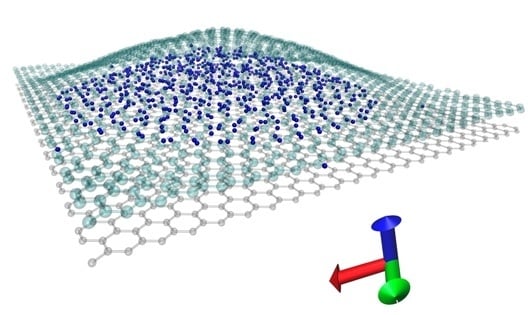
High-Density Hydrogen Storage in a 2D-Matrix from Graphene Nanoblisters: A Prospective Nanomaterial for Environmentally Friendly Technologies
Abstract
:1. Introduction
2. Methods
3. Results and Discussion
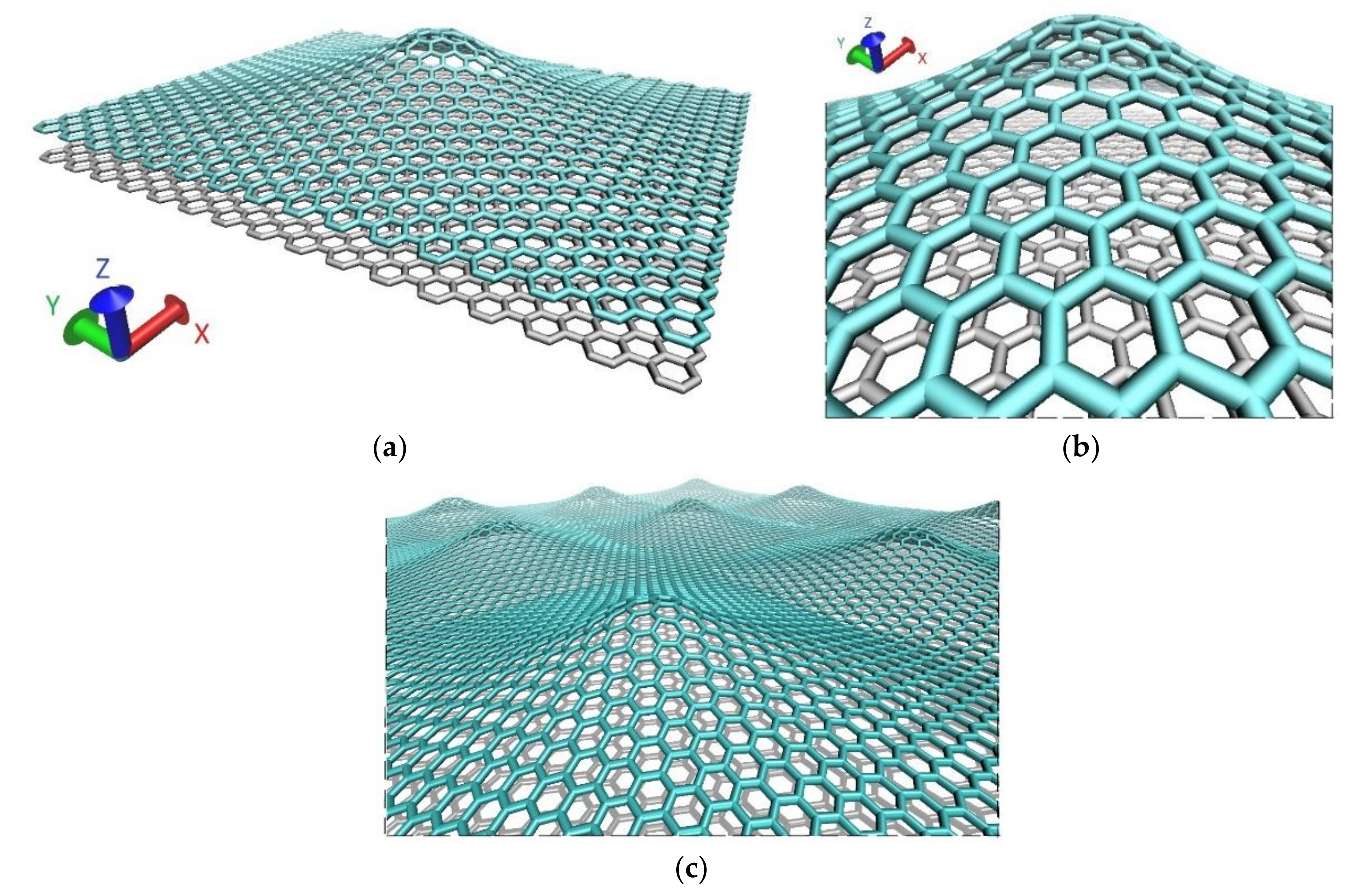
3.1. Mechanical Stability of the Atomic Network of Graphene Nanoblisters with Defects
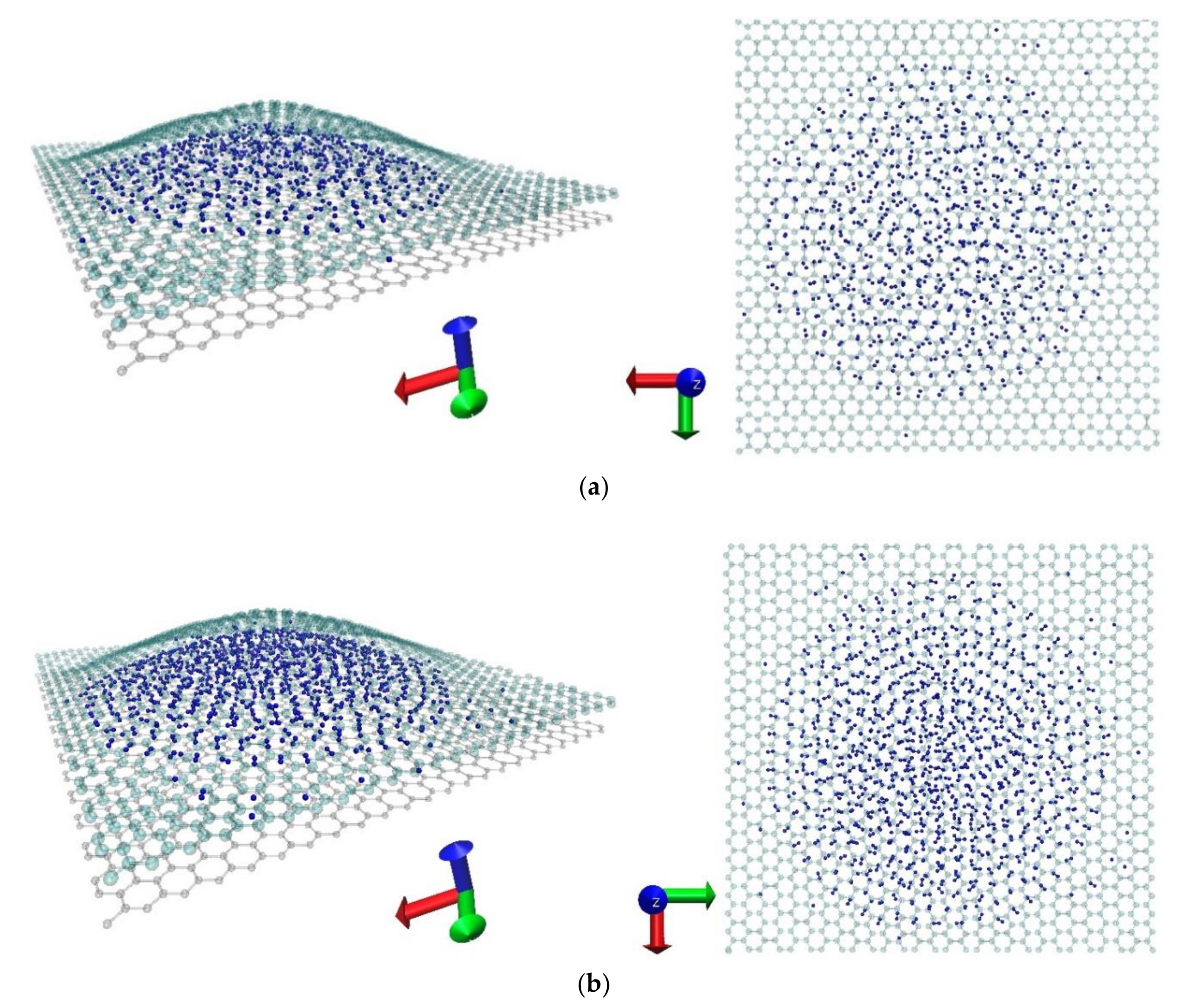
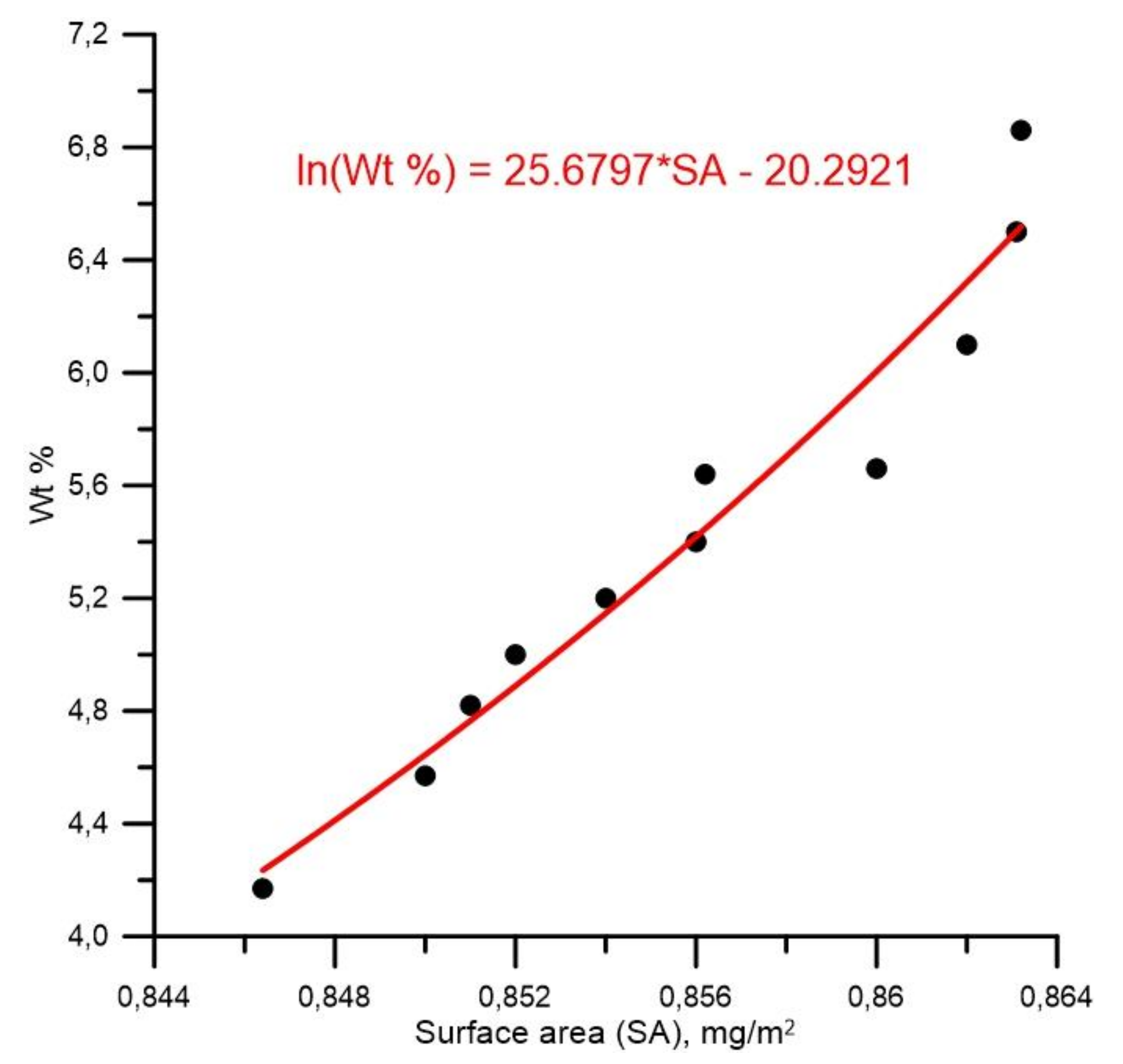
3.2. High-Density Hydrogen Storage in Graphene Nanoblisters
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nechaev, Y.S.; Veziroglu, T.N. Thermodynamic aspects of the stability of the graphene/graphane/hydrogensystems, relevance to the hydrogen on-board storage problem. Adv. Mater. Phys. Chem. 2013, 3, 255–280. [Google Scholar] [CrossRef]
