Energy Transfer in Dendritic Systems Having Pyrene Peripheral Groups as Donors and Different Acceptor Groups
Abstract
:1. Introduction
Optical and Photophysical Features of Pyrene
2. Dendritic Molecules Bearing Peripheral Pyrene Groups as Donors and a Porphyrin as an Acceptor
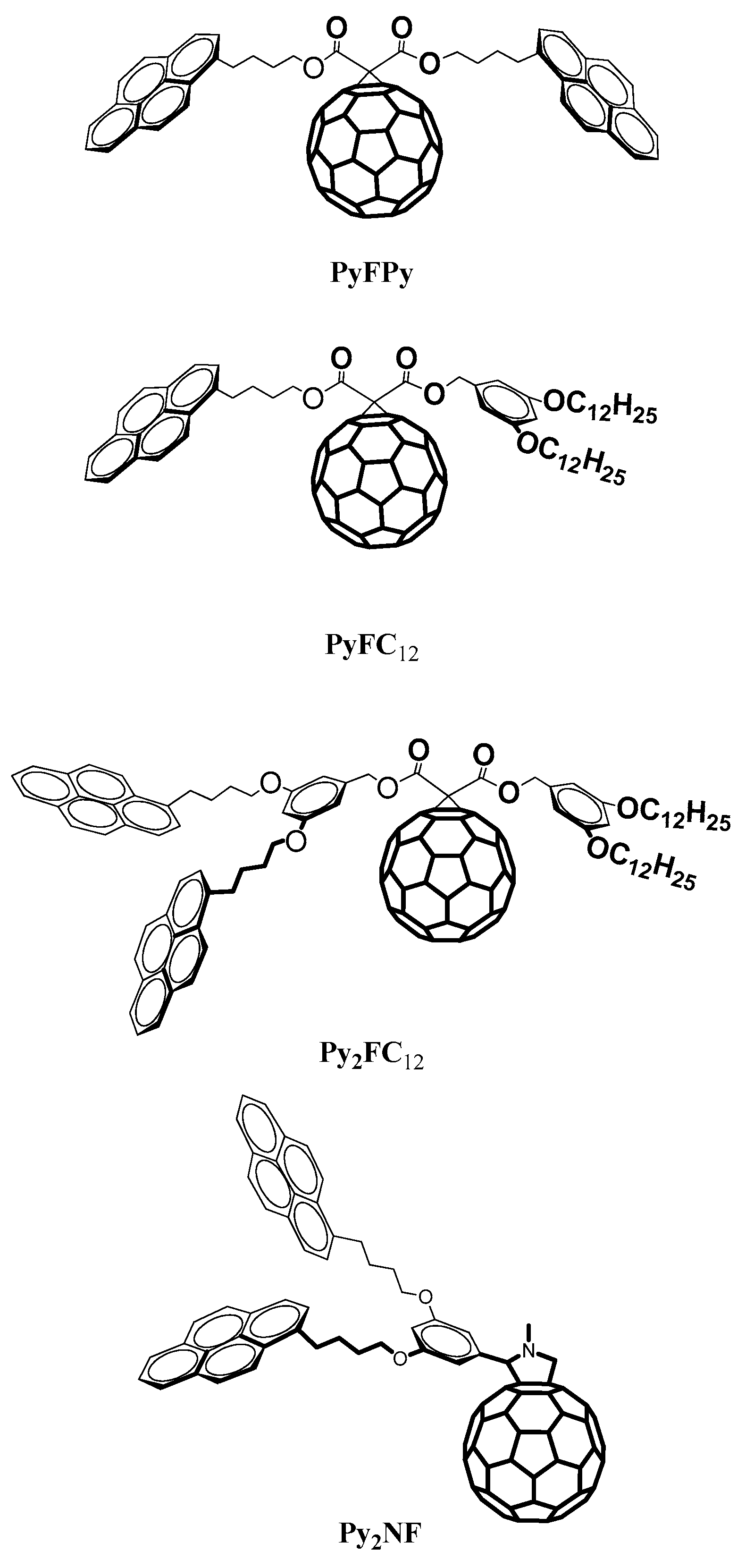
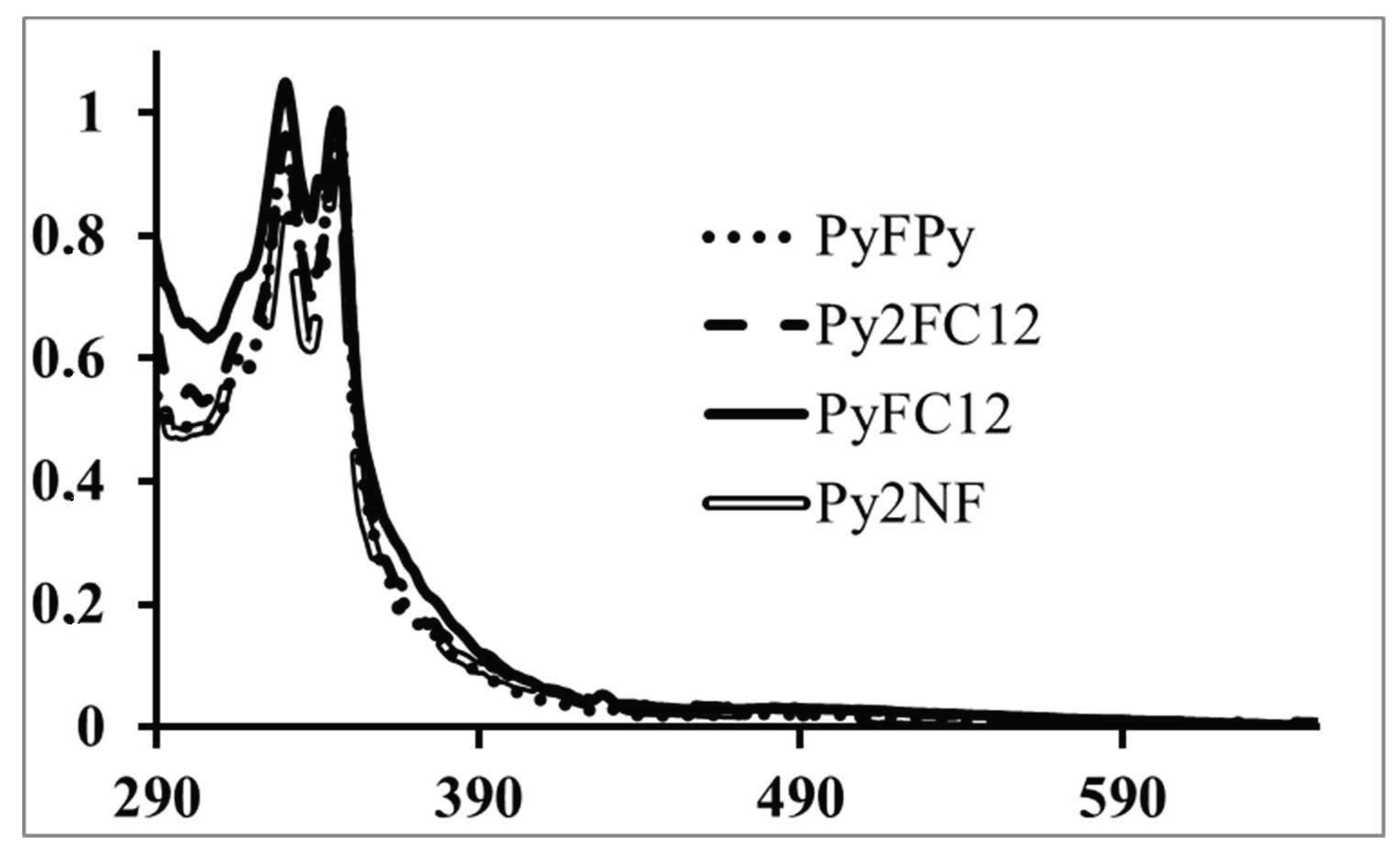
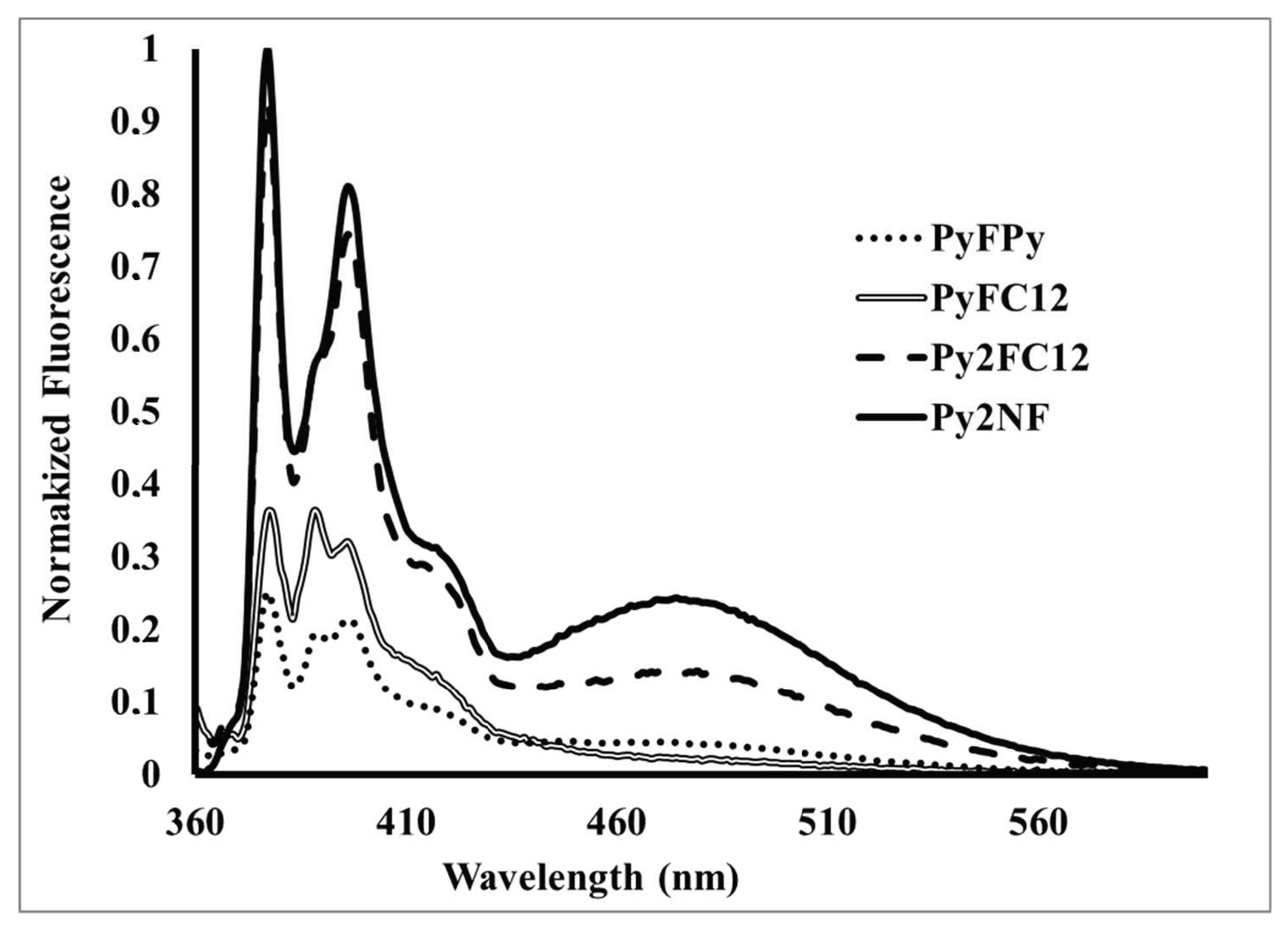
3. Dendritic Molecules Bearing Peripheral Pyrene Groups as Donors and a Fullerene as Acceptor
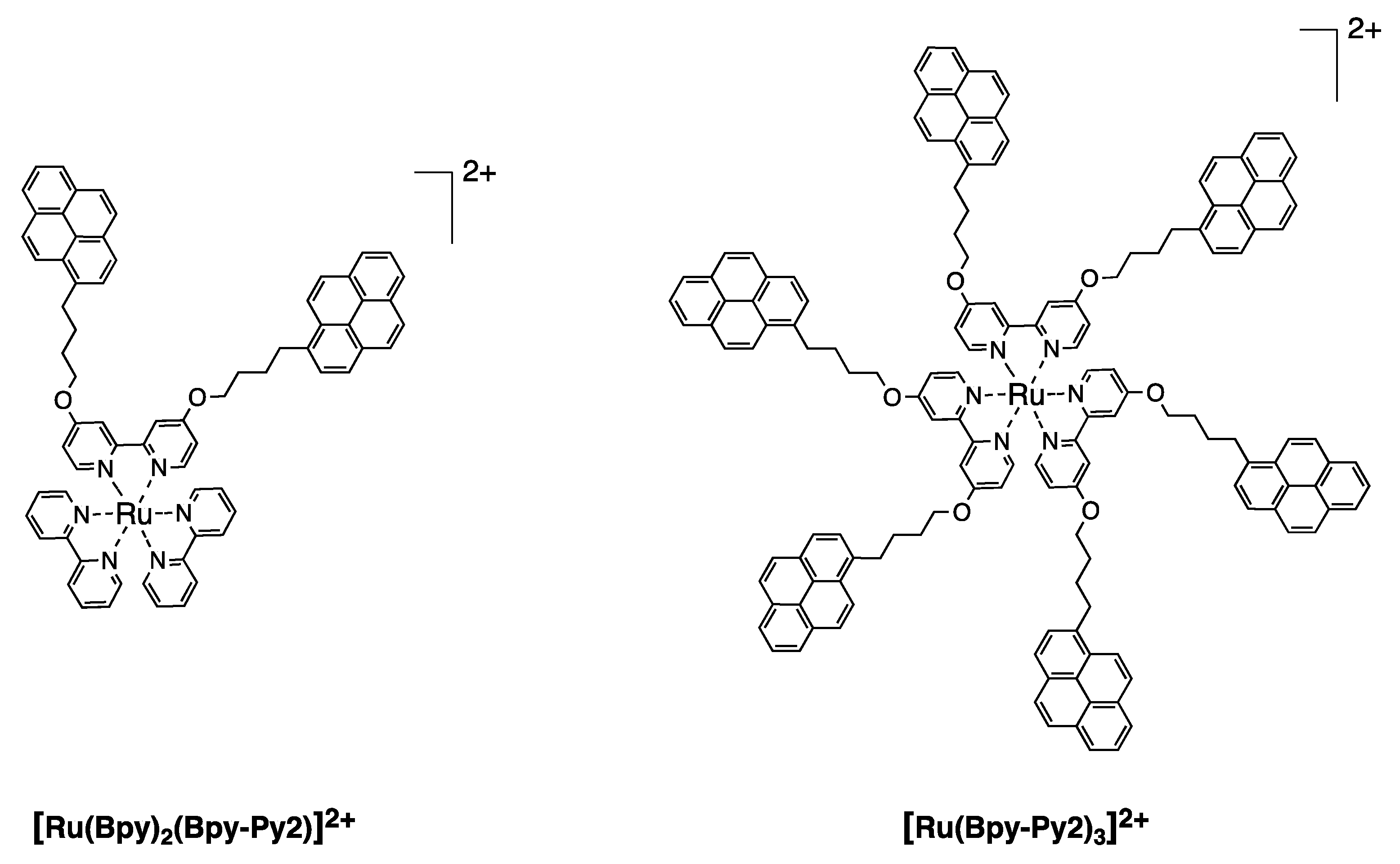
4. Dendritic Molecules Bearing Peripheral Pyrene Groups as Donors and an Organometallic Complex as Acceptor
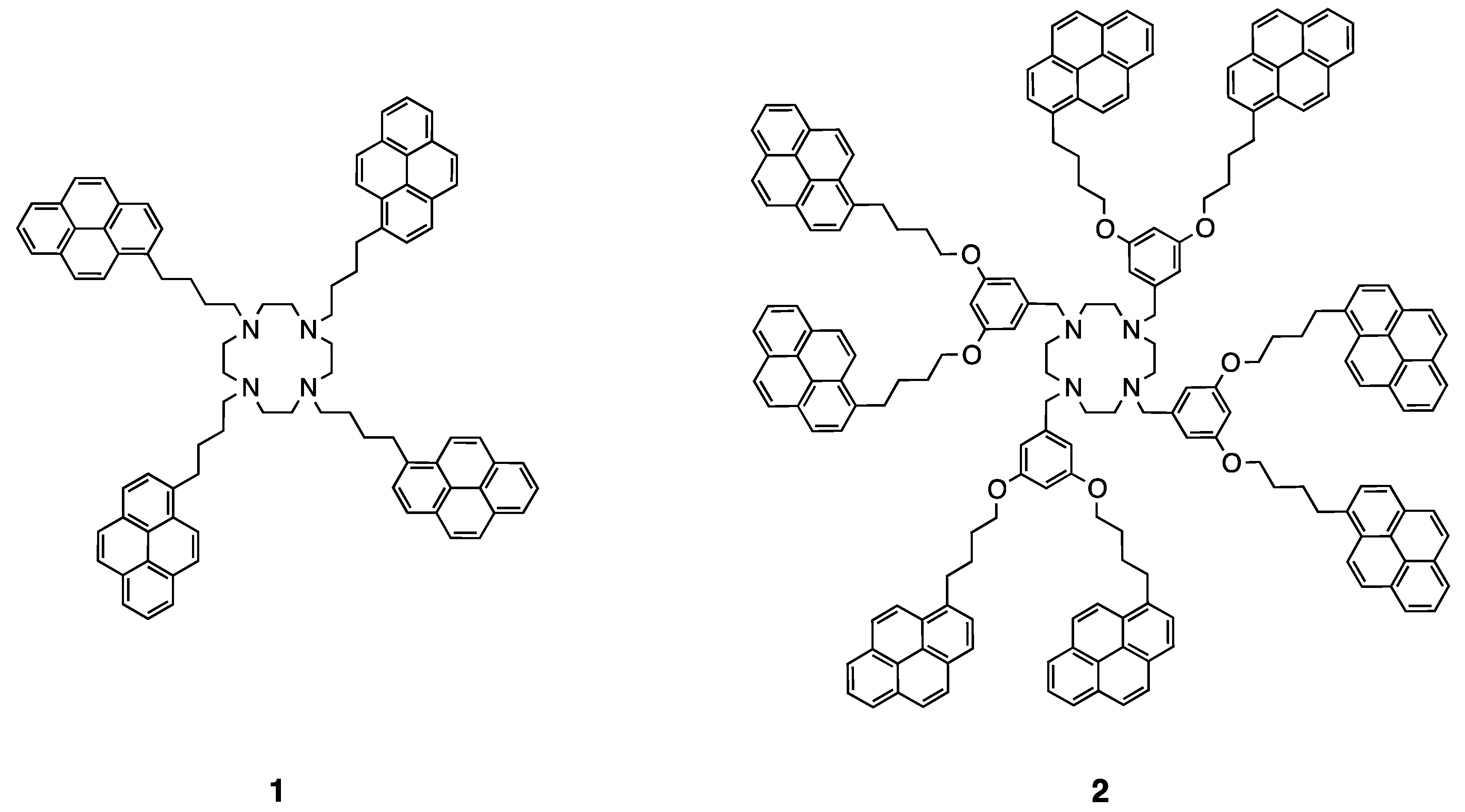
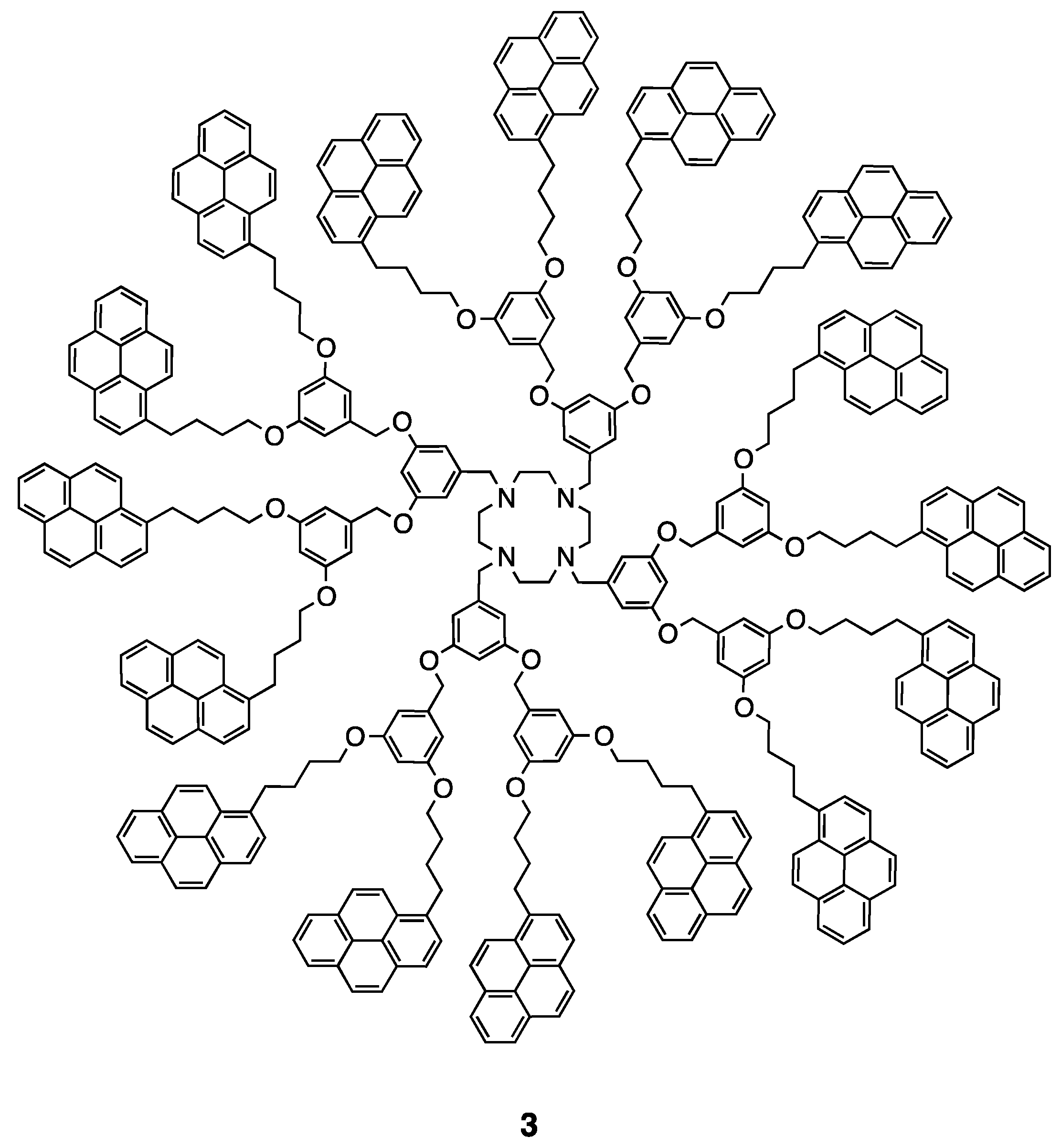
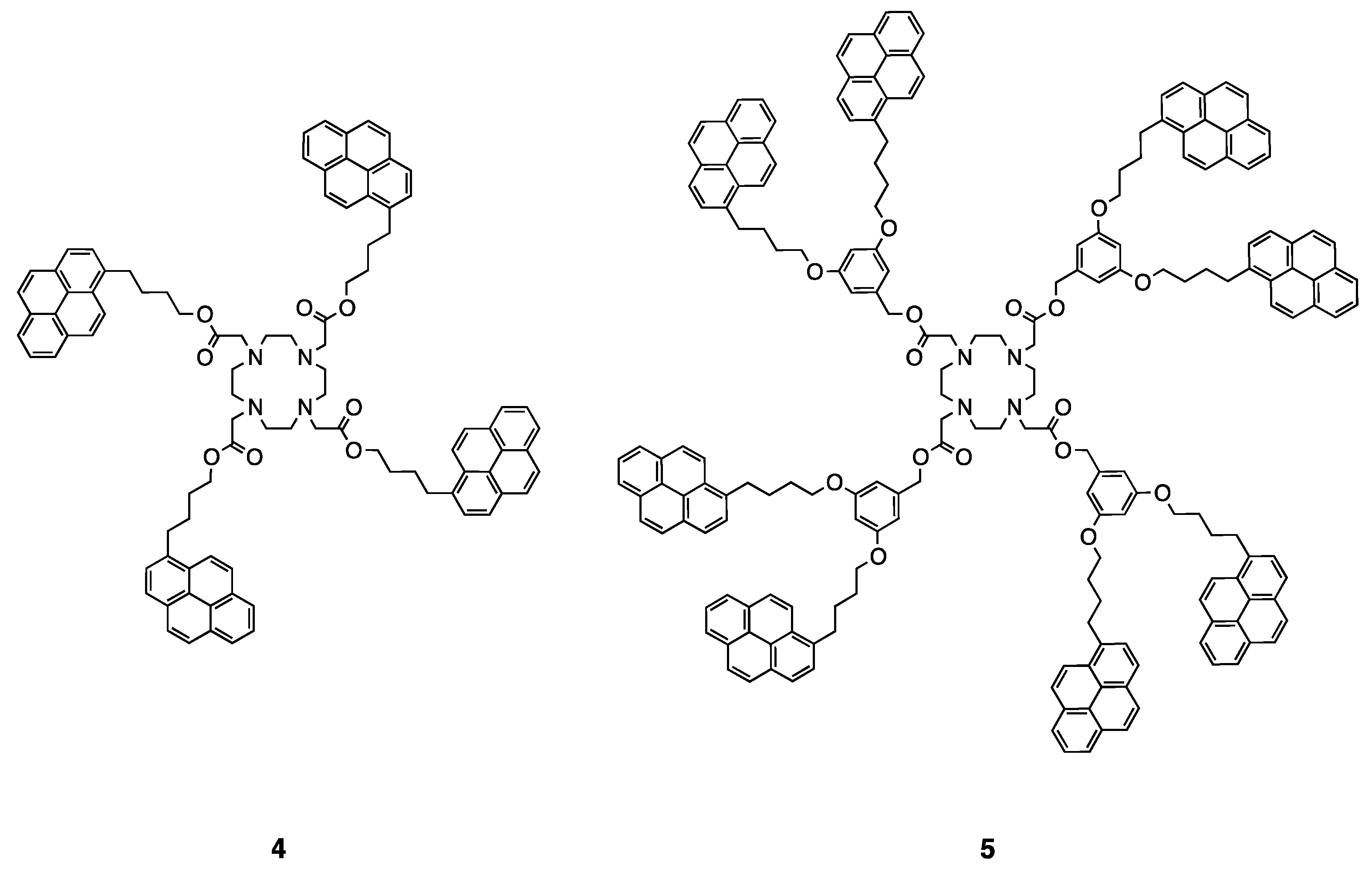
5. Dendritic Molecules Bearing Peripheral Pyrene Groups as Donors and Cyclen Core as Potential Ligands for Metal Ions
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vögtle, F.; Richardt, G.; Werner, N. Dendrimer Chemistry: Concepts, Syntheses, Properties, Applications; Wiley VCH: Weinheim, Germany, 2009; ISBN 9783527320660. [Google Scholar]
- Tomalia, D.A.; Naylor, A.M.; Goddard, W.A. Starburst Dendrimers: Molecular-Level Control of Size, Shape, Surface Chemistry, Topology, and Flexibility from Atoms to Macroscopic Matter. Angew. Chem. Int. Ed. 1990, 29, 138–175. [Google Scholar] [CrossRef]
- Newkome, G.R.; Yao, Z.; Baker, G.R.; Gupta, V.K. Micelles. Part 1. Cascade molecules: A new approach to micelles. A [27]-arborol. J. Org. Chem. 1985, 50, 2003–2004. [Google Scholar] [CrossRef]
- Nantalaksakul, A.; Reddy, D.R.; Bardeen, C.J.; Thayumanavan, S. Light harvesting dendrimers. Photosynth. Res. 2006, 87, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Mignani, S.; Rodrigues, J.; Tomas, H.; Zablocka, M.; Shi, X.; Caminade, A.M.; Majoral, J.P. Dendrimers in combination with natural products and analogues as anti-cancer agents. Chem. Soc. Rev. 2018, 47, 514–532. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.M.; Turrin, C.O. Dendrimers for drug delivery. J. Mater. Chem. B 2014, 2, 4055–4066. [Google Scholar] [CrossRef]
- Menjoge, A.R.; Kannan, R.M.; Tomalia, D.A. Dendrimer-based drug and imaging conjugates: Design considerations for nanomedical applications. Drug Discov. Today 2010, 15, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.J.; Zhang, S.; Percec, V. From structure to function via complex supramolecular dendrimer systems. Chem. Soc. Rev. 2015, 44, 3900–3923. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; He, C.; Tian, H.; Ding, J.; Hsiao, B.S.; Chu, B.; Chen, X. Polymeric nanostructured materials for biomedical applications. Prog. Polym. Sci. 2016, 60, 86–128. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Deraedt, C.; Ruiz, J.; Astruc, D. Magnetic and Dendritic Catalysts. Acc. Chem. Res. 2015, 48, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Harriman, A. Artificial light-harvesting arrays for solar energy conversion. Chem. Commun. 2015, 51, 11745–11756. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers designed for functions: From physical, photophysical, and supramolecular properties to applications in sensing, catalysis, molecular electronics, photonics, and nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef] [PubMed]
- Ceroni, P.; Bergamini, G.; Marchioni, F.; Balzani, V. Luminescence as a tool to investigate dendrimer properties. Prog. Polym. Sci. 2005, 30, 453–473. [Google Scholar] [CrossRef]
- Adronov, A.; Fréchet, J. Light-harvesting dendrimers. Chem. Commun. 2000, 18, 1701–1710. [Google Scholar] [CrossRef]
- Fréchet, J.M.J. Dendrimers and other dendritic macromolecules: From building blocks to functional assemblies in nanoscience and nanotechnology. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3713–3725. [Google Scholar] [CrossRef] [Green Version]
- Balzani, V.; Ceroni, P.; Maestri, M.; Vicinelli, V. Light-harvesting dendrimers. Curr. Opin. Chem. Biol. 2003, 7, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zeng, Y.; Yu, T.; Chen, J.; Yang, G.; Li, Y. Advances in photofunctional dendrimers for solar energy conversion. J. Phys. Chem. Lett. 2014, 5, 2340–2350. [Google Scholar] [CrossRef] [PubMed]
- Mula, S.; Frein, S.; Russo, V.; Ulrich, G.; Ziessel, R.; Barberá, J.; Deschenaux, R. Red and blue liquid-crystalline borondipyrromethene dendrimers. Chem. Mater. 2015, 27, 2332–2342. [Google Scholar] [CrossRef]
- Lo, S.C.; Burn, P.L. Development of dendrimers: Macromolecules for use in organic light-emitting diodes and solar cells. Chem. Rev. 2007, 107, 1097–1116. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, R.; Ramesh, M.; Padhy, H.; Chiang, I.H.; Chu, C.W.; Wei, K.H.; Lin, H.C. Novel metallo-dendrimers containing various Ru core ligands and dendritic thiophene arms for photovoltaic applications. Polym. Chem. 2014, 5, 5423–5435. [Google Scholar] [CrossRef]
