMed]
- Cury, J.A.; De Oliveira, B.H.; Dos Santos, A.P.P.; Tenuta, L.M.A. Are Fluoride Releasing Dental Materials Clinically Effective on Caries Control? Dent. Mater. 2016, 32, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, S.; Nicholson, J. A Review of Glass-Ionomer Cements for Clinical Dentistry. J. Funct. Biomater. 2016, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Lohbauer, U. Dental Glass Ionomer Cements as Permanent Filling Materials?—Properties, Limitations and Future Trends. Materials 2010, 3, 76–96. [Google Scholar] [CrossRef]
- Najeeb, S.; Khurshid, Z.; Zafar, M.S.; Khan, A.S.; Zohaib, S.; Martí, J.M.N.; Sauro, S.; Matinlinna, J.P.; Rehman, I.U. Modifications in Glass Ionomer Cements: Nano-Sized Fillers and Bioactive Nanoceramics. Int. J. Mol. Sci. 2016, 17, 1134. [Google Scholar] [CrossRef] [PubMed]
- Dinakaran, S. Sorption and Solubility Characteristics of Compomer, Conventional and Resin Modified Glass-Ionomer Immersed in Various Media. J. Dent. Med. Sci. 2014, 13, 41–45. [Google Scholar] [CrossRef]
- Ruse, N.D. What Is a “Compomer”? J. Can. Dent. Assoc. (Tor) 1999, 65, 500–504. [Google Scholar]
- Meyer, J.M.; Cattani-Lorente, M.A.; Dupuis, V. Compomers: Between Glass-Ionomer Cements and Composites. Biomaterials 1998, 19, 529–539. [Google Scholar] [CrossRef]
- Abdel-karim, U.M.; El-Eraky, M.; Etman, W.M. Three-Year Clinical Evaluation of Two Nano-Hybrid Giomer Restorative Composites. Tanta Dent. J. 2014, 11, 213–222. [Google Scholar] [CrossRef]
- Ferracane, J.L.; Hilton, T.J. Polymerization Stress—Is It Clinically Meaningful? Dent. Mater. 2016, 32, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Domarecka, M.; Sokołowski, K.; Krasowski, M.; Łukomska-Szymańska, M.; Sokołowski, J. The Shrinkage Stress of Modified Flowable Dental Composites. Dent. Med. Probl. 2015, 52, 424–433. [Google Scholar] [CrossRef]
- Bociong, K.; Szczesio, A.; Sokolowski, K.; Domarecka, M.; Sokolowski, J.; Krasowski, M.; Lukomska-Szymanska, M. The Influence of Water Sorption of Dental Light-Cured Composites on Shrinkage Stress. Materials 2017, 10, 1142. [Google Scholar] [CrossRef] [PubMed]
- Sokolowski, G.; Szczesio, A.; Bociong, K.; Kaluzinska, K.; Lapinska, B.; Sokolowski, J.; Domarecka, M.; Lukomska-Szymanska, M. Dental Resin Cements—The Influence of Water Sorption on Contraction Stress Changes and Hydroscopic Expansion. Materials 2018, 11, 973. [Google Scholar] [CrossRef] [PubMed]
- Domarecka, M.; Sokołowski, K.; Krasowski, M.; Szczesio, A.; Bociong, K.; Sokołowski, J.; Łukomska-Szymańska, M. Influence of Water Sorption on the Shrinkage Stresses of Dental Composites Wpływ Sorpcji Wody Na Naprężenia Skurczowe Materiałów Kompozytowych. J. Stomatol. 2016, 69, 412–419. [Google Scholar]
- PN-EN ISO 4049:2003. Warszawa 2003. Available online: http://sklep.pkn.pl/pn-en-iso-4049-2003p.html (accessed on 28 August 2018).