- Watcharinyanon, S.; Virojanadara, C.; Osiecki, J.R.; Zakharov, A.A.; Yakimova, R.; Uhrberg, R.I.G.; Johanssona, L.I. Hydrogen intercalation of graphene grown on 6H-SiC(0001). Surf. Sci. 2011, 605, 1662–1668. [Google Scholar] [CrossRef]
- Lim, C.H.Y.X.; Sorkin, A.; Bao, Q.; Li, A.; Zhang, K.; Nesladek, M.; Loh, K.P. A hydrothermal anvil made of graphene nanobubbles on diamond. Nat. Commun. 2013, 4, 1556. [Google Scholar] [PubMed]
- Huang, R. Graphene: Show of adhesive strength. Nat. Nanotechnol. 2011, 6, 537–538. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Koenig, S.P.; Boddeti, N.G.; Dunn, M.L.; Bunch, J.S. Ultrastrong adhesion of graphene membranes. Nat. Nanotechnol. 2011, 6, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Yuk, J.M.; Park, J.; Ercius, P.; Kim, K.; Hellebusch, D.J.; Crommie, M.F.; Lee, J.Y.; Zettl, A.; Paul, A. Alivisatos High-Resolution EM of Colloidal Nanocrystal Growth Using Graphene Liquid Cells. Science 2012, 336, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Tan, J.Y.; Toh, C.T.; Koenig, S.P.; Fedorov, V.E.; Castro Neto, A.H.; Özyilmaz, B. Nanometer Thick Elastic Graphene Engine. Nano Lett. 2014, 14, 2677–2680. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.; Xu, P. Effect of initial tension on mechanics of adhered graphene blisters. Appl. Phys. A 2015, 120, 1503–1509. [Google Scholar] [CrossRef]
- Glukhova, O.; Slepchenkov, M. Influence of the curvature of deformed graphene nanoribbons on their electronic and adsorptive properties: Theoretical investigation based on the analysis of the local stress field for an atomic grid. Nanoscale 2012, 4, 3335–3344. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, T.C.; Andzelm, J.; Robbins, M.O. AIREBO-M: A reactive model for hydrocarbons at extreme pressures. J. Chem. Phys. 2015, 142, 024903. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.J.; Holian, B.L. The Nose–Hoover thermostat. J. Chem. Phys. 1985, 83, 4069. [Google Scholar] [CrossRef]
- Grubmüller, H.; Heller, H.; Windemuth, A.; Schulten, K. Generalized Verlet Algorithm for Efficient Molecular Dynamics Simulations with Long-range Interactions. Mol. Simul. 1991, 6, 121–142. [Google Scholar] [CrossRef]
- Glukhova Research Group. Available online: http://nanokvazar.ru/en/kvazar (accessed on 15 June 2017).
- DFTB+. Available online: https://www.dftbplus.org/ (accessed on 20 July 2017).