- Krieger, A.; Fuenzalida Werner, J.P.; Mariani, G.; Gröhn, F. Functional Supramolecular Porphyrin-Dendrimer Assemblies for Light Harvesting and Photocatalysis. Macromolecules 2017, 50, 3464–3475. [Google Scholar] [CrossRef]
- Winnik, F. Photophysics of Preassociated Pyrenes in Aqueous Polymer Solutions and in Other Organized Media. Chem. Rev. 1993, 93, 587–614. [Google Scholar] [CrossRef]
- Winnik, M.A. End-to-End Cyclization of Polymer Chains. Acc. Chem. Res. 1985, 18, 73–79. [Google Scholar] [CrossRef]
- Duhamel, J. Polymer chain dynamics in solution probed with a fluorescence blob model. Acc. Chem. Res. 2006, 39, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Duhamel, J. Pyrene florescence to study polymeric systems. In Molecular Interfacial Phenomena of Polymers and Biopolymers; Woodhead Publishing: Cambridge, UK, 2005; pp. 214–248. ISBN 9781855739284. [Google Scholar]
- Duhamel, J. Internal dynamics of dendritic molecules probed by pyrene excimer formation. Polymers 2012, 4, 211–239. [Google Scholar] [CrossRef]
- Duhamel, J. New insights in the study of pyrene excimer fluorescence to characterize macromolecules and their supramolecular assemblies in solution. Langmuir 2012, 28, 6527–6538. [Google Scholar] [CrossRef] [PubMed]
- Figueira-Duarte, T.M.; Müllen, K. Pyrene-based materials for organic electronics. Chem. Rev. 2011, 111, 7260–7314. [Google Scholar] [CrossRef] [PubMed]
- Alqurashy, B.A.; Cartwright, L.; Iraqi, A.; Zhang, Y.; Lidzey, D.G. Pyrene–benzothiadiazole-based copolymers for application in photovoltaic devices. Polym. Adv. Technol. 2017, 28, 193–200. [Google Scholar] [CrossRef]
- Feng, X.; Hu, J.Y.; Redshaw, C.; Yamato, T. Functionalization of Pyrene To Prepare Luminescent Materials—Typical Examples of Synthetic Methodology. Chem. A Eur. J. 2016, 22, 11898–11916. [Google Scholar] [CrossRef] [PubMed]
- Salunke, J.K.; Wong, F.L.; Feron, K.; Manzhos, S.; Lo, M.F.; Shinde, D.; Patil, A.; Lee, C.S.; Roy, V.A.L.; Sonar, P.; et al. Phenothiazine and carbazole substituted pyrene based electroluminescent organic semiconductors for OLED devices. J. Mater. Chem. C 2016, 4, 1009–1018. [Google Scholar] [CrossRef]
- Ogawa, M.; Momotake, A.; Arai, T. Water-soluble poly(aryl ether) dendrimers as a potential fluorescent detergent to form micelles at very low CMC. Tetrahedron Lett. 2004, 45, 8515–8518. [Google Scholar] [CrossRef]
- Keyes-Baig, C.; Duhamel, J.; Wettig, S. Characterization of the behavior of a pyrene substituted gemini surfactant in water by fluorescence. Langmuir 2011, 27, 3361–3371. [Google Scholar] [CrossRef] [PubMed]
- Yip, J.; Duhamel, J.; Bahun, G.J.; Adronov, A. A study of the dynamics of the branch ends of a series of pyrene-labeled dendrimers based on pyrene excimer formation. J. Phys. Chem. B 2010, 114, 10254–10265. [Google Scholar] [CrossRef] [PubMed]
- Rivera, E.; Aguilar-Martínez, M.; Terán, G.; Flores, R.F.; Bautista-Martínez, J.A. Thermal, optical, electrochemical properties and conductivity of trans- and cis-poly(1-ethynylpyrene): A comparative investigation. Polymer 2005, 46, 4789–4798. [Google Scholar] [CrossRef]
- Illescas, J.; Caicedo, C.; Zaragoza-Galán, G.; Ramírez-Fuentes, Y.S.; Gelover-Santiago, A.; Rivera, E. Synthesis, characterization and optical properties of novel well-defined di(1-ethynylpyrene)s. Synth. Met. 2011, 161, 775–782. [Google Scholar] [CrossRef]
- Stewart, G.M.; Fox, M.A. Chromophore-labeled dendrons as light harvesting antennae. J. Am. Chem. Soc. 1996, 118, 4354–4360. [Google Scholar] [CrossRef]
- Cicchi, S.; Fabbrizzi, P.; Ghini, G.; Brandi, A.; Foggi, P.; Marcelli, A.; Righini, R.; Botta, C. Pyrene-excimers-based antenna systems. Chem. A Eur. J. 2009, 15, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Vanjinathan, M.; Lin, H.C.; Nasar, A.S. Synthesis, characterization and photophysical properties of DCM-based light-harvesting dendrimers. Macromol. Chem. Phys. 2011, 212, 849–859. [Google Scholar] [CrossRef]
- Fogel, Y.; Zhi, L.; Rouhanipour, A.; Andrienko, D.; Räder, H.J.; Müllen, K. Graphitic Nanoribbons with Dibenzo[e,l]pyrene Repeat Units: Synthesis and Self-Assembly. Macromolecules 2009, 42, 6878–6884. [Google Scholar] [CrossRef]
- Rivera, E.; Belletête, M.; Zhu, X.X.; Durocher, G.; Giasson, R. Novel polyacetylenes containing pendant 1-pyrenyl groups: Synthesis, characterization, and thermal and optical properties. Polymer 2002, 43, 5059–5068. [Google Scholar] [CrossRef]
- Rivera, E.; Wang, R.; Zhu, X.X.; Zargarian, D.; Giasson, R. Preparation of cis-poly(1-ethynylpyrene) using (1-Me-indenyl)(PPh3)Ni-C≡C-Ph/methylaluminoxane as catalyst. J. Mol. Catal. A Chem. 2003, 204–205, 325–332. [Google Scholar] [CrossRef]
- Belletête, M.; Rivera, E.; Giasson, R.; Zhu, X.X.; Durocher, G. UV-Vis and fluorescence study of polyacetylenes with pendant 1-pyrenyl groups: A comparative investigation of cis- and trans-poly(1-ethynyl-pyrene). Synth. Met. 2004, 143, 37–42. [Google Scholar] [CrossRef]
