Cry Protein Crystal-Immobilized Metallothioneins for Bioremediation of Heavy Metals from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Construction of the Cry3Aa-SmtA Fusion Plasmids
2.2. Expression and Purification of Cry3Aa and Cry3Aa-SmtA Fusion Crystals in Bt
2.3. Dynamic Light Scattering of Cry3Aa and Cry3Aa-SmtA Fusion Crystals
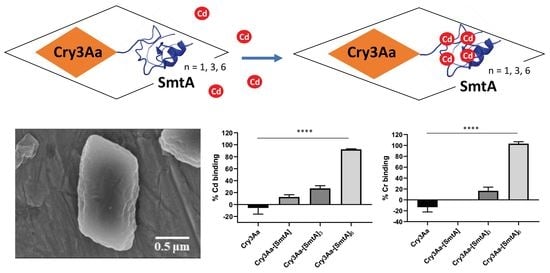
2.4. Scanning Electron Microscopy of Cry3Aa and Cry3Aa-SmtA Fusion Crystals
2.5. Metal Binding Capacity Studies by Atomic Absorption Spectrophotometer (AAS)
2.6. Statistical Analysis
3. Results
3.1. Production of Cry3Aa and Cry3Aa-SmtA Fusion Crystals
3.2. Characterization of Cry3Aa and Cry3Aa-SmtA Fusion Protein Crystals
3.3. Cadmium and Chromium Binding by Cry3Aa and Cry3Aa-SmtA Fusion Crystals
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whiteley, H.R.; Schnepf, H.E. The Molecular Biology of Parasporal Crystal Body Formation in Bacillus thuringiensis. Annu. Rev. Microbiol. 1986, 40, 549–576. [Google Scholar] [CrossRef] [PubMed]
- Schnepf, E.; Crickmore, N.; Van Rie, J.; Lereclus, D.; Baum, J.; Feitelson, J.; Zeigler, D.R.; Dean, D.H. Bacillus thuringiensis and Its Pesticidal Crystal Proteins. Microbiol. Mol. Boil. Rev. 1998, 62, 775–806. [Google Scholar]
- Park, H.-W.; Ge, B.; Bauer, L.S.; Federici, B.A. Optimization of Cry3A Yields in Bacillus thuringiensis by Use of Sporulation-Dependent Promoters in Combination with the STAB-SD mRNA Sequence. Appl. Environ. Microbiol. 1998, 64, 3932–3938. [Google Scholar] [PubMed]
- Sawaya, M.R.; Cascio, D.; Gingery, M.; Rodriguez, J.; Goldschmidt, L.; Colletier, J.-P.; Messerschmidt, M.M.; Boutet, S.; Koglin, J.E.; Williams, G.J. Protein crystal structure obtained at 2.9 Å resolution from injecting bacterial cells into an X-ray free-electron laser beam. Proc. Natl. Acad. Sci. USA 2014, 111, 12769–12774. [Google Scholar] [CrossRef]
- Shimada, N.; Miyamoto, K.; Kanda, K.; Murata, H. Bacillus thuringiensis insecticidal Cry1Ab toxin does not affect the membrane integrity of the mammalian intestinal epithelial cells: An in vitro study. In Vitro Cell. Dev. Biol.-Anim. 2006, 42, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, E.H.; Shimada, N.; Murata, H.; Mikami, O.; Sultana, P.; Miyazaki, S.; Yoshioka, M.; Yamanaka, N.; Hirai, N.; Nakajima, Y. Detection of Cry1Ab protein in gastrointestinal contents but not visceral organs of genetically modified Bt11-fed calves. Vet. Hum. Toxicol. 2003, 45, 72–75. [Google Scholar] [PubMed]
- Nair, M.S.; Lee, M.M.; Bonnegarde-Bernard, A.; Wallace, J.A.; Dean, D.H.; Ostrowski, M.C.; Burry, R.W.; Boyaka, P.N.; Chan, M.K. Cry Protein Crystals: A Novel Platform for Protein Delivery. PLoS ONE 2015, 10, e0127669. [Google Scholar] [CrossRef]
- Holmes, K.; Monro, R.; Holmes, K. Studies on the structure of parasporal inclusions from Bacillus thuringiensis. J. Mol. Boil. 1965, 14, 572-IN25. [Google Scholar] [CrossRef]
- Heater, B.S.; Lee, M.M.; Chan, M.K. Direct production of a genetically-encoded immobilized biodiesel catalyst. Sci. Rep. 2018, 8, 12783. [Google Scholar] [CrossRef] [PubMed]
- George, S.G.; Carpene, E.; Coombs, T.L.; Overnell, J.; Youngson, A. Characterisation of cadmium-binding proteins from mussels, Mytilus edulis (L), exposed to cadmium. Biochim. Biophys. Acta (BBA)-Protein Struct. 1979, 580, 225–233. [Google Scholar] [CrossRef]
- Lakherwal, D. Adsorption of heavy metals: A review. Int. J. Environ. Res. Dev. 2014, 4, 41–48. [Google Scholar]
- Srivastava, N.; Majumder, C. Novel biofiltration methods for the treatment of heavy metals from industrial wastewater. J. Hazard. Mater. 2008, 151, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Mazumder, M.J.; Al-Attas, O.; Husain, T. Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Sci. Total Environ. 2016, 569, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.E.; Olin, T.J.; Bricka, R.; Adrian, D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Demirbaş, A. Heavy metal adsorption onto agro-based waste materials: A review. J. Hazard. Mater. 2008, 157, 220–229. [Google Scholar] [CrossRef]
- Barakat, M. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Roy, D.; Greenlaw, P.N.; Shane, B.S. Adsorption of heavy metals by green algae and ground rice hulls. J. Environ. Sci. Health Part A Environ. Sci. Eng. Toxicol. 2008, 28, 37–50. [Google Scholar] [CrossRef]