- Skowron, S.T.; Lebedeva, I.V.; Popov, A.M.; Bichoutskaia, E. Energetics of atomic scale structure changes in graphene. Chem. Soc. Rev. 2015, 44, 3143–3176. [Google Scholar] [CrossRef] [PubMed]
- Zobelli, A.; Ivanovskaya, V.; Wagner, P.; Suarez-Martinez, I.; Yaya, A.; Ewels, C.P. A comparative study of density functional and density functional tight binding calculations of defects in graphene. Phys. Status Solidi B. 2012, 249, 276–282. [Google Scholar] [CrossRef]
- Larciprete, R.; Colonna, S.; Ronci, F.; Flammini, R.; Lacovig, P.; Apostol, N.; Politano, A.; Feulner, P.; Menzel, D.; Lizzit, S. Self-Assembly of Graphene Nanoblisters Sealed to a Bare Metal Surface. Nano Lett. 2016, 16, 1808–1817. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liechti, K.M.; Huang, R. Snap transitions of pressurized graphene blisters. J. Appl. Mech. 2016, 83, 071002. [Google Scholar] [CrossRef]
- Waqar, Z. Hydrogen accumulation in graphite and etching of graphite on hydrogen desorption. J. Mater. Sci. 2007, 42, 1169–1176. [Google Scholar] [CrossRef]
- Klechikov, A.G.; Mercier, G.; Merino, P.; Blanco, S.; Merino, C.; Talyzin, A.V. Hydrogen storage in bulk graphene-related materials. Microporous Mesoporous Mater. 2015, 210, 46–51. [Google Scholar] [CrossRef]

| Type and Number of Defects | Maximum Values of Bond Length dmax, Å | Maximum Values of Local Atomic Stress σmax, GPa |
|---|---|---|
| 2V (1) | 1.67 (2V) | 1.75 |
| 2V (2) | 1.67 (2V) | 1.72 |
| 2V (3) | 1.67 (2V) | 1.67 |
| AD (1) | 1.67 (AD) | 1.73 |
| AD (2) | 1.68 (AD) | 1.72 |
| AD (3) | 1.67 (AD) | 1.71 |
| SW (1) | 1.65 (SW) | 1.82 |
| SW (2) | 1.69 (SW) | 1.90 |
| SW (3) | 1.70 (SW) | 1.91 |
| AD (2) + 2V (1) | 1.67 (AD) 1.67 (2V) | 1.68 |
| AD (2) + SW (1) | 1.68 (AD) 1.70 (SW) | 1.89 |
| 2V (2) + SW (1) | 1.71 (2V) + 1.56 (SW) | 1.93 |
| 2V (2) + AD (1) | 1.67 (2V) + 1.64 (AD) | 1.67 |
| 2V (1) + SW (1) | 1.65(SW) + 1.67 (2V) | 1.79 |
| SW (1) + 2V (1) + AD (1) | 1.65 (SW) + 1.70 (2V) + 1.68 (AD) | 1.91 |
| SW (1) + AD (1) | 1.65 (SW) + 1.68 (AD) | 1.86 |
| 2V (1) + AD (1) | 1.67 (2V) + 1.65 (AD) | 1.70 |
| SW (2) + 2V (1) | 1.64 (SW) + 1.70 (2V) | 1.86 |
| SW (2) + AD (1) | 1.65 (SW) + 1.69 (AD) | 1.79 |
| 2V (3) | 1.67 (2V) | 1.67 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slepchenkov, M.M.; Barkov, P.V.; Glukhova, O.E. High-Density Hydrogen Storage in a 2D-Matrix from Graphene Nanoblisters: A Prospective Nanomaterial for Environmentally Friendly Technologies. Crystals 2018, 8, 161. https://doi.org/10.3390/cryst8040161
Slepchenkov MM, Barkov PV, Glukhova OE. High-Density Hydrogen Storage in a 2D-Matrix from Graphene Nanoblisters: A Prospective Nanomaterial for Environmentally Friendly Technologies. Crystals. 2018; 8(4):161. https://doi.org/10.3390/cryst8040161
Chicago/Turabian StyleSlepchenkov, Michael M., Pavel V. Barkov, and Olga E. Glukhova. 2018. "High-Density Hydrogen Storage in a 2D-Matrix from Graphene Nanoblisters: A Prospective Nanomaterial for Environmentally Friendly Technologies" Crystals 8, no. 4: 161. https://doi.org/10.3390/cryst8040161