- Müller, J.A.; Rohr, N.; Fischer, J. Evaluation of ISO 4049: Water Sorption and Water Solubility of Resin Cements. Eur. J. Oral Sci. 2017, 125, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, A.; Buchalla, W.; Attin, T. Review on Fluoride-Releasing Restorative Materials-Fluoride Release and Uptake Characteristics, Antibacterial Activity and Influence on Caries Formation. Dent. Mater. 2007, 23, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.B.; Gwinner, F.; Mitchell, J.C.; Ferracane, J.L. Ion Release from, and Fluoride Recharge of a Composite with a Fluoride-Containing Bioactive Glass. Dent. Mater. 2014, 30, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Hygroscopic and Hydrolytic Effects in Dental Polymer Networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L.; Palin, W.M. 10—Effects of Particulate Filler Systems on the Properties and Performance of Dental Polymer Composites. In Non-Metallic Biomaterials for Tooth Repair and Replacement; Vallittu, P., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2013. [Google Scholar]
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Study of Water Sorption, Solubility and Modulus of Elasticity of Light-Cured Dimethacrylate-Based Dental Resins. Biomaterials 2003, 24, 655–665. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Fukushima, T.; Horibe, T. Mechanical and Physical Properties of 2, 2′-Bis (4-Methacryloxy Polyethoxyphenyl) Propane Polymers. Dent. Mater. J. 1987, 6, 148–155, 224. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Kei, L.; Wei, S.H.Y.; Cheung, G.S.P.; Tay, F.R.; Pashley, D.H. The Influence of Hygroscopic Expansion of Resin-Based Restorative Materials on Artificial Gap Reduction. J. Adhes. Dent. 2002, 4, 61–71. [Google Scholar] [PubMed]
- McCabe, J.F.; Rusby, S. Water Absorption, Dimensional Change and Radial Pressure in Resin Matrix Dental Restorative Materials. Biomaterials 2004, 25, 4001–4007. [Google Scholar] [CrossRef] [PubMed]
- Hse, K.M.; Leung, S.K.; Wei, S.H. Resin-Ionomer Restorative Materials for Children: A Review. Aust. Dent. J. 1999, 44, 1–11. [Google Scholar] [PubMed]
- Eliades, G.; Kakaboura, A.; Palaghias, G. Acid-Base Reaction and Fluoride Release Profiles in Visible Light-Cured Polyacid-Modified Composite Restoratives (Compomers). Dent. Mater. 1998, 14, 57–63. [Google Scholar] [CrossRef]
- Davidson, C.L.; de Gee, A.J. Relaxation of Polymerization Contraction Stresses by Flow in Dental Composites. J. Dent. Res. 1984, 63, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Davidson, C.L.; de Gee, A.J.; Feilzer, A.; De Gee, A. The Competition between the Composite-Dentin Bond Strength and the Polymerization Contraction Stress. J. Dent. Res. 1984, 63, 1396–1399. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, R.L.; Peters, M.C.; Nelson, S.R.; Douglas, W.H.; Poort, H.W. Effects of Polymerization Contraction in Composite Restorations. J. Dent. 1992, 20, 178–182. [Google Scholar] [CrossRef]