- Pansini, M.; Colella, C.; De Gennaro, M. Chromium removal from water by ion exchange using zeolite. Desalination 1991, 83, 145–157. [Google Scholar] [CrossRef]
- Pérez-Candela, M.; Martín-Martínez, J.; Torregrosa-Maciá, R. Chromium(VI) removal with activated carbons. Water Res. 1995, 29, 2174–2180. [Google Scholar] [CrossRef]
- Rengaraj, S.; Yeon, K.-H.; Moon, S.-H. Removal of chromium from water and wastewater by ion exchange resins. J. Hazard. Mater. 2001, 87, 273–287. [Google Scholar] [CrossRef]
- Vašák, M. Advances in metallothionein structure and functions. J. Trace Elem. Med. Boil. 2005, 19, 13–17. [Google Scholar] [CrossRef]
- Romero-Isart, N.; Vašák, M. Advances in the structure and chemistry of metallothioneins. J. Inorg. Biochem. 2002, 88, 388–396. [Google Scholar] [CrossRef]
- Blindauer, C.A. Bacterial metallothioneins: Past, present, and questions for the future. JBIC J. Boil. Inorg. Chem. 2011, 16, 1011–1024. [Google Scholar] [CrossRef]
- Capdevila, M.; Atrian, S. Metallothionein protein evolution: A miniassay. JBIC J. Boil. Inorg. Chem. 2011, 16, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, M.; Bofill, R.; Palacios, Ò.; Atrian, S. State-of-the-art of metallothioneins at the beginning of the 21st century. Coord. Chem. Rev. 2012, 256, 46–62. [Google Scholar] [CrossRef]
- Blindauer, C.A.; Harrison, M.D.; Parkinson, J.A.; Robinson, A.K.; Cavet, J.S.; Robinson, N.J.; Sadler, P.J. A metallothionein containing a zinc finger within a four-metal cluster protects a bacterium from zinc toxicity. Proc. Natl. Acad. Sci. USA 2001, 98, 9593–9598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esser-Kahn, A.P.; Iavarone, A.T.; Francis, M.B. Metallothionein-Cross-Linked Hydrogels for the Selective Removal of Heavy Metals from Water. J. Am. Chem. Soc. 2008, 130, 15820–15822. [Google Scholar] [CrossRef] [PubMed]
- Freisinger, E.; Vašák, M. Cadmium in metallothioneins. In Cadmium: From Toxicity to Essentiality; Sigel, A., Sigel, H., Sigel, R.K., Eds.; Springer: Dordrecht, The Netherlands, 2013; Volume 11, pp. 339–371. [Google Scholar]
- Verma, N.; Singh, M. Biosensors for heavy metals. BioMetals 2005, 18, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Martín-Betancor, K.; Rodea-Palomares, I.; Muñoz-Martín, M.A.; Leganés, F.; Fernández-Piñas, F.; Palomares, I.M.R. Construction of a self-luminescent cyanobacterial bioreporter that detects a broad range of bioavailable heavy metals in aquatic environments. Front. Microbiol. 2015, 6, 186. [Google Scholar] [CrossRef] [PubMed]
- Bontidean, I.; Berggren, C.; Johansson, G.; Csöregi, E.; Mattiasson, B.; Lloyd, J.R.; Jakeman, K.J.; Brown, N.L. Detection of Heavy Metal Ions at Femtomolar Levels Using Protein-Based Biosensors. Anal. Chem. 1998, 70, 4162–4169. [Google Scholar] [CrossRef]
- Wu, C.-M.; Lin, L.-Y. Immobilization of metallothionein as a sensitive biosensor chip for the detection of metal ions by surface plasmon resonance. Biosens. Bioelectron. 2004, 20, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Gonzalezbellavista, A.; Atrian, S.; Muñoz, M.; Capdevila, M.; Fàbregas, E. Novel potentiometric sensors based on polysulfone immobilized metallothioneins as metal-ionophores. Talanta 2009, 77, 1528–1533. [Google Scholar] [CrossRef]
- Lim, A.P.; Aris, A.Z. A review on economically adsorbents on heavy metals removal in water and wastewater. Rev. Environ. Sci. Bio/Technol. 2013, 13, 163–181. [Google Scholar] [CrossRef]
- Babel, S. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]





| Construct | Length (nm) * | Width (nm) * |
|---|---|---|
| Cry3Aa | 1215 ± 197 | 912 ± 97 |
| Cry3Aa-[SmtA] | 1378 ± 86 | 871 ± 138 |
| Cry3Aa-[SmtA]3 | 1471 ± 195 | 1014 ± 151 |
| Cry3Aa-[SmtA]6 | 1405 ± 112 | 913 ± 48 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Q.; Cheng, S.W.; Cheung, K.; Lee, M.M.; Chan, M.K. Cry Protein Crystal-Immobilized Metallothioneins for Bioremediation of Heavy Metals from Water. Crystals 2019, 9, 287. https://doi.org/10.3390/cryst9060287
Sun Q, Cheng SW, Cheung K, Lee MM, Chan MK. Cry Protein Crystal-Immobilized Metallothioneins for Bioremediation of Heavy Metals from Water. Crystals. 2019; 9(6):287. https://doi.org/10.3390/cryst9060287
Chicago/Turabian StyleSun, Qian, Sze Wan Cheng, Kelton Cheung, Marianne M. Lee, and Michael K. Chan. 2019. "Cry Protein Crystal-Immobilized Metallothioneins for Bioremediation of Heavy Metals from Water" Crystals 9, no. 6: 287. https://doi.org/10.3390/cryst9060287