- Morales-Saavedra, O.G.; Rivera, E. Linear and nonlinear optical properties of trans- and cis-poly(1-ethynylpyrene) based sonogel hybrid materials. Polymer 2006, 47, 5330–5337. [Google Scholar] [CrossRef]
- Ramírez-Fuentes, Y.S.; Illescas, J.; Gelover-Santiago, A.; Rivera, E. Luminescent polymers containing pyrenyl groups prepared by frontal polymerization of di(ethylene glycol) ethyl ether acrylate using Trigonox-23 as initiator. Mater. Chem. Phys. 2012, 135, 772–779. [Google Scholar] [CrossRef]
- Illescas, J.; Ramírez-Fuentes, Y.S.; Zaragoza-Galán, G.; Porcu, P.; Mariani, A.; Rivera, E. PEGDA-based luminescent polymers prepared by frontal polymerization. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2890–2897. [Google Scholar] [CrossRef]
- Zaragoza-Galán, G.; Fowler, M.A.; Duhamel, J.; Rein, R.; Solladié, N.; Rivera, E. Synthesis and characterization of novel pyrene-dendronized porphyrins exhibiting efficient fluorescence resonance energy transfer: Optical and photophysical properties. Langmuir 2012, 28, 11195–11205. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza-Galán, G.; Ortíz-Palacios, J.; Valderrama, B.X.; Camacho-Dávila, A.A.; Chávez-Flores, D.; Ramos-Sánchez, V.H.; Rivera, E. Pyrene-fullerene C60 dyads as light-harvesting antennas. Molecules 2014, 19, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Vonlanthen, M.; Cevallos-Vallejo, A.; Aguilar-Ortíz, E.; Ruiu, A.; Porcu, P.; Rivera, E. Synthesis, characterization and photophysical studies of novel pyrene labeled ruthenium (II) trisbipyridine complex cored dendrimers. Polymer 2016, 99, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Cevallos-Vallejo, A.; Vonlanthen, M.; Porcu, P.; Ruiu, A.; Rivera, E. New cyclen-cored dendrimers functionalized with pyrene: Synthesis characterization, optical and photophysical properties. Tetrahedron Lett. 2017, 58, 1319–1323. [Google Scholar] [CrossRef]
- Bonnett, R.; Buckley, D.G.; Burrow, T.; Galia, A.B.B.; Seville, B.; Songca, S.P. Photobactericidal materials based on porphyrins and phthalocyanines. J. Mater. Chem. 1993, 3, 323–324. [Google Scholar] [CrossRef]
- Bozja, J.; Sherrill, J.; Michielsen, S.; Stojiljkovic, I. Porphyrin-based, light-activated antimicrobial materials. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 2297–2303. [Google Scholar] [CrossRef]
- Goldberg, I. Design strategies for supramolecular porphyrin-based materials. CrystEngComm 2002, 4, 109–116. [Google Scholar] [CrossRef]
- Suijkerbuijk, B.M.J.M.; Klein Gebbink, R.J.M. Merging Porphyrins with Organometallics: Synthesis and Applications. Angew. Chem. Int. Ed. 2008, 47, 7396–7421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senge, M.O.; Fazekas, M.; Notaras, E.G.A.; Blau, W.J.; Zawadzka, M.; Locos, O.B.; Ni Mhuircheartaigh, E.M. Nonlinear Optical Properties of Porphyrins. Adv. Mater. 2007, 19, 2737–2774. [Google Scholar] [CrossRef]
- Pawlicki, M.; Collins, H.A.; Denning, R.G.; Anderson, H.L. Two-photon absorption and the design of two-photon dyes. Angew. Chem. Int. Ed. 2009, 48, 3244–3266. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.L. Building molecular wires from the colours of life: Conjugated porphyrin oligomers. Chem. Commun. 1999, 23, 2323–2330. [Google Scholar] [CrossRef]
- Camps, X.; Dietel, E.; Hirsch, A.; Pyo, S.; Echegoyen, L.; Hackbarth, S.; Röder, B. Globular dendrimers involving a C60core and a tetraphenyl porphyrin function. Chem. A Eur. J. 1999, 5, 2362–2373. [Google Scholar] [CrossRef]
- Flamigni, L.; Talarico, A.M.; Ventura, B.; Rein, R.; Solladié, N. A versatile bis-porphyrin tweezer host for the assembly of noncovalent photoactive architectures: A photophysical characterization of the tweezers and their association with porphyrins and other guests. Chem. A Eur. J. 2006, 12, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Bell, T.D.M.; Bhosale, S.V.; Ghiggino, K.P.; Langford, S.J.; Woodward, C.P. Synthesis and photophysical properties of a conformationally flexible mixed porphyrin star-pentamer. Aust. J. Chem. 2009, 62, 692–699. [Google Scholar] [CrossRef]
- Solladie, N.; Hamel, A.; Gross, M. Synthesis of multiporphyrinic α-polypeptides: Towards the study of the migration of an excited state for the mimicking of the natural light harvesting device. Tetrahedron Lett. 2000, 41, 6075–6078. [Google Scholar] [CrossRef]
- Zhu, M.; Lu, Y.; Du, Y.; Li, J.; Wang, X.; Yang, P. Photocatalytic hydrogen evolution without an electron mediator using a porphyrin-pyrene conjugate functionalized Pt nanocomposite as a photocatalyst. Int. J. Hydrogen Energy 2011, 36, 4298–4304. [Google Scholar] [CrossRef]
- Kim, J.B.; Leonard, J.J.; Longo, F.R. Mechanistic study of the synthesis and spectral properties of meso-tetraarylporphyrins. J. Am. Chem. Soc. 1972, 94, 3986–3992. [Google Scholar] [CrossRef] [PubMed]
- Quimby, D.J.; Longo, F.R. Luminescence Studies on Several Tetraarylporphins and Their Zinc Derivatives. J. Am. Chem. Soc. 1975, 97, 5111–5117. [Google Scholar] [CrossRef]