- Suiter, E.A.; Watson, L.E.; Tantbirojn, D.; Lou, J.S.B.; Versluis, A. Effective Expansion: Balance between Shrinkage and Hygroscopic Expansion. J. Dent. Res. 2016, 95, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Manhart, J.; Hickel, R.; Kunzelmann, K. Polymerization Contraction Stress in Light-Cured Packable Composite Resins. Dent. Mater 2001, 17, 253–259. [Google Scholar] [CrossRef]
- Chen, H.Y.; Manhart, J.; Kunzelmann, K.H.; Hickel, R. Polymerization Contraction Stress in Light-Cured Compomer Restorative Materials. Dent. Mater. 2003, 19, 597–602. [Google Scholar] [CrossRef]
- Versluis, A.; Tantbirojn, D.; Lee, M.S.; Tu, L.S.; Delong, R. Can Hygroscopic Expansion Compensate Polymerization Shrinkage? Part I. Deformation of Restored Teeth. Dent. Mater. 2011, 27, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Cattani-Lorente, M.A.; Dupuis, V.; Payan, J.; Moya, F.; Meyer, J.M. Effect of Water on the Physical Properties of Resin-Modified Glass Ionomer Cements. Dent. Mater. 1999, 15, 71–78. [Google Scholar] [CrossRef]
- Momoi, Y.; McCabe, J.F. Hygroscopic Expansion of Resin Based Composites during 6 Months of Water Storage. Br. Dent. J. 1994, 176, 91–96. [Google Scholar] [CrossRef] [PubMed]


| Material | Manufacturer (Country) | Type | Composition | Curing Time [s] |
|---|---|---|---|---|
| Beautifil Bulk Fill Flow | Shofu (Japan) | Giomer | bis-GMA, UDMA, bis-MEPEPP, TEGDMA, multi-functional glass filler and S-PRG filler based fluoro-alumino-silicate glass (73 wt %) | 10 |
| Beautifil Flow Plus F00 | Shofu (Japan) | Giomer | bis-GMA, TEGDMA, multifunctional glass filler, improved S-PGR filler based on aluminofluoro-borosilicate glass, Al2O3 (67.3 wt %, 47.0 vol %) | 10 |
| Beautifil Flow F02 | Shofu (Japan) | Giomer | bis-GMA, TEGDMA, multifunctional glass filler, improved S-PGR filler based on fluoro-boroaluminosilicate glass (54.5 wt %/34.6 vol %) | 10 |
| Dyract eXtra | Dentsply Sirona (USA) | Compomer | UDMA, carboxylic acid modified, dimethacrylate resin, TEGDMA, BHT, strontium alimino-sodium-fluoro-silicate glass (50 vol %) | 10 |
| Compoglass Flow | Ivoclar Vivadent (Germany) | Compomer | UDMA, PEGDMA, cycloaliphate dicarbonic acid dimethacrylate, catalysts, stabilizers and pigments, mixed oxide—silanized, ytterbiumtrifluoride, Ba-Al-fluorosilikateglass-silanized (66.8 wt %) | 20 |
| Ionosit | DMG (Germany) | Compomer | acrylic resin, glass powder, silica, aliphatic dimethacrylate, aromatic dimethacrylate, polycarboxylic polymethacrylate (72 wt % 55 vol %) | 20 |
| Glasiosite | Voco (Germany) | Compomer | bis-GMA, UDMA, TEGDMA, BHT, SiO2, (Ba,B)AlSi, FAlSi (77.5 wt%) | 20 |
| TwinkiStar | Voco (Germany) | Compomer | bis-GMA, UDMA, carboxylic acid modified methacrylate, camphorquinone, BHT, Ba-Al- Str-fluorosilicate glass, Silicon dioxide (78 wt %) | 20 |
| Ionolux | Voco (Germany) | RMGI | polyacrylic acid, HEMA, bis-GMA, UDMA, fluoro-alumino-silicate glass | 20 |
| Fuji II LC | GC (USA) | RMGI | polyacrylic acid, HEMA, UDMA, camphorqunone, fluoro-alumino-silicate glass | 20 |