- Berlman, I.B. Handbook of Fluorescence Spectra of Aromatic Molecules; Academic Press: New York, NY, USA, 1971; ISBN 0120926563. [Google Scholar]
- Zaragoza-Galán, G.; Fowler, M.; Rein, R.; Solladié, N.; Duhamel, J.; Rivera, E. Fluorescence resonance energy transfer in partially and fully labeled pyrene dendronized porphyrins studied with model free analysis. J. Phys. Chem. C 2014, 118, 8280–8294. [Google Scholar] [CrossRef]
- Galván-Miranda, E.K.; Zaragoza-Galán, G.; Rivera, E.; Aguilar-Martínez, M.; Macías-Ruvalcaba, N.A. Electrochemical and spectroelectrochemical study of A4and A2B2pyrene dendronized porphyrins. Electrochim. Acta 2014, 148, 266–275. [Google Scholar] [CrossRef]
- Giacalone, F.; Martín, N. Fullerene polymers: Synthesis and properties. Chem. Rev. 2006, 106, 5136–5190. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, J.; Zhang, Z.-G.; Bai, H.; Li, Y.; Zhu, D.; Zhan, X. An Electron Acceptor Challenging Fullerenes for Efficient Polymer Solar Cells. Adv. Mater. 2015, 27, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Chiang, L.Y.; Upasani, R.B.; Swirczewski, J.W. Versatile Nitronium Chemistry for C60 Fullerene Functionalization. J. Am. Chem. Soc. 1992, 114, 10154–10157. [Google Scholar] [CrossRef]
- Mikami, K.; Matsumoto, S.; Tonoi, T.; Okubo, Y.; Suenobu, T.; Fukuzumi, S. Solid state photochemistry for fullerene functionalization: Solid state photoinduced electron transfer in the diels-alder reaction with anthracenes. Tetrahedron Lett. 1998, 39, 3733–3736. [Google Scholar] [CrossRef]
- Itami, K. Molecular catalysis for fullerene functionalization. Chem. Rec. 2011, 11, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Schlueter, J.A.; Cooper, A.C.; Smart, J.L.; Whitten, M.E.; Geiser, U.; Carlson, K.D.; Williams, J.M.; Welp, U.; Dudek, J.D.; et al. Fullerene derivatives and fullerene superconductors. J. Phys. Chem. Solids 1993, 54, 1655–1666. [Google Scholar] [CrossRef]
- Ball, Z.T.; Sivula, K.; Fréchet, J.M.J. Well-defined fullerene-containing homopolymers and diblock copolymers with high fullerene content and their use for solution-phase and bulk organization. Macromolecules 2006, 39, 70–72. [Google Scholar] [CrossRef]
- Guldi, D.M.; Prato, M. Excited-state properties of C60 fullerene derivatives. Acc. Chem. Res. 2000, 33, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Semenov, K.N.; Charykov, N.A.; Keskinov, V.A.; Piartman, A.K.; Blokhin, A.A.; Kopyrin, A.A. Solubility of light fullerenes in organic solvents. J. Chem. Eng. Data 2010, 55, 13–36. [Google Scholar] [CrossRef]
- Marcus, Y.; Smith, A.L.; Korobov, M.V.; Mirakyan, A.L.; Avramenko, N.V.; Stukalin, E.B. Solubility of C 60 Fullerene. J. Phys. Chem. B 2001, 105, 2499–2506. [Google Scholar] [CrossRef]
- Backer, S.A.; Sivula, K.; Kavulak, D.F.; Frechet, J.M.J. High efficiency organic photovoltaics incorporating a new family of soluble fullerene derivatives. Chem. Mater. 2007, 19, 2927–2929. [Google Scholar] [CrossRef]
- Sharma, P.S.; Dabrowski, M.; Noworyta, K.; Huynh, T.P.; KC, C.B.; Sobczak, J.W.; Pieta, P.; D’Souza, F.; Kutner, W. Fullerene derived molecularly imprinted polymer for chemosensing of adenosine-5′-triphosphate (ATP). Anal. Chim. Acta 2014, 844, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Haino, T.; Araki, H.; Fujiwara, Y.; Tanimoto, Y.; Fukazawa, Y. Fullerene sensors based on calix[5]arene. Chem. Commun. 2002, 2, 2148–2149. [Google Scholar] [CrossRef]
- Ciotta, E.; Paoloni, S.; Richetta, M.; Prosposito, P.; Tagliatesta, P.; Lorecchio, C.; Venditti, I.; Fratoddi, I.; Casciardi, S.; Pizzoferrato, R. Sensitivity to heavy-metal ions of unfolded fullerene quantum dots. Sensors 2017, 17, 2614. [Google Scholar] [CrossRef] [PubMed]
- Nalwa, K.S.; Cai, Y.; Thoeming, A.L.; Shinar, J.; Shinar, R.; Chaudhary, S. Polythiophene-fullerene based photodetectors: Tuning of spectral esponse and application in photoluminescence based (Bio)chemical sensors. Adv. Mater. 2010, 22, 4157–4161. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Mashino, T. Biological activities of water-soluble fullerene derivatives. J. Phys. Conf. Ser. 2009, 159, 012003. [Google Scholar] [CrossRef] [Green Version]
- Bosi, S.; Da Ros, T.; Spalluto, G.; Prato, M. Fullerene derivatives: An attractive tool for biological applications. Eur. J. Med. Chem. 2003, 38, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.G.; Glass, J.I.; Lartigue, C.; Noskov, V.N.; Chuang, R.Y.; Algire, M.A.; Benders, G.A.; Montague, M.G.; Ma, L.; Moodie, M.M.; et al. Creation of a bacterial cell controlled by a chemically synthesized genome. Science 2010, 329, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.-Y.; Huang, W.-Q.; Xu, L.; Yang, Y.-C.; Li, X.; Hu, W.; Peng, P.; Huang, G.-F. Enhanced photocatalytic performance of an Ag3PO4 photocatalyst via fullerene modification: First-principles study. Phys. Chem. Chem. Phys. 2016, 18, 2878–2886. [Google Scholar] [CrossRef] [PubMed]
- Mengele, A.K.; Kaufhold, S.; Streb, C.; Rau, S. Generation of a stable supramolecular hydrogen evolving photocatalyst by alteration of the catalytic center. Dalton Trans. 2016, 45, 6612–6618. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.-C.; Ciou, J.-H.; Zheng, J.-H.; Pan, J.; Hsiao, Y.-S.; Lee, K.-C.; Huang, J.-H. Fullerene C70 decorated TiO2 nanowires for visible-light-responsive photocatalyst. Appl. Surf. Sci. 2015, 355, 536–546. [Google Scholar] [CrossRef]
- Apostolopoulou, V.; Vakros, J.; Kordulis, C.; Lycourghiotis, A. Preparation and characterization of [60] fullerene nanoparticles supported on titania used as a photocatalyst. Colloids Surf. A Physicochem. Eng. Asp. 2009, 349, 189–194. [Google Scholar] [CrossRef]
- Cominetti, A.; Pellegrino, A.; Longo, L.; Po, R.; Tacca, A.; Carbonera, C.; Salvalaggio, M.; Baldrighi, M.; Meille, S.V. Polymer solar cells based on poly(3-hexylthiophene) and fullerene: Pyrene acceptor systems. Mater. Chem. Phys. 2015, 159, 46–55. [Google Scholar] [CrossRef]
- Matsuo, Y.; Morita, K.; Nakamura, E. Penta(pyrenyl)[60]fullerenes: Pyrene-pyrene and [60]fullerene-pyrene interactions in the crystal and in solution. Chem. Asian J. 2008, 3, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Sandanayaka, A.S.D.; Araki, Y.; Ito, O.; Deviprasad, G.R.; Smith, P.M.; Rogers, L.M.; Zandler, M.E.; D’Souza, F. Photoinduced electron transfer in fullerene triads bearing pyrene and fluorene. Chem. Phys. 2006, 325, 452–460. [Google Scholar] [CrossRef]
- Hwang, Y.L.; Hwang, K.C. Nonlinear Stern-Volmer fluorescence quenching of pyrene by C60/70. Fuller Sci. Technol. 1999, 7, 437–454. [Google Scholar] [CrossRef]
- Newkome, G.R.; He, E.; Moorefield, C.N. Suprasupermolecules with Novel Properties: Metallodendrimers. Chem. Rev. 1999, 99, 1689–1746. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D.; Ruiz, J. The Redox Functions of Metallodendrimers. J. Inorg. Organomet. Polym. Mater. 2015, 25, 2–11. [Google Scholar] [CrossRef]
- Méry, D.; Astruc, D. Dendritic catalysis: Major concepts and recent progress. Coord. Chem. Rev. 2006, 250, 1965–1979. [Google Scholar] [CrossRef]
- Albrecht, M.; Hovestad, N.J.; Boersma, J.; Van Koten, G. Multiple use of soluble metallodendritic materials as catalysts and dyes. Chem. A Eur. J. 2001, 7, 1289–1294. [Google Scholar] [CrossRef]
- D’Ambruoso, G.D.; McGrath, D.V. Energy harvesting in synthetic dendrimer materials. Adv. Polym. Sci. 2008, 214, 87–147. [Google Scholar] [CrossRef]
- Balzani, V.; Campagna, S.; Denti, G.; Juris, A.; Serroni, S.; Venturi, M. Designing Dendrimers Based on Transition-Metal Complexes. Light-Harvesting Properties and Predetermined Redox Patterns. Acc. Chem. Res. 1998, 31, 26–34. [Google Scholar] [CrossRef]
- Larsen, J.; Puntoriero, F.; Pascher, T.; McClenaghan, N.; Campagna, S.; Åkesson, E.; Sundström, V. Extending the light-harvesting properties of transition-metal dendrimers. ChemPhysChem 2007, 8, 2643–2651. [Google Scholar] [CrossRef] [PubMed]
- Vögtle, F.; Plevoets, M.; Nieger, M.; Azzellini, G.C.; Credi, A.; De Cola, L.; De Marchis, V.; Venturi, M.; Balzani, V. Dendrimers with a photoactive and redox-active [Ru(bpy)3]2+-type core: Photophysical properties, electrochemical behavior, and excited-state electron-transfer reactions. J. Am. Chem. Soc. 1999, 121, 6290–6298. [Google Scholar] [CrossRef]
- Issberner, J.; Vogtle, F.; De Cola, L.; Balzani, V. Dendritic bipyridine ligands and their tris(bipyridine)ruthenium(II) chelates—Syntheses, absorption spectra, and photophysical properties. Chem. A Eur. J. 1997, 3, 706–712. [Google Scholar] [CrossRef]
- Kimura, M.; Shiba, T.; Muto, T.; Hanabusa, K.; Shirai, H. Energy transfer within ruthenium-cored rigid metallodendrimers. Tetrahedron Lett. 2000, 41, 6809–6813. [Google Scholar] [CrossRef]
- Tyson, D.S.; Luman, C.R.; Castellano, F.N. Photodriven electron and energy transfer from a light-harvesting metallodendrimer. Inorg. Chem. 2002, 41, 3578–3586. [Google Scholar] [CrossRef] [PubMed]
- Juris, A.; Balzani, V.; Barigelletti, F.; Campagna, S.; Belser, P.; von Zelewsky, A. Ru(II) polypyridine complexes: Photophysics, photochemistry, eletrochemistry, and chemiluminescence. Coord. Chem. Rev. 1988, 84, 85–277. [Google Scholar] [CrossRef]
- Crosby, G.A. Spectroscopic Investigations of Excited States of Transition-Metal Complexes. Acc. Chem. Res. 1975, 8, 231–238. [Google Scholar] [CrossRef]
- Crosby, G.A.; Elfring, W.H. Excited states of mixed ligand chelates of ruthenium(II) and rhodium(III). J. Phys. Chem. 1976, 80, 2206–2211. [Google Scholar] [CrossRef]
- Tyson, D.S.; Gryczynski, I.; Castellano, F.N. Long-Range Resonance Energy Transfer to [Ru(bpy)3]2+. J. Phys. Chem. A 2000, 104, 2919–2924. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Klein, C.; Liska, P.; Grätzel, M. Synthesis of novel ruthenium sensitizers and their application in dye-sensitized solar cells. Coord. Chem. Rev. 2005, 249, 1460–1467. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- Howarth, A.J.; Majewski, M.B.; Wolf, M.O. Photophysical properties and applications of coordination complexes incorporating pyrene. Coord. Chem. Rev. 2015, 282–283, 139–149. [Google Scholar] [CrossRef]