| Material | Manufacturer (Country) | Dedicated Restorative Material | Composition | Curing Time [s] |
|---|---|---|---|---|
| BeautiBond | Shofu (Japan) | Beautifil Flow, Beautifil Bulk Fill Flow, Beautifil Flow Plus F00 | bis-GMA, TEGDMA, phosphoric acid monomer, carboxylic acid monomer | 10 |
| XP Bond | Dentsply Sirona (USA) | Dyract eXtra, Fuji II LC | TCB, PENTA, UDMA, TEGDMA, HEMA, butylated benzenediol (stabilizer), ethyl-4-dimethylaminobenzoate, camphorquinone | 10 |
| Monobond Plus | Ivoclar Vivadent (Germany) | Compoglass Flow | 10-MDP, silane methacrylate, ethanol, sulfide methacrylate | 10 |
| Ecosite-Bond | DMG (Germany) | Ionosit | dental resins, ethanol, water, additives and catalysts | 10 |
| Futurabond M+ | Voco (Germany) | Glasiosite, TwinkyStar, Ionolux | HEMA, bis-GMA, etanol, acidic adhesive monomer | 10 |
| Material | Stress State [MPa] | Absolute Values of Stress Changes [MPa] | Contraction Stress Drop [%] | Sorption [µg/mm3] | Solubility [µg/mm3] | |
|---|---|---|---|---|---|---|
| 0.5 h | 2016 h | |||||
| Beautifil Flow F02 | 16.7 ± 0.9 | −4.7 ± 0.8 | 21.4 | 128 * | 45.9 ± 2.1 | 0.2 ± 0.1 |
| Beautifil Bulk Fill Flow | 11.1 ± 0.4 | 4.2 ± 0.9 | 6.9 | 62 | 13.6 ± 0.4 | 0.9 ± 0.3 |
| Beautifil Flow Plus F00 | 12.8 ± 0.4 | 0.0 ± 0.2 | 12.8 | 100 | 26.4 ± 1.0 | 0.5 ± 0.1 |
| Dyract eXtra | 7.8 ± 0.1 | 1.6 ± 0.1 | 6.2 | 79 | 16.5 ± 0.5 | 2.9 ± 0.7 |
| Compoglass Flow | 10.4 ± 0.9 | −0.4 ± 0.2 | 10.8 | 104 * | 28.9 ± 0.8 | 2.3 ± 0.5 |
| Ionosit | 13.4 ± 1.1 | −4.7 ± 0.2 | 18.1 | 135 * | 103.8 ± 0.9 | 3.0 ± 0.2 |
| Glasiosite | 9.5 ± 0.2 | −0.5 ± 0.2 | 10.0 | 105 * | 16.4 ± 0.8 | 1.3 ± 0.1 |
| TwinkiStar | 7.9 ± 0.2 | 0.0 ± 0.2 | 7.9 | 100 | 17.7 ± 0.4 | 1.6 ± 0.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokolowski, K.; Szczesio-Wlodarczyk, A.; Bociong, K.; Krasowski, M.; Fronczek-Wojciechowska, M.; Domarecka, M.; Sokolowski, J.; Lukomska-Szymanska, M. Contraction and Hydroscopic Expansion Stress of Dental Ion-Releasing Polymeric Materials. Polymers 2018, 10, 1093. https://doi.org/10.3390/polym10101093
Sokolowski K, Szczesio-Wlodarczyk A, Bociong K, Krasowski M, Fronczek-Wojciechowska M, Domarecka M, Sokolowski J, Lukomska-Szymanska M. Contraction and Hydroscopic Expansion Stress of Dental Ion-Releasing Polymeric Materials. Polymers. 2018; 10(10):1093. https://doi.org/10.3390/polym10101093
Chicago/Turabian StyleSokolowski, Krzysztof, Agata Szczesio-Wlodarczyk, Kinga Bociong, Michal Krasowski, Magdalena Fronczek-Wojciechowska, Monika Domarecka, Jerzy Sokolowski, and Monika Lukomska-Szymanska. 2018. "Contraction and Hydroscopic Expansion Stress of Dental Ion-Releasing Polymeric Materials" Polymers 10, no. 10: 1093. https://doi.org/10.3390/polym10101093
APA StyleSokolowski, K., Szczesio-Wlodarczyk, A., Bociong, K., Krasowski, M., Fronczek-Wojciechowska, M., Domarecka, M., Sokolowski, J., & Lukomska-Szymanska, M. (2018). Contraction and Hydroscopic Expansion Stress of Dental Ion-Releasing Polymeric Materials. Polymers, 10(10), 1093. https://doi.org/10.3390/polym10101093