- Balzani, V.; Juris, A. Photochemistry and photophysics of Ru(II)-polypyridine complexes in the Bologna group. From early studies to recent developments. Coord. Chem. Rev. 2001, 211, 97–115. [Google Scholar] [CrossRef]
- Le Goff, A.; Gorgy, K.; Holzinger, M.; Haddad, R.; Zimmerman, M.; Cosnier, S. Tris(bispyrene-bipyridine)iron(II): A supramolecular bridge for the biofunctionalization of carbon nanotubes via π-stacking and pyrene/β-cyclodextrin host-guest interactions. Chem. A Eur. J. 2011, 17, 10216–10221. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.J. Cyclic Polyethers and Their Complexes with Metal Salts. J. Am. Chem. Soc. 1967, 89, 7017–7036. [Google Scholar] [CrossRef]
- Delgado, R.; Félix, V.; Lima, L.M.P.; Price, D.W. Metal complexes of cyclen and cyclam derivatives useful for medical applications: A discussion based on thermodynamic stability constants and structural data. Dalton Trans. 2007, 0, 2734–2745. [Google Scholar] [CrossRef] [PubMed]
- Austin, C.A.; Chen, Y.; Rodgers, M.T. Alkali metal cation-cyclen complexes: Effects of alkali metal cation size on the structure and binding energy. Int. J. Mass Spectrom. 2012, 330–332, 27–34. [Google Scholar] [CrossRef]
- Gunnlaugsson, T.; Leonard, J.P.; Mulready, S.; Nieuwenhuyzen, M. Three step vs one pot synthesis and X-ray crystallographic investigation of heptadentate triamide cyclen (1,4,7,10-tetraazacyclododecane) based ligands and some of their lanthanide ion complexes. Tetrahedron 2004, 60, 105–113. [Google Scholar] [CrossRef]
- Aime, S.; Botta, M.; Garda, Z.; Kucera, B.E.; Tircso, G.; Young, V.G.; Woods, M. Properties, solution state behavior, and crystal structures of chelates of DOTMA. Inorg. Chem. 2011, 50, 7955–7965. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yoon, J.; Spring, D.R. Fluorescent chemosensors for Zn2+. Chem. Soc. Rev. 2010, 39, 1996–2006. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, M.; Katayev, E.; Stadlbauer, S.; Nonaka, H.; Ojida, A.; Hamachi, I.; König, B. Rigid luminescent bis-zinc(II)-bis-cyclen complexes for the detection of phosphate anions and non-covalent protein labeling in aqueous solution. Eur. J. Org. Chem. 2011, 2011, 2807–2817. [Google Scholar] [CrossRef]
- Plush, S.E.; Gunnlaugsson, T. Luminescent sensing of dicarboxylates in water by a bismacrocyclic dinuclear Eu(III) conjugate. Org. Lett. 2007, 9, 1919–1922. [Google Scholar] [CrossRef] [PubMed]
- Gunnlaugsson, T.; Leonard, J.P. Responsive lanthanide luminescent cyclen complexes: From switching/sensing to supramolecular architectures. Chem. Commun. 2005, 0, 3114–3131. [Google Scholar] [CrossRef] [PubMed]
- Kimura, E.; Aoki, S.; Koike, T.; Shiro, M. A tris(Zn(II)-1,4,7,10-tetraazacyclododecane) complex as a new receptor for phosphate dianions in aqueous solution. J. Am. Chem. Soc. 1997, 119, 3068–3076. [Google Scholar] [CrossRef]
- Borbas, K.E.; Bruce, J.I. Synthesis of asymmetrically substituted cyclen-based ligands for the controlled sensitisation of lanthanides. Org. Biomol. Chem. 2007, 5, 2274–2282. [Google Scholar] [CrossRef] [PubMed]
- Pillai, Z.S.; Ceroni, P.; Kubeil, M.; Heldt, J.M.; Stephan, H.; Bergamini, G. Dendrimers as Nd3+ ligands: Effect of generation on the efficiency of the sensitized lanthanide emission. Chem. Asian J. 2013, 8, 771–777. [Google Scholar] [CrossRef] [PubMed]
- González Ortega, J.C. Síntesis Y Caracterización De Nuevos Sistemas Dendriticos Que Contienen Grupos Donador-Aceptor Pireno-Cicleno Metalado: Estudio De Las Propiedades Ópticas Y Fotofisicas. Master’s Thesis, Universidad Nacional Autonoma De Mexico, Mexico City, Mexico, 2017. [Google Scholar]

 ) Por-(Py2G1)4, (green
) Por-(Py2G1)4, (green  )Por-(Py2G1)3, (red
)Por-(Py2G1)3, (red  ) Por-(Py2G1)2, and (blue
) Por-(Py2G1)2, and (blue  ) Por-(Py2G1)1; (B) (black
) Por-(Py2G1)1; (B) (black  ) Zn-Por-(Py2G1)4 and (red
) Zn-Por-(Py2G1)4 and (red  ) Zn-Por-(Py2G1)2; (C) (black
) Zn-Por-(Py2G1)2; (C) (black  ) Py4-TMEG2 and (red
) Py4-TMEG2 and (red  ) Py2-TMEG1; (D) (black
) Py2-TMEG1; (D) (black  ) Zn-Py4-TMEG2 and (red
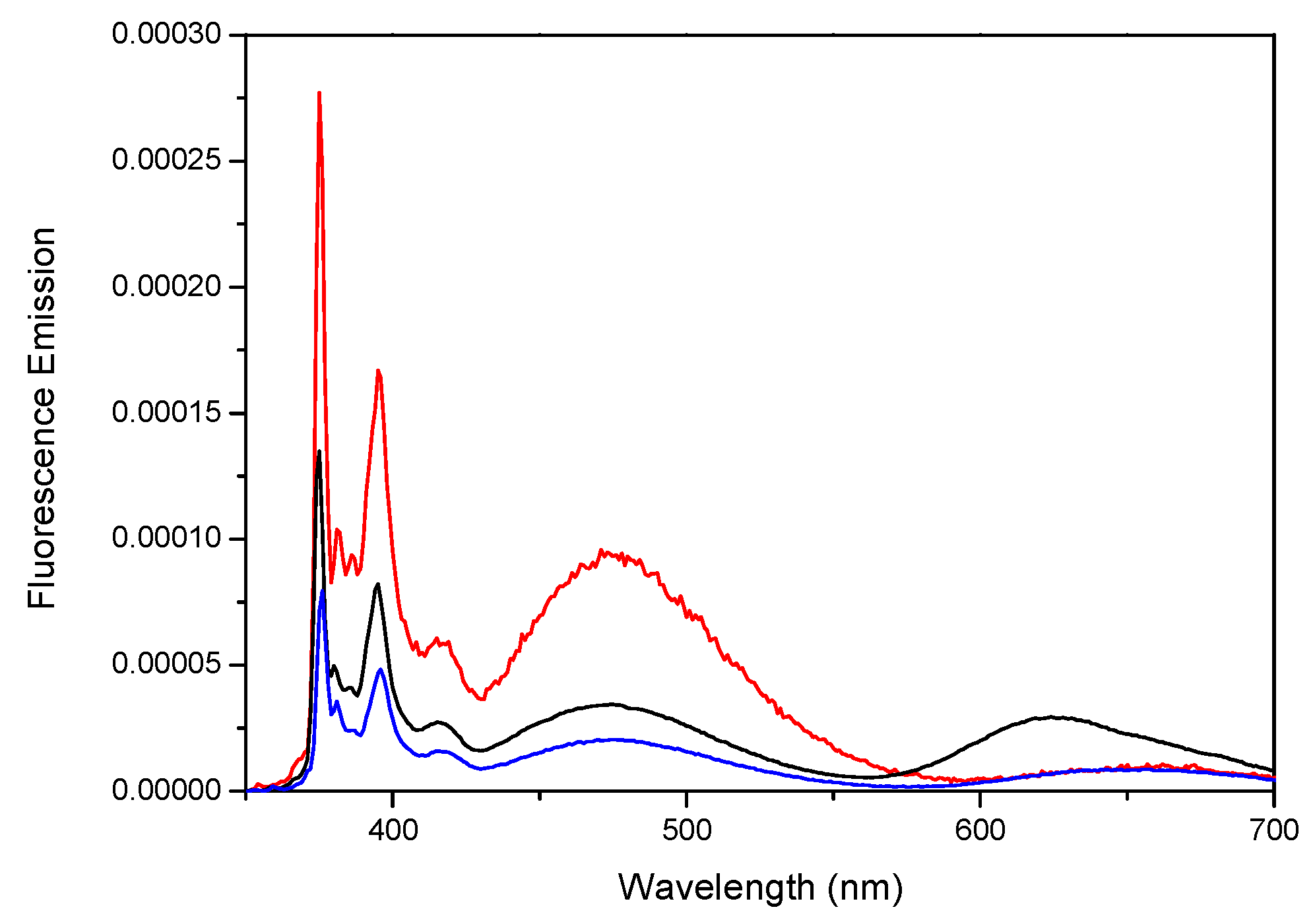
) Zn-Py4-TMEG2 and (red  ) Zn-Py2-TMEG1; (E,F) plot of Abs(344 nm)/Abs(Soret) versus the number of pyrenyl units per porphyrinic construct for the free and metallated porphyrins, respectively. Reprinted with permission from (Zaragoza-Galán, G., Fowler, M., Rein, R., Solladié, N., Duhamel, J., & Rivera, E. (2014). Fluorescence Resonance Energy Transfer in Partially and Fully Labelled Pyrene Dendronized Porphyrins Studied with Model Free Analysis. The Journal of Physical Chemistry C, 118(16), 8280–8294). Copyright (2014) American Chemical Society [66].
) Zn-Py2-TMEG1; (E,F) plot of Abs(344 nm)/Abs(Soret) versus the number of pyrenyl units per porphyrinic construct for the free and metallated porphyrins, respectively. Reprinted with permission from (Zaragoza-Galán, G., Fowler, M., Rein, R., Solladié, N., Duhamel, J., & Rivera, E. (2014). Fluorescence Resonance Energy Transfer in Partially and Fully Labelled Pyrene Dendronized Porphyrins Studied with Model Free Analysis. The Journal of Physical Chemistry C, 118(16), 8280–8294). Copyright (2014) American Chemical Society [66].
 ) Por-(Py2G1)4, (green
) Por-(Py2G1)4, (green  )Por-(Py2G1)3, (red
)Por-(Py2G1)3, (red  ) Por-(Py2G1)2, and (blue
) Por-(Py2G1)2, and (blue  ) Por-(Py2G1)1; (B) (black
) Por-(Py2G1)1; (B) (black  ) Zn-Por-(Py2G1)4 and (red
) Zn-Por-(Py2G1)4 and (red  ) Zn-Por-(Py2G1)2; (C) (black
) Zn-Por-(Py2G1)2; (C) (black  ) Py4-TMEG2 and (red
) Py4-TMEG2 and (red  ) Py2-TMEG1; (D) (black
) Py2-TMEG1; (D) (black  ) Zn-Py4-TMEG2 and (red
) Zn-Py4-TMEG2 and (red  ) Zn-Py2-TMEG1; (E,F) plot of Abs(344 nm)/Abs(Soret) versus the number of pyrenyl units per porphyrinic construct for the free and metallated porphyrins, respectively. Reprinted with permission from (Zaragoza-Galán, G., Fowler, M., Rein, R., Solladié, N., Duhamel, J., & Rivera, E. (2014). Fluorescence Resonance Energy Transfer in Partially and Fully Labelled Pyrene Dendronized Porphyrins Studied with Model Free Analysis. The Journal of Physical Chemistry C, 118(16), 8280–8294). Copyright (2014) American Chemical Society [66].
) Zn-Py2-TMEG1; (E,F) plot of Abs(344 nm)/Abs(Soret) versus the number of pyrenyl units per porphyrinic construct for the free and metallated porphyrins, respectively. Reprinted with permission from (Zaragoza-Galán, G., Fowler, M., Rein, R., Solladié, N., Duhamel, J., & Rivera, E. (2014). Fluorescence Resonance Energy Transfer in Partially and Fully Labelled Pyrene Dendronized Porphyrins Studied with Model Free Analysis. The Journal of Physical Chemistry C, 118(16), 8280–8294). Copyright (2014) American Chemical Society [66].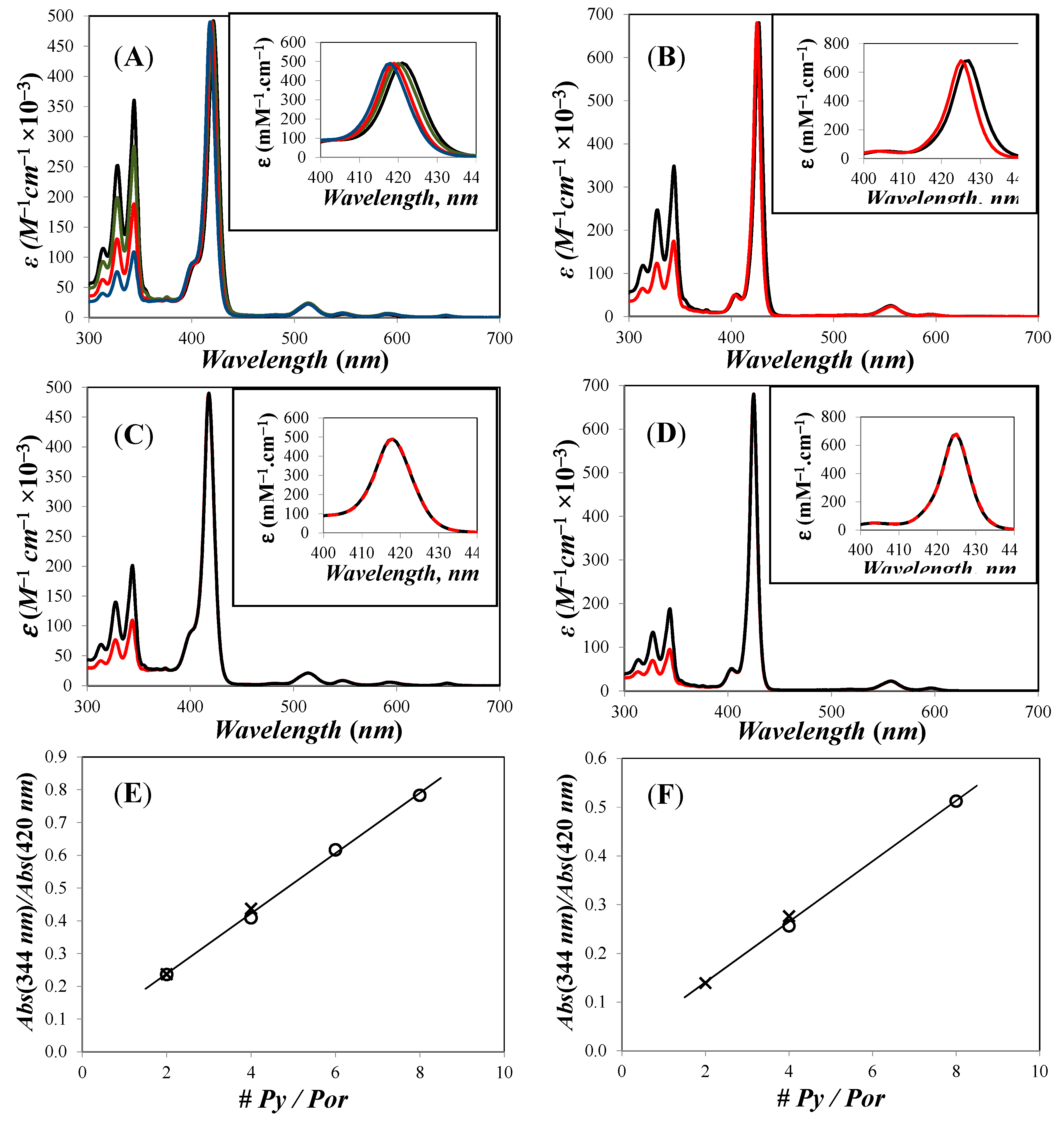
 ) Por-(Py2G1)4, (green
) Por-(Py2G1)4, (green  ) Por-(Py2G1)3, (red
) Por-(Py2G1)3, (red  ) Por-(Py2G1)2, and (blue
) Por-(Py2G1)2, and (blue  ) Por-(Py2G1)1; (B) (black
) Por-(Py2G1)1; (B) (black  ) Zn-Por-(Py2G1)4 and (red
) Zn-Por-(Py2G1)4 and (red  ) Zn-Por-(Py2G1)2; (C) (black
) Zn-Por-(Py2G1)2; (C) (black  ) Py4-TMEG2 and (red
) Py4-TMEG2 and (red  ) Py2-TMEG1; (D) (black
) Py2-TMEG1; (D) (black  ) Zn-Py4-TMEG2 and (red
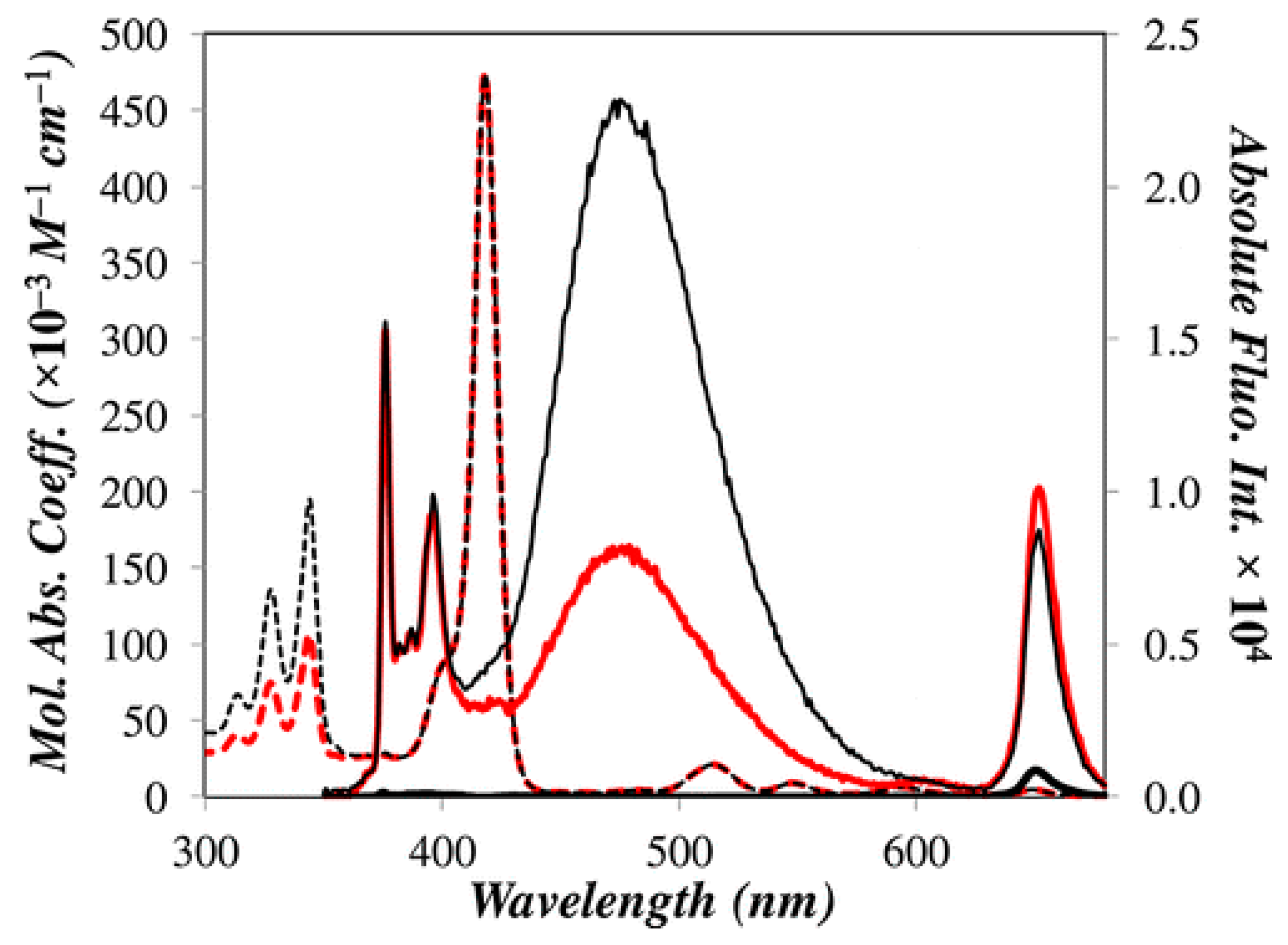
) Zn-Py4-TMEG2 and (red  ) Zn-Py2-TMEG1; The absolute fluorescence spectrum of 1-pyrenebutanol (
) Zn-Py2-TMEG1; The absolute fluorescence spectrum of 1-pyrenebutanol (  ) is shown in all figures with its intensity reported on the secondary axis on the right hand side. Reprinted with permission from (Zaragoza-Galán, G., Fowler, M., Rein, R., Solladié, N., Duhamel, J., & Rivera, E. (2014). Fluorescence Resonance Energy Transfer in Partially and Fully Labelled Pyrene Dendronized Porphyrins Studied with Model Free Analysis. The Journal of Physical Chemistry C, 118(16), 8280–8294). Copyright (2014) American Chemical Society [66].
) is shown in all figures with its intensity reported on the secondary axis on the right hand side. Reprinted with permission from (Zaragoza-Galán, G., Fowler, M., Rein, R., Solladié, N., Duhamel, J., & Rivera, E. (2014). Fluorescence Resonance Energy Transfer in Partially and Fully Labelled Pyrene Dendronized Porphyrins Studied with Model Free Analysis. The Journal of Physical Chemistry C, 118(16), 8280–8294). Copyright (2014) American Chemical Society [66].
 ) Por-(Py2G1)4, (green
) Por-(Py2G1)4, (green  ) Por-(Py2G1)3, (red
) Por-(Py2G1)3, (red  ) Por-(Py2G1)2, and (blue
) Por-(Py2G1)2, and (blue  ) Por-(Py2G1)1; (B) (black
) Por-(Py2G1)1; (B) (black  ) Zn-Por-(Py2G1)4 and (red
) Zn-Por-(Py2G1)4 and (red  ) Zn-Por-(Py2G1)2; (C) (black
) Zn-Por-(Py2G1)2; (C) (black  ) Py4-TMEG2 and (red
) Py4-TMEG2 and (red  ) Py2-TMEG1; (D) (black
) Py2-TMEG1; (D) (black  ) Zn-Py4-TMEG2 and (red
) Zn-Py4-TMEG2 and (red  ) Zn-Py2-TMEG1; The absolute fluorescence spectrum of 1-pyrenebutanol (
) Zn-Py2-TMEG1; The absolute fluorescence spectrum of 1-pyrenebutanol (  ) is shown in all figures with its intensity reported on the secondary axis on the right hand side. Reprinted with permission from (Zaragoza-Galán, G., Fowler, M., Rein, R., Solladié, N., Duhamel, J., & Rivera, E. (2014). Fluorescence Resonance Energy Transfer in Partially and Fully Labelled Pyrene Dendronized Porphyrins Studied with Model Free Analysis. The Journal of Physical Chemistry C, 118(16), 8280–8294). Copyright (2014) American Chemical Society [66].
) is shown in all figures with its intensity reported on the secondary axis on the right hand side. Reprinted with permission from (Zaragoza-Galán, G., Fowler, M., Rein, R., Solladié, N., Duhamel, J., & Rivera, E. (2014). Fluorescence Resonance Energy Transfer in Partially and Fully Labelled Pyrene Dendronized Porphyrins Studied with Model Free Analysis. The Journal of Physical Chemistry C, 118(16), 8280–8294). Copyright (2014) American Chemical Society [66].



| Compound | Quantum Yield (Φ) | Quantum Yield (Φ) | EFRET c |
|---|---|---|---|
| Pyrene Units a | Porphyrin Units b | ||
| λex = 344 nm | λex = 344 nm | ||
| PyBuOH | 0.52 | - | - |
| (±error) d | (0.03) | ||
| TME | - | 0.0015 | - |
| (±error) d | (0.0001) | ||
| Py2-G1OH | 0.63 | - | - |
| (±error) d | (0.02) | ||
| Py4-G2OH | 0.60 | - | - |
| (± error) d | (0.03) | ||
| Py2-TMEG1 | 0.008 | 0.0015 | 0.99 |
| (±error) d | (0.001) | (0.00005) | |
| Py4-TMEG2 | 0.018 | 0.0014 | 0.97 |
| (±error) d | (0.003) | (0.0001) |
| Without Zn | With Zn | |||||
|---|---|---|---|---|---|---|
| Compound | R0 (Å) | ϕPy (×104) | ϕPor (×104) | R0 (Å) | ϕPy (×104) | ϕPor (×104) |
| Por-(Py2G1)4 | 51.4 | 6 | 15 | 48.3 | 1 | 27 |
| Por-(Py2G1)3 | 51.8 | 16 | 15 | |||
| Por-(Py2G1)2 | 51.5 | 12 | 15 | 48.5 | 2 | 34 |
| Por-(Py2G1)1 | 51.8 | 25 | 15 | |||
| Py2-TMEG1 | 52.0 | 13 | 14 | 48.9 | 12 | 39 |
| Py4-TMEG2 | 52.0 | 18 | 14 | 49.0 | 23 | 38 |
| Without Zn | With Zn | |||||
|---|---|---|---|---|---|---|
| Compound | EFRET (SS) | EFRET (SPC) | dPor–PyEXT (Å) | EFRET (SS) | EFRET (SPC) | dPor–PyEXT (Å) |
| Por-(Py2G1)4 | 0.999 | 1.000 | 18.2 | 1.000 | 1.000 | 17.8 |
| Por-(Py2G1)3 | 0.997 | 1.000 | 18.2 | 17.8 | ||
| Por-(Py2G1)2 | 0.998 | 1.000 | 18.2 | 1.000 | 0.999 | 17.8 |
| Por-(Py2G1)1 | 0.995 | 1.000 | 18.2 | 17.8 | ||
| Py2-TMEG1 | 0.997 | 0.998 | 30.2 | 0.998 | 0.998 | 29.9 |
| Py4-TMEG2 | 0.998 | 0.997 | 34.9 | 0.996 | 0.996 | 34.7 |
| Compound | Relative Quantum Yield a | % Quenching |
|---|---|---|
| 1-Pyrenbutanol | 1 (Donor) | - |
| PyFC12 | 0.01 (Dyad) | 99% |
| PyFPy | 0.01 (Dyad) | 99% |
| Py2FC12 | (Dyad) | 99% |
| Py2NF | 0.04 (Dyad) | 96% |
| Compound | λmax abs (nm) ε(M−1 cm−1) | λmax (nm) | Φ Pyrene unit λex = 344 nm c | Φ Ru (II) unit λex = 344 nm d | Φ Ru (II) unit λex = 452 nm d | EFRET e |
|---|---|---|---|---|---|---|
| [Ru(Bpy)2 | 342/69’000 | 625 | - | 0.0032 a | 0.0052 a | 0.98 |
| (Bpy-Py2)]2+ | 462/10’600 | 0.008 b | 0.0174 b | 0.0277 b | ||
| [Ru(Bpy-Py2)3]2+ | 344/210’900 | 661 | - | 0.0016 a | 0.0028 a | 0.99 |
| 480/11’400 | 0.004 b | 0.0026 b | 0.0053 b | |||
| [Ru(BpyG1-Py4)3]2+ | 344/411’000 | 661 | - | 0.0025 a | 0.0029 a | 0.96 |
| 480/11’200 | 0.014 b | 0.0031 b | 0.0050 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porcu, P.; Vonlanthen, M.; Ruiu, A.; González-Méndez, I.; Rivera, E. Energy Transfer in Dendritic Systems Having Pyrene Peripheral Groups as Donors and Different Acceptor Groups. Polymers 2018, 10, 1062. https://doi.org/10.3390/polym10101062
Porcu P, Vonlanthen M, Ruiu A, González-Méndez I, Rivera E. Energy Transfer in Dendritic Systems Having Pyrene Peripheral Groups as Donors and Different Acceptor Groups. Polymers. 2018; 10(10):1062. https://doi.org/10.3390/polym10101062
Chicago/Turabian StylePorcu, Pasquale, Mireille Vonlanthen, Andrea Ruiu, Israel González-Méndez, and Ernesto Rivera. 2018. "Energy Transfer in Dendritic Systems Having Pyrene Peripheral Groups as Donors and Different Acceptor Groups" Polymers 10, no. 10: 1062. https://doi.org/10.3390/polym10101062






